Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Development of catalyst complexes for upgrading biomass into ester-based biolubricants for automotive applications: a review
Md. Anwar Hossainab,
Mohammad Anwar Mohamed Iqbalc,
Nurhidayatullaili Muhd Julkaplia,
Pei San Kongde,
Juan Joon Chinga and
Hwei Voon Lee *a
*a
aNanotechnology & Catalysis Research Centre (NANOCAT), Institute of Postgraduate Studies, Universiti Malaya, 50603 Kuala Lumpur, Malaysia. E-mail: leehweivoon@um.edu.my; Fax: +603-7957-6956; Tel: +603-7967-6954
bDepartment of Chemistry, Rajshahi University of Engineering & Technology, Rajshahi 6204, Bangladesh
cSchool of Chemical Sciences, Universiti Sains Malaysia, 11800 Penang, Malaysia
dDepartment of Chemical Engineering, Faculty of Engineering, Universiti Malaya, 50603 Kuala Lumpur, Malaysia
eLaboratoire de Génie Chimique (Labège), BP84234 Campus INP-ENSIACET, 4 allée Emile Monso, 31432 Toulouse Cedex 4, France
First published on 1st February 2018
Abstract
Biomass-derived oils are recognised as the most promising renewable resources for the production of ester-based biolubricants due to their biodegradable, non-toxic and metal adhering properties. Homogeneous acid catalysts have been conventionally used in catalytic esterification and transesterification for the synthesis of ester-based biolubricants. Although homogeneous acid catalysts encounter difficulty during phase separation, they exhibit superior selectivity and good stereochemistry and regiochemistry control in the reaction. Consequently, transition metal complex catalysts (also known as homogeneous organometallic catalysts) are proposed for biolubricant synthesis in order to achieve a higher selectivity and conversion. Herein, the potential of both homogeneous transition metal complexes and heterogeneous supported metal complexes towards the synthesis of biolubricants, particularly, in esterification and transesterification, as well as the upgrading process, including hydrogenation and in situ hydrogenation–esterification, is critically reviewed.
1 Introduction
Automotive-type lubricants are utilised to reduce friction and to moderate the heat produced when two surfaces are in mutual contact.1 The lubricants can form a thin film between the two sliding parts, which generates a slippery surface for the metal parts to move easily.2 Generally, lubrication can prolong the wear and tear duration of machinery and significantly reduce the surface deformation of the parts. Besides acting as a coolant for the sliding surfaces, lubricants also act as a transport medium for foreign particles and forces on the parts of machinery.Generally, petroleum-based oils or mineral oils have been used as lubricants in automobiles and machinery for several decades. The continuous depletion of mineral oils has raised the attention of scientists towards the utilisation of renewable biomass-based lubricants.3 It has been widely reported that the petroleum-based oils can lead to serious groundwater pollution,4 soil pollution, surface water contamination, air pollution, and agricultural material and food contamination.5 Moreover, gaseous CO, CO2, NOx and SOx are liberated together with nanoparticle traced metals (Hg, Ca, P, Zn, Mg, and Fe) during the combustion of mineral oil, which results in a negative impact on the environment.6 It is reported that mineral oil is carcinogenic as continuous inhalation of this lubricant emission may cause inflammatory and analgesic effects on the human respiratory system.7,8 In addition, by-products derived from the degradation of the lubricants are toxic and can lead to soil infertility.
Ester-based biolubricants are greener, renewable, non-toxic and emit zero greenhouse gas.9 They can be used as additives for anti-oxidants, viscosity index promoters, pour point mitigation, detergents and emulsion stabilisers. For industrial application, these biolubricants are suitable to be applied for complete fluid lubrication, boundary lubrication, and extreme pressure lubrication.
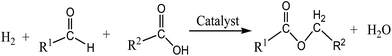
Notably, bioester-based lubricants are conventionally synthesised by reacting long-chain fatty acids (derived from hydrolysis of plant oil) with polyols (e.g. trimethylolpropane, neopentyl glycol, pentaerythritol) in the presence of liquid acid or Lewis acid catalysts by esterification. In contrast, the reaction between triglyceride-based feedstock (plant oil or animal fat) and alcohols/polyols occurs via the transesterification process in the presence of acid or base catalyst.10–12 Recently, a new technology has been developed to upgrade the performance of ester-based biolubricants (especially in oxidation stability) via hydrogenation, and/or in situ hydrogenation–esterification in which molecular hydrogen is used at a high temperature and pressure in the presence of homogeneous or heterogeneous catalysts. These types of reactions aim to reduce the unsaturated bonding presence in the bioester into a saturated product for better stability of the product under extreme conditions and long-term storage.
Recent development focused on the production of various characteristics of biolubricant products has provided potential substitutes to mineral-based lubricants for automotive applications.13,14 Nevertheless, to the best of our knowledge, there is a lack of comprehensive study on the usage of homogeneous transition complex catalysts in biolubricant synthesis.15,16 As derived from the literature, a homogeneous-type transition metal complex catalyst is a potential catalyst for ester production, mainly due to its simple preparation step, large surface area, good molecular dispersion between reactants, and sufficient pore volume.17 In contrast, the heterogeneous-type metal complex catalyst is scarce in the catalytic synthesis of biolubricants when long-chain/polyhydric alcohols are employed in the reaction due to the occurrence of undesirable side products, low selectivity and soap formation in the process.18 Consequently, the catalytic activity of homogeneous transition metal complexes and the supported metal complexes (heterogeneous) towards the production of biolubricants is critically reviewed in this study. In addition, this review outlines the future prospects of and global demand for biolubricants.
1.1 Global demand for lubricant usage
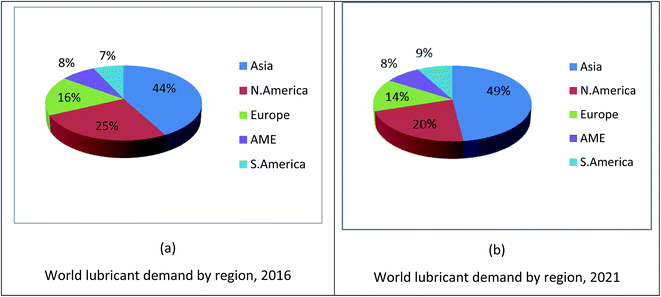
The global demand for lubricants is expected to increase 2.0% per year to 45.40 million metric tons by 2020.6 This growth is projected to increase as the demand for engine oils for new motor vehicles increases. The fastest demand is projected for the Asia and Pacific regions, where number of motor vehicles and industrialization are increasing. The demand for lubricants in Central and South America and the Middle East/Africa region is also expected to increase due to the increasing number of manufacturing units, rising motor vehicle manufacturing and proprietary rates. Fig. 1 shows the world demand of lubricant usage in 2016 (a) and world lubricant demand forecast for the year 2021 (b).19,20 According to a published report by Kline & Company, the collected data in 2016 indicated that the demand for lubricants is increasing in Asia and South America (S. America), whereas, it is decreasing in North America (N. America) and Europe and is constant in Africa/Middle East (AME). The reasons are the strong economic growth and increase in vehicle ownership rate and industrialisation, which result in an increase in demand for lubricants. Besides, another analysis reported by RPS Energy (a multinational energy and environmental consultancy company) stated that the demand of lubricants is increasing continuously in the Asian region due to economic prospects, vehicle ownership and large-scale GDP growth. As GDP growth per capita income is rising in Asia and Middle East/Africa regions compared to that in North America and Europe, there is an increase in vehicle ownership rate, thus leading to an increase in lubricant demand (Table 1).19,20| Asia Pacific | Middle East & Africa | North America | Europe | ||||
|---|---|---|---|---|---|---|---|
| GDP growth (avg) | 4.0% | GDP growth (avg) | 4.0% | GDP growth (avg) | 2.4% | GDP growth (avg) | 1.7% |
| Per capita (avg) | 3.6% | Per capita (avg) | 2.3% | Per capita (avg) | 1.6% | Per capita (avg) | 1.5% |
| Lubes growth | 1–2% | Lubes growth | 0.5–2% | Lubes growth | (1%)–1% | Lubes growth | (1%)–0.5% |
1.2 Type of automotive lubricants
Besides providing a slippery surface for the moving parts, a good automotive lubricant must be able to remain in the liquid state within a wide range of temperatures. For instance, the lubricant oil should have a high boiling temperature with low freezing points, so that it can stay on the metallic surface in the liquid form for long periods. Indeed, the viscosity index of lubricant oils is the most important property to determine it's application in automotive usage such as resistant to flow, adherence on the surfaces, and changes in temperature and pressure. Thermal and oxidation stability indicates the stability of the lubricating oils at higher temperatures and pressures. Tables 2 and 3 show the categories for different types of automotive lubricants that are measured by various factors.| Sources | Category of base oils | Sulphur (%) | Saturation (%) | Viscosity index |
|---|---|---|---|---|
| a Synthetic oils have high a viscosity index (130–210) and high saturation (>92%) and contain a smaller amount of sulphur (0.003 wt%).26 | ||||
| Categories of base oils, API | ||||
| Mineral | Group-I base oils are prepared through solvent extraction, hydro-finishing and catalytic dewaxing processes at temperature ranges of 305–423 °C | Greater than 0.03 | Less than 90 | 80–120 |
| Group-II (hydrotreated) base oils are highly stable and have better anti-oxidation properties, since all the carbon molecules are highly saturated | Less than 0.03 | Greater than 90 | 80–120 | |
| Group-III (hydrocracked) base oils are synthesized by special processes called isohydromerization and are severely hydrocracked at elevated temperature and pressure | Less than 0.03 | Greater than 90 | Greater than 120 | |
| Synthetica | Group-IV base oils are prepared by a reaction called manufacturing or synthesizing. These oils can be used in a wide range of temperatures such as in crucial cold conditions, extreme heat applications and also suitable under extreme pressure | PAO synthetic lubricants | ||
| Group-V base oils including silicone, polyolester, phosphate ester, biolubes and polyalkylene glycol. Currently, these oils are mixed with other base stocks for improving the oil's performance | All other base oils rather than groups I, II, III, or IV | |||
| Physical appearance | |
| Solid | Lubricants form a thin film of material on the metal surface. They comprise organic or inorganic compounds, for example, graphite, molybdenum disulphide and cadmium disulphide |
| Semi-solid | The liquid is dissolved in solid and sometimes additives are added, for example, grease |
| Liquid | The examples of liquid oils are as follows: vegetable oils, petroleum oils, and synthetic and animal oils |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Resources of base oil | |
| Natural sources | These base oils are obtained from vegetable oils and/or animal fats and are called natural oils |
| Refined sources | Oils obtained from crude petroleum sources; examples are paraffinic oils and aromatic and naphthenic oils |
| Synthetic oils sources | They are highly synthesized as final reaction products, such as synthetic esters, silicones, and polyalphaolefins |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| On the basis of application | |
| Automotive oils/fluids | These oils are commonly used for automobile application and transportation sectors, e.g., engine oils, gear-box oils, transmission fluids, and hydraulic brake fluids |
| Industrial instrument oils | They are used for industrial goals such as compressor fluids, machine oils, hydraulic fluids, and metal-working oils |
| Special fluids | These fluids are used in special cases according to definite applications such as white fluids, process fluids, and instrumental fluids |
2 Biolubricants
Biolubricants are generally obtained from biomass or naturally occurring sources like soybean, palm, sunflower, coconut, and rapeseed oils.16 A biolubricant is an ester of glycerides, which is still limited for application as a lubricant as their freezing point is quite high, and they are easily oxidised. Biolubricants can be prepared using long-chain alcohols/polyols with fatty acids that fulfil the toxicity and biodegradability criteria (refer to Table 4). Typically, biolubricants are biodegradable and release less carbon and greenhouse gases compared to petroleum-based lubricants. As a result, biolubricants evaporate slowly compared to petroleum oil and adhere to metal surfaces strongly even under extreme conditions for a long time. A biolubricant's adhering properties on metal surfaces and performance can also be increased by additives and can be used under abnormal conditions. For instance, fatty acid such as oleic acid, linoleic acid, butanoic acid, levulinic acid and palmitic acid react with long-chain/polyhydric alcohols forming mono-, di-, triester-based biolubricants. In general, conventional biolubricant oils found in the market are derived from biomass, such as castor oil, olive oil, coconut oil, sperm oil, rapeseed oil, rosin oil, palm oil, neatsfoot oil, and seal and whale oils. Commonly, mineral oil is blended with biolubricant base oil to increase the lube performance.| Polyol | Fatty acids | Viscosity index | Pour point (°C) | Flash point (°C) | Oxidation stability | Biodegradability (%) |
|---|---|---|---|---|---|---|
| NPG | Oleic acid | 207 | −24 | 272 | 175 | 98 |
| Acetic acid | 135 | −22 | 275 | 181 | 97 | |
| TMP | Oleic acid | 190 | −39 | 289 | 189 | 95 |
| Levulinic acid | 150 | −25 | 280 | 184 | 99 | |
| Caprylic acid | 114 | −45 | 285 | 178 | 94 | |
| PE | Oleic acid | 141 | −21 | >300 | 177 | 98 |
| Gly | Oleic acid | 180 | −28 | 278 | 176 | 96 |
| Butanoic acid | 160 | −25 | 275 | 179 | 98 |
2.1 Synthesis of biolubricants
2.2 Properties of synthetic biolubricants
2.3 Industrial application of biolubricants
Ester-based biolubricants have been used as alternative lubricants in industries and automotives as they reduce friction and wear and operating noise and improve heat transfer. They have a longer adherence period on metal surfaces, as they have polar-based chemical structures, whereas petroleum-based lubricants are non-polar hydrocarbons and do not exhibit adhering properties on metal surfaces (Fig. 2). The application of biolubricant products in automotive industry are including hydraulic fluids, metal working oils, two-stroke and three stroke engine oils,37 and chainsaw fluids.38 In addition, biolubricants are extensively used as engine oils, transmission fluids, gear box oils, and brake and hydraulic fluids and are able to provide a slippery surface, reduce metallic corrosion and enhance performance of the machinery (Table 5).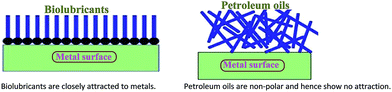 | ||
| Fig. 2 Bio-based lubricants possess a polar attraction to metals; by contrast, petroleum-based fluids have no polarity and no affinity to metals. | ||
| Maintenance areas | Specific applications |
|---|---|
| In automotives | Engine oils, brake fluids, gear oils, gasoline engine oils, greases |
| In aviation | Turbine fluids, hydraulic fluids, piston engine fluids, lubricating greases |
| In industry | Gas turbine fluids, hydraulic oils, circulation and bearing oils, air compressor fluids, gas compressor oils, metal working fluids, lubricating greases and heat transfer oils |
2.4 Advantages of biolubricants
The benefits of biolubricants are that they produce a cleaner, safer, less toxic environment and cause fewer skin problems for those who are working with engines and hydraulic systems. The physical and chemical properties of biolubricants are safer owing to their higher flash point, low pour point, constant viscosity, and less vapor emissions and oil mist.41 Moreover, they produce a low emission due to the high range of boiling temperature of esters. They can reduce pollution in stormwater from leaks in engines, hydraulic systems, and brake lines. These biolubricants are highly biodegradable42 and their cost is less over the product's life-cycle, as less maintenance43 and storage and disposal systems are needed. However, they are 3–5 times more expensive than petroleum oils (Table 6).44| Properties | Standard test method | Biolubricant | Petroleum oil |
|---|---|---|---|
| Density at 20 °C (kg m−3) | ASTM D445-15a | 930–950 | 880 |
| Viscosity index (VI) | ASTM D 445 | 150–200 | 100 |
| Pour point, °C | ASTM D97-12 | −20 | −15 |
| Flash point | ASTM D92-12b | Good | Poor |
| Cold flow behaviour | ASTM D5949 | Good | Poor |
| Oiliness | ASTM D6079 | Good | Poor |
| Miscibility with petroleum oils | ASTM 17025 | Good | Poor |
| Oxidation stability | ASTM D2440 | Moderate | Good |
| Biodegradability | EN 45000 | Good | Poor |
| Sludge forming affinity | ASTM D2070 | Poor | Good |
| Price, Euro per L | — | 3–5 | 1 |
3 Catalytic reaction for synthesis of biolubricants
The homogeneous catalysts are molecularly dispersed in the reacting fluids; hence, pore diffusion limitations are absent and bulk phase mass transfer limitations usually occur. Homogenous catalytic reactions are used not only for the biolubricant production46,47 but also for many conventional organic transformation reactions (Table 7).| Type of catalyst | Advantages | Disadvantages |
|---|---|---|
| Homogeneous catalyst | ||
| Acid catalyst | ||
| Liquid acid, e.g. H2SO4, HCl, H3PO4 | Easily react with fatty acids and alcohols, forming ester based-biolubricants | Product neutralization and separation problem due to corrosive nature of these acids |
| Metal salt (Lewis acid), e.g. Sn, Cu, Co, Ni salts | Easily react with feedstock and alcohols in solution, giving better yield | Catalyst separation from product is difficult due to high solubility in the solution |
| Ligand metal complex (Lewis acid), e.g. Sn, Co, Ni, Fe, Ru complexes | Molecularly dispersed in the reacting fluids, giving better yield with high product selectivity. Hence, reaction mechanism and kinetics are easy to understand | Catalyst separation is difficult and requires high technology, which is sometimes expensive |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Base catalyst | ||
| Schiff base complex (Lewis base), e.g. Co, Ni, Cd, Zn complexes | Transesterification and hydrogenation reactions are influenced by these catalysts | As they are a little basic and soluble in reacting fluids, they are used in the presence of another base |
The transition metal complexes (present in homogeneous form) are acidic and thus potentially catalyse many organic reactions (e.g. esterification, transesterification, and ester hydrolysis). Transition metal complexes are potentially applied as acid catalysts for ester-based lubricant synthesis. It exhibits properties of chemoselectivity, regioselectivity,48 and enantioselectivity in which the reaction mechanism and kinetics are easy to understand. On the other hand, heterogenous catalyst are poorly defined in the reacting fluids, and their synthesis problem and reaction mechanism are rarely understood. In case of heterogeneous catalysts, the reaction mixture is not well dispersed because of their larger particle, so a low yield with low selectivity is usually observed. In this context, as a homogeneous catalyst is well dispersed within the reacting fluid, it is chosen for the production of biolubricants. In fact, transition metal complexes in the homogeneous form are reviewed for further study. Table 13 briefly illustrates these catalysts.
3.1 Catalytic esterification reaction
Ester-based biolubricants are generally prepared by the esterification reaction between long-chain carboxylic acids (C4–C14) (e.g. butanoic acid, pentanoic acid, levulinic acid, oleic acid and linoleic acid)49 with polyols including glycerol (Gly), neopentyl glycol (NPG), trimethylolpropane (TMP), pentaerythritol (PE) and n-octanol, and n-butanol in the presence of liquid acid (e.g. H2SO4, HCl, HNO3) or Lewis acid catalysts (e.g. metal salts or transition metal complexes). Basically, the liquid acid catalyst used for the esterification reaction causes major corrosion of the reacting system and produces a salt as a result of the neutralisation reaction with base chemicals. Besides, additional cost is required for product washing and water separation from the final liquid ester product.50 In this part, a comparative study on the homogeneous51 and heterogeneous52 catalysed esterification reactions are reviewed herein.Noor et al.53 studied the catalytic performance of perchloric acid, sulfuric acid, hydrochloric acid, and nitric acid (liquid acid) for biolubricant production. The process involved the esterification between Jatropha curcas oil with trimethylolpropane (TMP) at 150 °C, reaction time of 3 h and molar ratio of FA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMP was 4
TMP was 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with 2% w/w concentrated catalyst. They revealed a higher TMP ester yield, which was dependent on the esterification catalysts's acidity strength; here perchloric acid had the strongest acidity, giving a maximum of 70% TMP–ester. By contrast, sulfuric acid, p-toluenesulfonic acid, hydrochloric acid and nitric acid gave 46%, 42%, 41% and 37% ester yield, respectively.
1 with 2% w/w concentrated catalyst. They revealed a higher TMP ester yield, which was dependent on the esterification catalysts's acidity strength; here perchloric acid had the strongest acidity, giving a maximum of 70% TMP–ester. By contrast, sulfuric acid, p-toluenesulfonic acid, hydrochloric acid and nitric acid gave 46%, 42%, 41% and 37% ester yield, respectively.
Abiney et al.54 assessed the influence of SnCl2 as the catalyst on the conversion of oleic acid to ethyl oleate in C2H5OH solution; the oleic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst ratio was 100
catalyst ratio was 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/w), under 80 °C of temperature for 6 h reflux. The use of SnCl2 has more advantages than the use of mineral acid catalysts, as the former is less corrosive, less expensive, shows Lewis acid characteristics, is required in low amounts, and is able to avoid unnecessary neutralisation of the products. The SnCl2 has shown a better catalytic activity than mineral H2SO4 acid and a better conversion rate with high selectivity (Fig. 3).
1 (v/w), under 80 °C of temperature for 6 h reflux. The use of SnCl2 has more advantages than the use of mineral acid catalysts, as the former is less corrosive, less expensive, shows Lewis acid characteristics, is required in low amounts, and is able to avoid unnecessary neutralisation of the products. The SnCl2 has shown a better catalytic activity than mineral H2SO4 acid and a better conversion rate with high selectivity (Fig. 3).
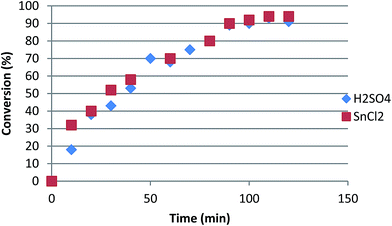 | ||
| Fig. 3 Conversion of oleic acid alcoholysis with ethanol catalyzed by H2SO4 and SnCl2.54 | ||
Ieda et al.55 studied the catalytic performance of a series of metal salts for the esterification process of fatty acids with alcohols, as shown in Fig. 4. Metal salts have Lewis acid character and are highly soluble in reacting fluids, and are used as the catalyst for the esterification reaction. Long-chain fatty acids including C10–C18 and alcohols were examined in equimolar ratios in the catalysts, substrate/catalyst = 200 (v/w) and reaction period = 6 h in mesitylene solution. The salts including chlorides, sulfates, nitrates and acetates of Al3+, Fe3+, In3+, ZrO2+, HfO2+, Zn2+, Ni2+, Co2+, Cr3+, Cu2+, Mg2+ and Mn3+ were used. The conversion rate and selectivity depended on the amount of the catalyst used and the reaction time. Ferric salts, particularly, FeCl3·6H2O was the most active and 90% yield was attained in this case.
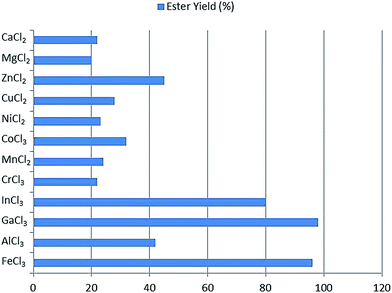 | ||
| Fig. 4 Catalytic activities of various metal chlorides for esterification of palmitic acid with cetyl alcohol.55 | ||
Shivankar et al.56 synthesised the Co(II) and Ni(II) mixed ligand complexes using 8-hydroxyquinoline (Q) as the primary and amino acids (HA) as secondary ligands for ester hydrolysis. The hydrolysis of ethyl acetate and methyl acetate was studied using metal complexes as homogeneous catalysts. The amount of ester was produced under a reaction temperature of 40–50 °C) and substrate/catalyst ratio = 100–500 (v/w) to study the reaction mechanism and kinetics. The reaction mixture (5 mL) was withdrawn at regular intervals for titration against standard NaOH solution to measure the acid values. It was evident from their experiment that mixed ligand metal complexes are more efficient catalysts for ester hydrolysis. The rate constant (k) value increased (2) with an increase in the amount of the catalyst (0.05 g) at 40 °C and ethyl acetate was 5.0 cm3 as shown in Fig. 5.
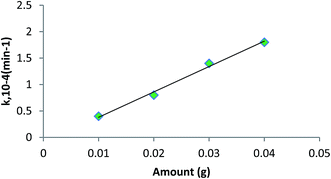 | ||
| Fig. 5 Plot of rate constant (k) vs. amount of catalyst loading; [Co(Q)(Val)]·H2O.56 | ||
The plot between k and the amount of catalyst (Fig. 5) used is a straight line, showing that the rate constant (k) is proportional to the amount of catalyst used for the hydrolysis process.
Shivankar et al.57 in another report investigated the chiral mixed ligand complexes of 8-hydroxyquinoline as primary ligands, while dextrose, fructose, mannitol and tartaric acid were secondary ligands for ester hydrolysis. The hydrolysis of ethyl acetate and methyl acetate was studied using metal complexes as the homogeneous catalysts. The hydrolysis of ester is the opposite of the esterification reaction and is catalysed by transition metal complexes; researchers would like to determine the change in enthalpy (ΔH), entropy (ΔS) and Gibbs free energy (ΔG) in an activated state. In this case, the amount of ester at a temperature of 30–50 °C was being constant when the amount of catalysts were varied, with substrate/catalyst = 100–500 (v/w). Table 8 summarises that the rate constants are highly dependent on the type of catalyst at constant temperature (40 °C), where changes in Gibb's free energy was almost similar, while the reaction entropy largely varied in an activated state. The value of rate constant (k) increased with the type of catalyst used; [Co(Q)(Val)]·H2O was found to have obtained the highest kinetic constant. The amount of catalyst was strongly dependent on the kinetic constant summarised in Table 8. For instance, [Co(Q)(Val)]·H2O consumed the least amount of catalyst as the rate constant was directly related to the amount of catalyst involved in the reaction.
| Complex | T, (°C) | k × 10−2, (min−1) | ΔH#, (kJ mol−1) | ΔS#, (kJ mol−1) | ΔG#, (kJ mol−1) |
|---|---|---|---|---|---|
| [Co(Q)(Val)]·H2O | 40 | 1.64 | 36.62 | −226.30 | 107.48 |
| [Co(Q)(Man)]·H2O | 40 | 1.11 | 48.46 | −191.35 | 108.35 |
| [Co(Q)(Phe)]·H2O | 40 | 1.27 | 47.54 | −192.2 | 107.69 |
| [Co(Q)(Fru)]·H2O | 40 | 1.45 | 50.16 | −183.20 | 107.50 |
| [Ni(Q)(Phe)]·H2O | 40 | 0.69 | 30.81 | −248.09 | 109.19 |
| [Ni(Q)(Dex)]·H2O | 40 | 0.79 | 32.89 | −244.09 | 109.29 |
Oliveira et al.51 carried out an experiment using bivalent tin chelate of 3-hydroxy-2-methyl-4-pyrone (HMP) complex (Fig. 6) as a homogeneous catalyst for poly-esterification of neopentyl glycol (NPG), terephthalic acid (TFA) and trimethylolpropane (TMP) by using acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) alcohol ratio of 4.8
alcohol ratio of 4.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.2
4.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.1 at 150–230 °C for 5 h reflux. The Sn(IV) with Lewis acid character together with its chelate complexes can enhance the esterification reaction by expanding the metal's d orbitals to increase the accessibility towards substract molecules via an associative exchange with ligands. By increasing the amount of catalyst loading, they observed a decrease in the reaction time, although the productivity was higher for reactions with lower catalyst loading. So, compared with other properties, they concluded that the behaviour was not linear (Table 9).
2.1 at 150–230 °C for 5 h reflux. The Sn(IV) with Lewis acid character together with its chelate complexes can enhance the esterification reaction by expanding the metal's d orbitals to increase the accessibility towards substract molecules via an associative exchange with ligands. By increasing the amount of catalyst loading, they observed a decrease in the reaction time, although the productivity was higher for reactions with lower catalyst loading. So, compared with other properties, they concluded that the behaviour was not linear (Table 9).
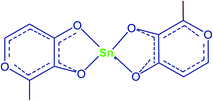 | ||
| Fig. 6 Chelate structure of tin-HMP.51 | ||
| Entry no. | Substrate/catalyst ratio (v/w) | Reaction time (h) | Viscosity (CP) | Productivity | Water (g) |
|---|---|---|---|---|---|
| 01 | 100 | 18.31 | 485 | 364.40 | 142.29 |
| 02 | 200 | 13.25 | 355 | 250.80 | 163.93 |
| 03 | 300 | 13.20 | 133 | 235.00 | 131.58 |
| 04 | 400 | 11.30 | 330 | 154.80 | 161.03 |
| 05 | 500 | 12.55 | 525 | 104.30 | 146.44 |
| 06 | 600 | 9.35 | 385 | 117.00 | 170.22 |
Meneghetti et al.58 reported on Sn(IV)-based complexes as catalysts to produce alkyl esters from alcohol and carboxylic acid. The researchers proposed two types of reaction mechanisms; Lewis acid and exchange/insertion mechanism by introducing labile ligands, which can be exchanged between the feedstock and catalyst (Fig. 7). The Sn metal having vacant 5d orbitals could expand its coordination numbers by insertion with subtract molecules through non-bonding electron pairs and enhance the esterification reactions. First, Sn(IV) complexes bonded with alcohol to form an intermediate product. This intermediate product was then reacted with carboxylic acid, forming another transition compound with the ester product and water as by-products.
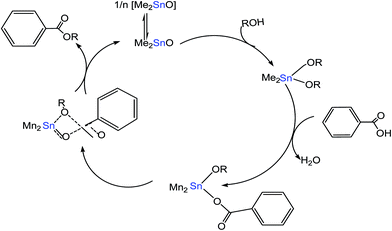 | ||
| Fig. 7 Schematic pattern of organotin(IV)-based catalyst for esterification using Me2SnO as a catalyst.58 | ||
Zendehdel et al.59 reported that the utilisation of NaY zeolite supported 2,6-diformyl-4-methylphenol (DFP) complexes (Fig. 8) as the heterogeneous organic catalyst for esterification of acetic acid with different alcohols (e.g. 2-pentanol, isoamyl alcohol). The results indicated that the Schiff based complexes60 had a better catalytic activity towards esterification. The NaY zeolite catalyst supports the Schiff base complexes with available of Na ions on the zeolite Y matrix, which gives NaY–NH2 a strong basic character even without organic bases. Hence, the fixation of Schiff-based complexes over the NaY–zeolite surface could enhance the catalyst activity61 towards the esterification reactions. The esterification was carried out at 70 °C for 2 h with 50 mg of catalyst loading and a maximum of 90% conversion of acetic acid.
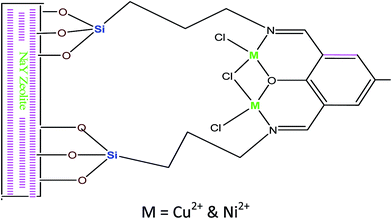 | ||
| Fig. 8 Illustration pattern of immobilization DFP and related complexes.59 | ||
Oh et al.62 reported on the preparation of biolubricants using long-chain alcohols (8 carbon atoms or more) with saturated and unsaturated fatty acids over sulphated zirconia as the potential heterogeneous catalyst.63 Sulphated zirconia is a solid acid catalyst for developing environmentally benign and friendly processes for esterification, as they have acidic sites and the so-called solid superacid catalyst. Sulphated zirconia compounds consist of monoclinic and tetragonal zirconia phases, and the transition from the monoclinic phase to the tetragonal of zirconia is attributed to sulfate content in the Zr–O–S framework. The incorporation of SO42− into the ZrO2 matrix is achieved by the wet impregnation method by varying the SO42− content. The alcohol structure is found to have greatly affected the conversion rate and the product yield. The yield of the product and rate of conversion for the esterification of stearic acid and unsaturated acids (e.g., oleic acid, linolenic acid and linoleic acid) with various alcohols using 6.25 mmol fatty acid, 7.5 mmol alcohol and 100 mg of catalyst at 140 °C for 4 hours is shown in Table 10.
| Alcohols | Free fatty acids (FFA) | Conversion of FFA (%) | Yield of product (%) |
|---|---|---|---|
| 1-OcOH | Stearic acid (StA) | 97.80 | 93.90 |
| 1-OcOH | Oleic acid (OA) | 90.40 | 88.60 |
| 1-OcOH | Linolenic acid (LinOA) | 86.30 | 84.60 |
| Tetradecanol | Oleic acid (OA) | 87.30 | 83.20 |
| Hexadecanol | Oleic acid (OA) | 85.70 | 81.70 |
| 2-OcOH | Oleic acid (OA) | 85.20 | 82.20 |
| 3-OcOH | Oleic acid (OA) | 31.00 | 28.10 |
Adam et al.64 described the kinetic study for the esterification of ethyl alcohols and acetic acid over the heterogeneous L-phenylalanine–Ru(III) complex immobilisation on silica. Initially, the reaction was performed with 0.10 g catalyst using ethyl alcohol to acetic acid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 at 85 °C and the conversion of ethyl alcohol in 1 h was 34% and the conversion reached 94% over the next 12 hours. As silica was grafted on Ru(III) site, the complex catalyst rendered an acidic character capable of enhancing the esterification reaction. This catalyst could be regenerated by washing with ethanol and further be used without significant loss of reactivity.
3 at 85 °C and the conversion of ethyl alcohol in 1 h was 34% and the conversion reached 94% over the next 12 hours. As silica was grafted on Ru(III) site, the complex catalyst rendered an acidic character capable of enhancing the esterification reaction. This catalyst could be regenerated by washing with ethanol and further be used without significant loss of reactivity.
Kotwal et al.18 reported that the biolubricants were synthesised using three-dimensional compounds of titanosilicates, Ti-SBA-12 and Ti-SBA-16. The incorporation of Ti in the silica framework produced Lewis acid sites that were active for the esterification reaction. The higher catalytic performances of these catalysts were due to Lewis acidic Ti sites and the mesoporous character of their structure. In a typical reaction containing 0.12 g catalyst, the oleic acid to polyol (NPG, TMP and PE) ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and reaction temperature was 180 °C with 1 h continuous reflux condition; the following results of these complexes were found (Table 11).
1 and reaction temperature was 180 °C with 1 h continuous reflux condition; the following results of these complexes were found (Table 11).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18
18
| Polyolacs | Catalysts | OA conversion (mol%) | Ester selectivity (mol%) | ||
|---|---|---|---|---|---|
| Mono | Di | Tri | |||
| Trimethylolpropane (TMP) | Ti-SBA-12 | 75.40 | 62.80 | 36.20 | 1.10 |
| Ti-SBA-16 | 71.10 | 70.20 | 28.10 | 1.50 | |
| Neopentyl glycol (NPG) | Ti-SBA-12 | 52.40 | 87.90 | 12.10 | — |
| Ti-SBA-16 | 62.70 | 86.60 | 13.40 | — | |
| Pentaerythritol (PE) | Ti-SBA-12 | 36.60 | 72.60 | 18.40 | 9.00 |
| Ti-SBA-16 | 31.10 | 77.60 | 18.80 | 3.60 | |
Maki et al.65 compared the catalytic activity of N-alkyl-4-boronopyridinium halides and boric acid (H3BO3) via esterification of α-hydroxycarboxylic acids with different alcohols (methanol, ethanol, propanol, butanol). The results suggested that N-methyl-4-boronopyridinium iodide showed a better reactivity than H3BO3 catalyst with a maximum yield of 90%, which was attained for 6 h reflux. However, boric acid catalyst was more effective for dehydrating esterification between an equimolar ratio of α-hydroxycarboxylic acids and alcohols. N-Polystyrene bound with 4-boronopyridinium chloride to form a heterogeneous catalyst was also effective and could be reused after filtration.
Nandiwale et al.66 reported the production of octyl levulinate biolubricants by the esterification process over the modified H-ZSM-5 (Meso-HZ-5) catalyst (where Si/Al ratio = 37) of biomass-derived levulinic acid with n-octanol and achieved a 99% yield at 100–120 °C with 10–30 wt% (LA) catalyst. This was an efficient catalytic process for conversion of agricultural waste feedstock to valuable chemicals. The ZSM-5 zeolite is composed of several pentasil units linked together by oxygen bridges to form a pentasil chains and is a medium-pore zeolite with channels defined by ten-membered rings; H-ZSM-5 is in protonated form. The ZSM-5 zeolite catalyst exhibits Lewis acidity that enhances the esterification reaction (Table 12).
| Catalyst | Catalyst type | Specific catalyst | Reaction conditions | Feedstock | Conv. (%) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Homogeneous | 3.1 Liquid acid | HClO4, H2SO4, HCl, HNO3 | T = 150 °C, 2 w/w catalyst at 3 h reflux | Jatropha curcas oil | 80 | 70 | 53 |
| H3BO3 | Alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acid = 1 acid = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, catalyst = 5–10 mol% with reflux 1, catalyst = 5–10 mol% with reflux |
α-Hydroxy carboxylic acids | 90 | 90 | 65 | ||
| 3.2 Metal salt | (1) SnCl2 | (1) Substrate/catalyst = 120, SnCl2 = 0.01–0.4 mmol followed by reflux | Oleic acid | 95 | 90 | 54 | |
| (2) FeCl3 | (2) Substrate/catalyst = 200, reflux in mesitylene, time = 6 h | Stearic, myristic, and capric acids | 90 | 95 | 55 | ||
| (3) ZrOCl2 | (3) Substrate/catalyst = 1, 5 mol% salt, T = 50 °C, time = 24 h | Acrylic acids, carboxylic acids | 80 | 75 | 67 | ||
| 3.3 Metal complex | (1) Co(II), Ni(II) complexes | Ester in DMF, catalyst = 0.01–0.04 g, T = 30, 40 and 50 °C | Methyl acetate and ethyl acetate | 90 | 89 | 56 | |
| (2) Co(II), Ni(II) chiral complexes | Ester in DMF, catalyst = 0.01–0.04 g, T = 30, 40 and 50 °C | Methyl acetate and ethyl acetate | 85 | 90 | 57 | ||
| (3) Tin chelate, Sn(C6H5O3)2 complex | TFA, NPG and TMP are 4.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.2 4.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.1, T = 150–230 °C, time = 5 h 2.1, T = 150–230 °C, time = 5 h |
Terephthalic acid (TFA) | 83 | 78 | 51 | ||
| (4) Sn(IV) organometallic complexes | MeOH/EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil oil![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst = 400 catalyst = 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; T = 80 °C; stirring = 1000 rpm; OG reactor 1; T = 80 °C; stirring = 1000 rpm; OG reactor |
Soybean oils | 89 | 75 | 58 | ||
| Heterogeneous | 3.4 Supported metal complex | (1) Cu(II), Ni(II) complex with zeolite support | Catalyst = 50 mg, CH3COOH = 50 mmol, alcohol = 100 mmol, T = 70 °C, time = 2 h | Acetic acid | 60–92 | 93 | 59 |
| (2) Sulphated zirconia, Zr(OCH2, CH2CH3)4 | Fatty acid = 6.25 mmol, alcohol = 7.5 mmol, catalyst = 100 mg, T = 140 °C, time = 4 h, stirring = 300 rpm | Oleic acid | 90 | 84 | 62 | ||
| (3) Ru(III) complex on silica support | Ethanol = 0.20 mol, catalyst = 0.10 g, acetic acid = 0.20 mol, T = 85 °C, time = 9 h | Acetic acid | 80 | 94 | 64 | ||
| (4) Ti-SBA-12 & Ti-SBA-16, titanosilicates | OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) polyol = 3 polyol = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1 1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, catalyst = 3 wt% of OA, T = 180 °C, time = 1–10 h 4, catalyst = 3 wt% of OA, T = 180 °C, time = 1–10 h |
Oleic acid | 82 | 92 | 18 | ||
| (5) Modified H-ZSM-5 | n-Octanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LA molar = 4–10, catalyst = 10–30 wt% (LA), T = 100–120 °C, time = 5 h LA molar = 4–10, catalyst = 10–30 wt% (LA), T = 100–120 °C, time = 5 h |
Levulinic acid | 90 | 99 | 66 |
3.2 Transesterification reaction
Transesterification pathway is another conventional process to synthesise ester-based biolubricants using triglyceride-based feedstock and alcohol with the aid of a catalyst. Transesterification is more advantageous compared to general acid-catalysed esterification processes from carboxylic acids and alcohols. For example, some carboxylic acids are sparingly soluble in organic solvents and homogenise esterification with great difficulty, while esters are generally soluble in most organic solvents. The ester-to-ester transformation is highly suitable when the parent carboxylic acids are changeable and difficult to isolate. The most frequently used acid catalysts are sulfuric, sulfonic, phosphoric, and hydrochloric acids. Base-catalysed transesterification is another conventional method, and a wide variety of metal alkoxides, acetates, oxides, and carbonates work in this method. The most popular bases are sodium and potassium alkoxides. Acid catalysed transesterification is much slower than alkali catalysed transesterification and typically requires a higher temperature. However, acid-catalysed transesterifications are more favourable than base catalysed transesterifications, since acid catalysis is not highly affected by the presence of free fatty acids in the feedstock. Practically, acid catalysts can simultaneously catalyse both the esterification and transesterification reactions. The most commonly used alcohol for this purpose is methanol and the process is sometimes called methanolysis. Here we intend to describe the Lewis acidity of catalysts that enhance the transesterification reaction. For this, we include some metal salts, especially Sn(II) and Sn(IV) based complexes with different ligands, which are reviewed. The main advantages of these catalysts are that they are highly soluble in the oil phase where the reaction occurs and give the maximum yield, but catalyst separation is quite difficult.Hao et al.68 utilised Sn(IV), Hf(IV) and Yb(III) bis(perfluorooctanesulfonyl) amide complexes in the transesterification reaction between methyl butyrate (MB) and n-octanol (n-OcOH). The catalytic activity for transesterification was strongly affected by the Lewis acid character of the catalyst complexes. The findings revealed that Sn[N(SO2C8F17)2]4 catalyst exhibits an excellent yield of 89% with 99% selectivity for transesterification at an equimolar ratio of methyl butyrate with n-octanol in a fluorous biphase system (FBS). All the catalytic activity of Sn(IV), Hf(IV) and Yb(III) was evaluated using a fluorous solvent, which enhances the immobilisation of the catalyst and phase separation at the end of reaction (also known as FBS system). Table 13 shows the catalytic performances of metal complex catalysts, which were investigated in the FBS system under 80 °C for 15 h. It was revealed that Sn[N(SO2C8F17)2]4 was the outperformed catalyst due to the catalyst being completely immobilised in the fluorous phase for the transesterification and the fluorous solution being directly used in the subsequent reaction, i.e., it being recyclable.
| Entry no. | Catalysts | Yield (%) |
|---|---|---|
| a Results of conversions are not provided by the study. | ||
| 1 | Sn[N(SO2C8F17)2]4 | 89 |
| 2 | Sn[N(SO2C8F17)2]2 | 84 |
| 3 | Sn(OSO2CF3)2 | 83 |
| 4 | Hf[N(SO2C8F17)2]4 | 76 |
| 5 | Hf(OSO2CF3)4 | 65 |
| 6 | Yb(OSO2CF3)3 | 12 |
Abreu et al.69 tested three homogeneous catalysts, (i) Sn(HMP)2(H2O)2, (ii) Pb(HMP)2(H2O)2, and (iii) Zn(HMP)2(H2O)2 (HMP = 3-hydroxy-2-methyl-4-pyrone) for the transesterification reaction of triglycerides with methanol. Andiroba, babassu, piqui, cumaru, palm and soybean oil methanolysis were performed at 60 °C with a reaction condition of methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil
oil![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst ratio of 400
catalyst ratio of 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v/w) with a reaction time of 1 h. Table 14 depicts the summary of catalytic performances of Sn-, Pb- and Zn-based complexes, and the H2SO4 catalyst. It has been concluded in the study that catalytic activity decreases in the order Sn2+ ≫ Zn2+ ≫ Pb2+ with decreasing Lewis acid character. Sn has a stronger Lewis acid character due to it having vacant 5d orbitals that can expand its coordination numbers by insertion with subtract molecules, which gives a higher yield.
1 (v/v/w) with a reaction time of 1 h. Table 14 depicts the summary of catalytic performances of Sn-, Pb- and Zn-based complexes, and the H2SO4 catalyst. It has been concluded in the study that catalytic activity decreases in the order Sn2+ ≫ Zn2+ ≫ Pb2+ with decreasing Lewis acid character. Sn has a stronger Lewis acid character due to it having vacant 5d orbitals that can expand its coordination numbers by insertion with subtract molecules, which gives a higher yield.
| Vegetable oils | Catalysts | Yield (%) | Composition of fatty acids | |
|---|---|---|---|---|
| Unsaturation (%) | Chain size (% C) | |||
| a Results of conversions are not provided by the study. | ||||
| Soybean | H2SO4 | 1.40 | 76 | 14 |
| Sn(HMP)2(H2O)2 | 37.10 | |||
| Pb(HMP)2(H2O)2 | 4.20 | |||
| Zn(HMP)2(H2O)2 | 15.50 | |||
| Andiroba | H2SO4 | 3.80 | 66 | 28 |
| Sn(HMP)2(H2O)2 | 23.30 | |||
| Pb(HMP)2(H2O)2 | 5.20 | |||
| Zn(HMP)2(H2O)2 | 11.20 | |||
| Palm | H2SO4 | 8.50 | 58 | 35 |
| Sn(HMP)2(H2O)2 | 16.20 | |||
| Pb(HMP)2(H2O)2 | 5.40 | |||
| Zn(HMP)2(H2O)2 | 11.30 | |||
Moreover, Serra et al.71 also reported on the methanolysis of castor oil and soybean oil using homogeneous Sn(IV)-based catalysts. The Sn(IV)-based catalysts are dibutyltin diacetate ((C4H9)2Sn(C2H3O2)2), di-n-butyl-oxo-stannane ((C4H9)2SnO), butylstannoic acid ((C4H9)SnO(OH)) and dibutyltin dilaurate ((C4H9)2Sn(C12H23O2)2), which were taken as Lewis acid catalysts for transesterification reactions. Two types of reactors were used, open glass reactor (OG), where the reactions were performed under methanol reflux at atmospheric pressure (65 °C), and closed steel reactor (CS), where the reactions were carried out at 80 °C and 120 °C. The reaction conditions for both reactors were MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil
oil![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst = 400
catalyst = 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at constant magnetic stirring of 1000 rpm. The results implied that the methanolysis of the castor oil led to lower yields than soybean oil although Sn(IV) catalyst was employed due to the influence of the chemical composition of the triglycerides on the activity of the catalysts based on the Lewis acid sites. Another important observation was the use of a CS reactor rather than an OG reactor, which increased the reaction temperature, leading to greater reaction yields for all Sn(IV) complexes. The FAMEs (% yield) by methanolysis of castor and soybean oil in the presence of Sn(IV) catalysts, using OG and CS reactors, are depicted in Table 15. The (C4H9)2SnO catalyst gave the highest yield in the closed steel reactor because this was attained under high reaction temperature with high methanol concentration in the liquid phase, giving high reaction rates during methanolysis.
1 at constant magnetic stirring of 1000 rpm. The results implied that the methanolysis of the castor oil led to lower yields than soybean oil although Sn(IV) catalyst was employed due to the influence of the chemical composition of the triglycerides on the activity of the catalysts based on the Lewis acid sites. Another important observation was the use of a CS reactor rather than an OG reactor, which increased the reaction temperature, leading to greater reaction yields for all Sn(IV) complexes. The FAMEs (% yield) by methanolysis of castor and soybean oil in the presence of Sn(IV) catalysts, using OG and CS reactors, are depicted in Table 15. The (C4H9)2SnO catalyst gave the highest yield in the closed steel reactor because this was attained under high reaction temperature with high methanol concentration in the liquid phase, giving high reaction rates during methanolysis.
| Reactor temperature (°C) | Reaction time (h) | (C4H9)2Sn(C2H3O2)2 | (C4H9)2Sn(C12H23O2)2 | (C4H9)2SnO | |||
|---|---|---|---|---|---|---|---|
| Soybean oil | Castor oil | Soybean oil | Castor oil | Soybean oil | Castor oil | ||
| OG | 1 | 8 | <5 | 7 | <5 | <5 | <5 |
| 2 | 13 | <5 | 11 | <5 | <5 | 6 | |
| 4 | 23 | <5 | 20 | <5 | 7 | <5 | |
| CS 80 °C | 1 | 32 | <5 | 47 | 6 | 35 | <5 |
| 2 | 63 | <5 | 48 | 7 | 48 | 12 | |
| 4 | 75 | <5 | 62 | 8 | 64 | 16 | |
| CS 120 °C | 1 | 56 | 28 | 70 | 19 | 45 | 8 |
| 2 | 73 | 47 | 77 | 23 | 83 | 23 | |
| 4 | 77 | 64 | 76 | 36 | 85 | 46 | |
Ferreira et al.72 investigated the methanolysis of soybean oil in the presence of tin(IV) complexes including FASCAT® 4100, 4201, and 4350 and LIOCAT® 118 catalysts (Table 16) under mild conditions at 80 °C, methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil
oil![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst ratio of 400
catalyst ratio of 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v/w), and reaction time of 10 h. Results indicated that the dibutyltin dilaurate catalyst offered the best reactivity in terms of reaction yield, approximately 43% of FAMEs after a 10 h reaction period.
1 (v/v/w), and reaction time of 10 h. Results indicated that the dibutyltin dilaurate catalyst offered the best reactivity in terms of reaction yield, approximately 43% of FAMEs after a 10 h reaction period.
| Catalysts | Chemical formula | Commercial name | Yield (%) |
|---|---|---|---|
| FASCAT® 4100 | (C4H9)SnO(OH) | Butylstannoic acid | 7 |
| FASCAT® 4201 | (C4H9)2SnO (modified) | Di-n-butyl-oxo-stannane | 19 |
| FASCAT® 4350 | (C4H9)2SnO (98%) | Stannane (98%) | 14 |
| LIOCAT® 118 | (C4H9)2Sn(C12H23O2)2 | Dibutyltin dilaurate | 43 |
The tin(IV)-based compounds have the potential to act as heterogeneous or homogeneous precursors for esterification, transesterification and/or polycondensation reactions. Meneghetti et al.58 proposed that the key role of organotin(IV) complexes for transesterification reactions was due to the Lewis acidity. Tin(IV) atoms can coordinate with many molecules in solution and associative exchanges in certain mobile ligands with other compounds. The proposed catalytic transesterification mechanisms involving organotin(IV) complexes including ligand association or exchange processes are shown in Fig. 9. In the case of exchange mechanism, Sn(IV) molecule were bonded with acid and alcohols to form an intermediate product. For coordination, Sn(IV) molecule was bonded with ester and alcohols to form a transition compound. In both cases, these products immediately decomposed, leading to ester and alcohols.
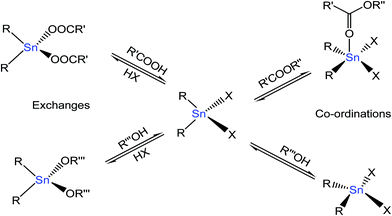 | ||
| Fig. 9 Coordination mode of additional ligands and associative exchange.58 | ||
The transesterification reactivity of Sn(IV) compounds including Bu2Sn(Lau)2, BuSn(O)OH, and Bu2SnO was investigated by Meneghetti et al.58 In addition, the effect of the reaction parameters (reactor type, substrate to alcohol ratio, reaction time, temperature and catalyst loading) for the transesterification process is reported in Table 17. The finding reveals that the Bu2Sn(Lau)2 catalyst exhibits an excellent yield (98%) at 150 °C when MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) soybean oil
soybean oil![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst = 400
catalyst = 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v/w) with constant stirring at 1000 rpm. Bu2Sn(Lau)2 is the best catalyst compared to Bu2SnO and BuSn(O)OH due to its higher degree of solubility and activation, which can be reached at higher temperatures.
1 (v/v/w) with constant stirring at 1000 rpm. Bu2Sn(Lau)2 is the best catalyst compared to Bu2SnO and BuSn(O)OH due to its higher degree of solubility and activation, which can be reached at higher temperatures.
| Reactor | Temperature (°C) | Time (h) | Catalysts | ||
|---|---|---|---|---|---|
| Bu2Sn(Lau)2 | Bu2SnO | BuSn(O)OH | |||
| Closed steel reactor | 80 | 1 | 47 | 35 | — |
| 2 | 48 | 48 | 10 | ||
| 4 | — | 64 | — | ||
| 120 | 1 | 70 | 45 | 40 | |
| 2 | 77 | 83 | 76 | ||
| 4 | 76 | 83 | 60 | ||
| 150 | 1 | 98 | 75 | 70 | |
| 2 | 98 | 95 | 73 | ||
| 4 | 80 | 74 | 74 | ||
Serio et al.74 reported that the most effective catalysts (e.g., Cd, Mn, Zn, Pb carboxylic salts) have been individuated and a correlation of the activities with the cation acidity has been obtained. These catalysts are active in the presence of high FFA concentrations, whereas homogeneous alkaline catalysts pose great difficulties due to the presence of large amounts of free fatty acids (FFA). The study reveals that the catalysts activity depends on the metal acidity and the structures of ester and alcohols; hence, every ester–alcohol couple will have a specific metal choice that will give the maximum activity.75 Besides, these catalysts are more efficient in the presence of high free fatty acid concentrations with a 5 × 10−3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio of catalyst to oil at 200–250 °C when 2.0 g (0.2% w/w of FFA) of soybean oil and 0.88 g of methanol is used (Table 18). The results show that the Cd(OAc)2 catalyst successfully achieved a maximum of 89% conversion contrary to that of Ba(OAc)2, which generated a lower conversion under the same reaction conditions. Cd(OAc)2 is the most promising catalyst in this study due to it's resistant to deactivation during by-product water formation from esterification of FFA process. It is possible to obtain high FAME yields (96%) and a low final FFA concentration (<1%) in a relatively short reaction time (200 min) and low catalyst concentration (4 × 10−4
1 weight ratio of catalyst to oil at 200–250 °C when 2.0 g (0.2% w/w of FFA) of soybean oil and 0.88 g of methanol is used (Table 18). The results show that the Cd(OAc)2 catalyst successfully achieved a maximum of 89% conversion contrary to that of Ba(OAc)2, which generated a lower conversion under the same reaction conditions. Cd(OAc)2 is the most promising catalyst in this study due to it's resistant to deactivation during by-product water formation from esterification of FFA process. It is possible to obtain high FAME yields (96%) and a low final FFA concentration (<1%) in a relatively short reaction time (200 min) and low catalyst concentration (4 × 10−4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio of catalyst to oil).
1 weight ratio of catalyst to oil).
| Entry | Triglycerides | Alcohols | Catalysts | Conversion (%) | Yield (%) |
|---|---|---|---|---|---|
| 01 | Soybean oil | Methanol | Ba(OAc)2 | 73 | 78 |
| 02 | Ca(OAc)2 | 73 | 82 | ||
| 03 | Mg(OAc)2·4H2O | 72 | 73 | ||
| 04 | Cd(OAc)2 | 89 | 96 | ||
| 05 | Mn(OAc)2 | 62 | 68 | ||
| 06 | Ni(OAc)2·4H2O | 66 | 75 | ||
| 07 | Co(OAc)2·4H2O | 81 | 85 |
The most recommended and best catalyst for transesterification reaction from the above study with respect to economic prospects, reaction conditions and the conversion rate are Sn(II) and Sn(IV) complexes. Since Sn has a vacant 5d orbital, showing a Lewis acid character, it is the most promising catalyst in this study due to it being lowered by the water formation during transesterification of FFA using a low amount of catalyst. The main advantage of Sn-based complex catalyst is that it is highly soluble in the oil phase. Tin(IV) atoms can coordinate with many molecules in solution and be associatively exchanged in certain mobile ligands with other compounds. They are cheaper, easily available, form complexes with other electron donating groups, and are useful for biolubricant production through transesterification process. In fact, transesterification is ester-to-ester transformation, and the most commonly used alcohol is methanol and hence transesterification is sometimes called methanolysis (Table 19).
| Catalyst type | Specific catalyst | Reaction conditions | Feedstocks | Conv. (%) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| Metal complex | Sn(IV), Hf(IV) | Ester/alcohol = 1, catalyst = 0.05 mmol, reflux, T = 50 °C, time = 8 h | Methyl butyrate | 90 | 89 | 68 |
| Sn(II), Pb(II), Zn(II) | Alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) vegetable oil vegetable oil![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst = 400 catalyst = 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and reflux at T = 60 °C, time = 1 h 1, and reflux at T = 60 °C, time = 1 h |
Soybean, babassu, piqui, palm oils | 85 | 90 | 69 | |
| DBTDA and DBTDL | Methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil oil![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst = 400 catalyst = 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with stirring = 1000 rpm 1 with stirring = 1000 rpm |
Castor oil and soybean oil | 80 | 70 | 71 | |
| FASCAT® 4100, 4201 and 4350 | MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oil oil![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst = 400 catalyst = 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with reflux at constant stirring 1 with reflux at constant stirring |
Soybean oil | 80 | 43 | 72 | |
| BuSn(O)OH, Bu2SnO, and Bu2Sn(Lau)2 | MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) soybean oil soybean oil![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst = 400 catalyst = 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and reflux at 1000 rpm 1 and reflux at 1000 rpm |
Soybean oil | 79 | 98 | 73 | |
| Cd, Mn, Pb, Zn carboxylic salts | MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) soybean oil soybean oil![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst is 400 catalyst is 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and reflux at T = 150–200 °C, time = 1 h 1 and reflux at T = 150–200 °C, time = 1 h |
Soybean oil | 78 | 96 | 74 |
3.3 Hydrogenation
The hydrogenation of esters is crucial to the chemical industry76,77 for the synthesis of biolubricants or surfactants that have broad applications in agrochemicals, pharmaceuticals, fine chemicals and consumer goods. The reduction of aldehydes, esters and ketones by employing molecular H2 at higher temperatures and pressures for producing ester-based lubricants is important to our daily life. The normal ester content of high oxygen is not suitable for them to be used as fine chemicals and engine oils as they are not biodegradable. Moreover, after hydrogenation of ester-based lubricants, the oxygen content decreases and the hydrogen content increases; hence, these lubricants can be potentially used as engine oils, which satisfies all types of lubrication.The potential catalysts that influence the reduction process of carbonyl group present in ester products include Ru, Rh, Os, or other valuable metal based complexes, like Pt, Au, Pd, Ag, Ir, In.78,79 However, the high price and limited availability of the precious metal-based catalysts has led to the development of less expensive active metals. Transition metal (Cu, Ni, Co, Cr and Fe) based complexes80 have gained recent interest. To date, organometallic complexes as catalysts for hydrogenation of esters are broadly used for creating a homogeneous phase. The ester product becomes highly hydrogenated, but catalyst separation is quite difficult. The main challenges of ligand-based catalysts are the stability of the catalysts,81 catalytic reactivity, and limitation for overcoming electron transfer routes that can be found in transition metal complexes.82
Valencia et al.83 investigated the hydrogenation of β-enamino esters, which was catalysed by cobalt complexes with a chiral (R-BINAP) ligand, bidentate phosphine, or achiral (PPh3) ligand, monodentate phosphine. It was observed that the [Co2(CO)8/rac-BINAP] catalytic system exhibited a higher reactivity at 120 °C with 7–15 h of reaction time. Moreover, the catalytic efficiency of cobalt complexes in asymmetric hydrogenation reaction of β-enamines was evaluated by altering racemic ligands and is summarised in Table 20. Fig. 10 implies how one molecule of hydrogen reacted with β-enamino ester, forming a hydrogenated ester in the presence of the Co2(CO)8 catalyst and racemic ligand at 450 psi.
| Entry no. | Metal complex | Temp. (°C) | Time (h) | Ligands | Yield (%) |
|---|---|---|---|---|---|
| a Results of conversions are not provided by the study. | |||||
| 01 | Co2(CO)8 | 120 | 24 | Race-BINAP | 90 |
| 02 | 120 | 15 | Race-BINAP | 89 | |
| 03 | 120 | 7 | Race-BINAP | 90 | |
| 04 | 120 | 7 | (R)-Tolu-BINAP | 92 | |
| 05 | 120 | 7 | (R)-H8-BINAP | 89 | |
| 06 | 120 | 7 | (R,R)-DIOP | 71 | |
| 07 | 120 | 7 | (R,R)-Met-DuPHOS | 81 | |
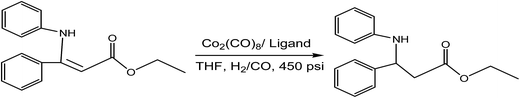 | ||
| Fig. 10 Hydrogenation of β-enamino esters under various conditions.83 | ||
Chakraborty et al.84 studied iron-based compounds as catalysts for the hydrogenation of esters to corresponding ester based-lubricants, surfactants or plasticisers bearing a PNP-pincer ligand. The PNP-pincer ligand contains a pyridine type molecule having donor atoms P, N, and P, which form complexes with iron acting as a catalyst for the hydrogenation of esters to ester-based lubricants at 115 °C under 63 psi per g of H2 pressure in toluene. In this study, the hydrogenation of CE-1270, derived from coconut oil, containing methyl laurate (C12, 73%), methyl myristate (C14, 26%), and a small amount of C10 and C16 methyl esters, was performed at 135 °C under 750 psig of H2 pressure and the iron complex was used as the catalyst (1 mol%). CE-1270 (typically 1.6–1.7 g) was fully converted in 3 h, producing ester-based oil that was confined by GC (yield; 98.6%).
Karamé et al.85 carried out an experiment on the asymmetric hydrogenation of acetophenone using N4-Schiff based chiral complexes containing sulfonamide or amine functionalities of ruthenium. The asymmetric hydrogenation (AH) was conducted at room temperature at 30 bar hydrogen pressure in the presence of a chiral catalyst (whereby the catalyst was synthesised by introducing the chiral ligand to the metallic precursor in absolute media). The asymmetric hydrogenation reaction of acetophenone with some metals of Rh, In, and Ru was also performed under the same conditions. The hydrogenation with the Ru species was usually carried out under solvent conditions (e.g. tert-butanol, iso-propanol), and the mixture was stirred with H2 for 24 hours (Table 21). Fig. 11 indicates that in the presence of metal complexes and i-PrOH and t-BuOH, the ester was converted to the corresponding hydrogenated ester (lubricant) under the mentioned reaction conditions.
| Catalysts | Solvents | Bases | Conversion (%) | Yield (%) |
|---|---|---|---|---|
| [Rh(COD)2]OTf | MeOH | — | 74 | 00 |
| [Ir(COD)2]BF4 | MeOH | — | 56 | 43 |
| [Ru(COD)Cl2]x | i-PrOH | t-BuOK | 100 | 41 |
| [Ir(COD)Cl]2 | i-PrOH | t-BuOK | 99 | 20 |
| [Ru(PPh3)3Cl2] | i-PrOH | t-BuOK | 100 | 54 |
| [Ru(C6H6)Cl2]2 | i-PrOH | t-BuOK | 100 | 20 |
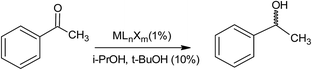 | ||
| Fig. 11 Asymmetric hydrogenation (AH) of acetophenone.85 | ||
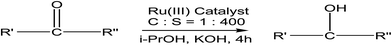
Prabhu et al.86 described the transfer hydrogenation (TH) process of ketones using Ru(II) carbonyl complexes bearing benzoylhydrazone ligands. The TH process of aromatic, aliphatic, heterocyclic, and cyclic ketones87 was studied by using Ru(III) complex and iso-PrOH/KOH (base) at 82 °C and the results are summarized in Table 22. The following reaction shows the reaction scheme of the Ru(III) complex catalysed transfer hydrogenation process of ketones to the corresponding hydrogenated ester. In this case, 4-nitro ketone and 4-cyano ketone (entry 1 and 2) were converted to their corresponding hydrogenated esters (about 99%) efficiently in the presence of Ru(III) complexes in basic media (i-PrOH/KOH) (Fig. 12).
| Entry no. | Ketones | Hydrogenated ester | Conversion (%) | TON | TOF |
|---|---|---|---|---|---|
| a TOF = turn over frequency, TON = turn over number. | |||||
| 1 | 4-Nitro ketone | 4-Nitro ester | 99.50 | 796 | 199 |
| 2 | 4-Cyano ketone | 4-Cyano ester | 99.00 | 792 | 198 |
| 3 | 4-Bromo ketone | 4-Bromo ester | 98.4 | 787 | 197 |
| 4 | Acetophenone | Benzyl ester | 97.5 | 780 | 195 |
| 5 | 4-Methyl ketone | 4-Methyl ester | 96.3 | 770 | 193 |
| 6 | 4-Hydroxy ketone | 4-Hydroxy ester | 92.7 | 742 | 185 |
 | ||
| Fig. 12 Transfer hydrogenation of ester to hydrogenated ester.86 | ||
Shimazu et al.88 esterified esters of α,β-unsaturated fatty acids through asymmetric hydrogenation using supported Rh(I)–phosphine on smectites and supported Rh hectorite (H) complex in acetonitrile/H2O solution. The study found that the asymmetric selectivity depends on the solvents, bulkiness of the ester groups and the interaction of substrate molecules. Hectorites could be incorporated into metal complexes as well as many organic compounds. Since tempered clays have certain interlayer sites, the interaction of chiral ligands with the substrates could occur. Such potential interaction may increase the selectivity in the asymmetric reactions. The proposed mechanism for hydrogenation is shown in Fig. 13. Itaconate has two forms: trans isomer of itaconate is known as mesaconate and cis-isomer is known as citraconate. cis-Isomer (i.e., citraconate on hydrogenation) gives the corresponding saturated hydrogenated ester. The high selectivity was found for the hectorite catalyst in all the solvents compared to that for the homogeneous catalysts, since hectorite still maintained its layer structure even after being swollen with the solvents. The most recommended operating parameters for the mentioned hydrogenation process in the suggested catalyst were hydrogen pressure (PH2) = 1.01 × 105 Pa; temperature (T) = 30 °C; substrate concentration = 6.25 × 10−4 mol; and substrate/Rh = 100 (Table 23).
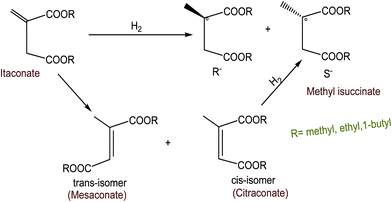 | ||
| Fig. 13 Asymmetric hydrogenation of itaconates.88 | ||
| Catalysts | Substrates | Solvents | Time (h) | Conversion (%) |
|---|---|---|---|---|
| a Reaction parameter: PH2 = 1.01 × 105 Pa, T = 30 °C, L2 = cyclooctadiene Et = ethanol, Cy = cyclohexane, Et/Cy = 2, Mesa = mesaconate, Citra = citraconate. | ||||
| [Rh((S1)-BINAP)L2]+/H | Methyl | MeOH | 2 | 100 |
| 1-Butyl | MeOH | 24 | 98 | |
| [Rh((S1)-BINAP)L2]CIO4 | Methyl | MeOH | 2 | 100 |
| 1-Butyl | MeOH | 12 | 93 | |
| [Rh((S1)-BINAP)L2]+/H | 1-Butyl (Mesa) | Et/Cy | 312 | 100 |
| [Rh((S1)-BINAP)L2]CIO4 | 1-Butyl (Mesa) | Et/Cy | 74 | 100 |
| [Rh((S1)-BINAP)L2]+/H | Methyl (Citra) | Et/Cy | 46 | 96 |
| [Rh((S1)-BINAP)L2]CIO4 | Methyl (Citra) | Et/Cy | 2.5 | 100 |
Table 24 summarises different catalysts used for the hydrogenation process in order to produce hydrogenated esters. The potential catalysts for this purpose are valuable metals like Ru, Rh, Os, Pt, Au, Pd, and Ir based complexes. In fact, the high price and limited availability of these metal-based catalysts leads to the development of highly abundant and less expensive active metals, i.e., transition metal (e.g., Cu, Ni, Co, Mn and Fe) based complexes are gaining recent interest. Nowadays, transition metal complexes as catalysts for hydrogenation of esters are widely used for producing ester-based lubricants.
| Catalyst type | Specific catalyst | Reaction conditions | Feedstock | Conv. (%) | Ref. |
|---|---|---|---|---|---|
| Homogeneous | Co2(CO)8 | Enamine = 0.37 mmol, ligand = 0.0075 mmol, THF = 10 mL, ratio of H2/CO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 450 psi) at 120 °C 3, 450 psi) at 120 °C |
β-Enamino ester | 90 | 83 |
| Iron based pincer complexes | T = 135 °C, PH2 = 750 psi per g, catalyst = 1 mol, CE-1270 = 1.6–1.7 g, time = 3 h | Methyl laurate and methyl myristate | 95 | 84 | |
| Ru(PPh3)3Cl2 | [S] = 0.3 M & S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k-BuOK k-BuOK![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M = 100 M = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; PH2 = 30 bar T = 50 °C for 16 h 1; PH2 = 30 bar T = 50 °C for 16 h |
Acetophenone | 100 | 85 | |
| Ru(L)(CO)(EPh3)2 | Ketone = 2.4 mmol, complex 2 = 3 μmol, KOH = 12 μmol and m-xylene = 0.24 mmol in i-PrOH, T = 82 °C and reflux time = 4 h | Acetophenone | 94 | 86 | |
| Heterogeneous | [Rh((S1)-BINAP)(COD)]+/H & [Rh((S1)-(R)-BPPFA)(COD)]+/h | Solvent = 3 mL; PH2 = 1.01 × 105 Pa; and T = 30 °C; substrate = 6.25 × 10−4 mol; subs/Rh = 100; L2 = COD | Carboxylic acid esters | 95 | 88 |
3.4 Hydrogenation–esterification reaction
In the one-step hydrogenation–esterification (OHE) process, biooils are converted into biofuels (biolubricants) simultaneously in the same reaction vessels employing the same catalyst and reaction conditions. The final products become highly suitable for combustible hydrocarbons as they are hydrogenated to form esters via basic reactions such as esterification89,90 and hydrogenation.91,92 The main constituents of biooils normally employed for the one-step hydrogenation–esterification process are fatty acids, aldehydes and ketones, and phenols, which affect the properties of biooils negatively. For instance, acetic acid, levulinic acid, furfural, hydroxyacetone, phenol, and ethanediol are considered as the recommended raw materials93 for production of biolubricants. The OHE process utilises different bifunctional metals-based catalysts,94 such as RANEY® Ni (RN) or Lewis acid catalyst under solvent conditions (methanol/ethanol) in order to generate combustible and stable compounds, i.e. hydrogenated esters.Xu et al.95 investigated a method to convert biooils into the hydrogenated ester using RN catalyst for the hydrogenation–esterification reaction. Acetic acid, furfural and hydroxyacetone were used as model compounds and 100% conversion of hydroxyacetone and furfural were attained over Ni catalysts modified with Mo, Sn, Fe, and Cu in the presence of methanol. The rate of conversion of CH3COOH was 35.1% when methanol was not added, but when 6 g/8 g methanol/biooil was added, the rate of conversion of acetic acid increased to 81.1%. Table 25 indicates that when Mo–RN was modified by 5% Fe, the conversion of methanol decreased from 35.6% to 28.6%. The degree of decrease was not as significant as the one of Fe–RN. However, the conversion of phenol decreased significantly by addition of Fe as compared to Mo–RN.
| Model compounds | Conversion (%) | ||
|---|---|---|---|
| Fe–Mo–RN (0%) | Fe–Mo–RN (1%) | Fe–Mo–RN (5%) | |
| a Reaction conditions: 0.5 g of Mo–Ni, temp. = 180 °C, H2 pressure = 5 MPa, and batch reaction time = 4 h. | |||
| Methanol | 35.60 | 37.10 | 28.60 |
| Acetic acid | 81.30 | 83.90 | 82.70 |
| Phenol | 76.90 | 49.90 | 51.90 |
| Furfural | 100 | 100 | 100 |
| Ethanediol | 7.00 | 20.70 | 9.50 |
| Hydroxy acetone | 100 | 100 | 100 |
Yu et al.96 described the bio-oil upgrading by the hydrogenation–esterification process using acetic acid and furfural as model compounds over bifunctional Pd. The OHE was performed in a 100 mL stainless steel autoclave with an equimolar ratio of furfural and acetic acid, with 0.40 g catalyst (0.40 Pd/C + 0.40 g Al2(SiO3)3 for mixed bifunctional catalyst) by adding toluene solvent. Reaction time of 4 h at 1.0–4.0 MPa of H2 and 80–200 °C with a stirring speed of 800 rpm and a particle size of 400 meshes were adopted to evaluate the catalytic performances of the tested catalysts. The catalytic performances of the catalysts used for OHE reaction of FAL and HAc is listed; among the tested catalysts, 5% (v/w) Pd/Al2(SiO3)3 showed the best catalytic activity. The better OHE activities over the composite bifunctional catalysts may be attributed to their better cooperative effects among metal sites and acid sites.
Yu et al.97 studied Al-SBA-15 supported Pd bifunctional catalysts (Pd/C, Pd, etc.) to upgrade bio-oils using acetic acid and furfural as the model compounds via the OHE process. The OHE experiments were conducted with an equimolar mixture of 0.10 mol furfural and 0.10 mol acetic acid, which were dissolved in 10.0 mL toluene and 0.40 g of Pd/Al-SBA-15 (5%) or 0.40 g Pd/C (5%) + 0.40 g Al-SBA-15 catalyst was added to the reactor. The reaction was performed under optimum condition of 2.0 MPa H2 pressure, 150 °C of reaction temperature, stirring speed of 800 rpm for 4 h of reaction time. It was important that the synergistic effect between the metal sites and the acid sites on bifunctional Pd/Al-SBA-15 (5%) favoured the OHE reaction, and the following results were obtained. Table 26 summarises the effects of the acidity on the support materials, as the reaction activity was studied over 5% Pd/Al-SBA-15(X) with different Si/Al ratios and the catalytic results are also shown. It is illustrated that with increasing Si/Al of Al-SBA-15 (decrease in acidity of the supports), Z(FAL) (conversion of furfural) decreases but X(D) (yield) increases.
| Catalysts (5%) | Z(FAL) (%) | Y(FOL) (%) | Y(FA) (%) | Y(BP) (%) | X(D) (%) |
|---|---|---|---|---|---|
| a Z(FAL) – conversion of furfural, Y(FOL) – selectivity of furfuryl alcohol, Y(FA) – selectivity of furfuryl acetate, Y(BP) – selectivity of by-products, X(D) – yield to desired products. | |||||
| Pd/C + Al-SBA-15(300) | 70.70 | 43.10 | 16.00 | 40.90 | 41.80 |
| Pd/Al-SBA-15(100) | 71.90 | 56.90 | 16.50 | 26.60 | 52.80 |
| Pd/Al-SBA-15(22) | 73.20 | 49.30 | 15.80 | 34.90 | 47.70 |
| Pd/Al-SBA-15(300) | 70.30 | 61.80 | 18.20 | 20.00 | 56.20 |
| Pd/SBA-15 | 35.20 | 92.20 | 3.30 | 4.50 | 33.60 |
| Pd/Al-SBA-15(500) | 48.60 | 79.60 | 9.60 | 10.80 | 43.40 |
| Pd/C + Al2(SiO3)3 | 69.40 | 19.70 | 9.10 | 71.20 | 20.0 |
Tang et al.98 investigated the catalytic activity of bifunctional Pd and Pt catalyst loaded with acidic supports by upgrading the bio-oils through the OHE route using fatty acid and aldehyde as the starting material. Acetic acid and butyl aldehyde were selected as model compounds for the OHE process using Pt catalysts with supported HZSM-5 and/or amorphous aluminium silicate as the bifunctional catalysts, which means they exhibited the properties of hydrogenation and esterification. The catalysts with a large surface area, high pore volume, small particle size, and strong acidic nature may be favourable for OHE reaction. The reaction was performed at 150 °C with hydrogen pressure of 15 atm, 0.2 g of catalyst, and reaction time of 4 h with a stirring speed of 750 rpm.
From the above study, the most recommended catalyst for the hydrogenation–esterification process of bio-oil upgrading are Pt catalysts with acidic supports e.g., HZSM-5 or amorphous aluminium silicate. These catalysts are bifunctional, have a large surface area, high pore volume, small particle size, and strong acidic nature and are the most favourable for the one-step hydrogenation–esterification (OHE) reaction. The better OHE activities of the bifunctional catalysts may be attributed to its better cooperative effect with metal sites and acid sites. The OHE reaction between aldehyde and acid to ester is feasible over a bifunctional catalyst under high temperature and pressure for producing biolubricants (Table 27).
| Catalyst type | Specific catalyst | Reaction conditions | Feedstock | Conv. (%) | Ref. |
|---|---|---|---|---|---|
| RANEY® Ni | RANEY® Ni with Mo, Sn, Fe, Cu | Mo–RN = 0.5 g, T = 180 °C, PH2 = 5 MPa, reaction time = 4 h | Acetic acid and furfural | 80 | 95 |
| Bifunctional Pd | Pd/Al2(SiO3)3 (5%) and Pd/C + Al2(SiO3)3 (5%) | T = 150 °C, PH2 = 2.0 MPa, rpm = 800, time = 4 h, 9.60 g FAL + 6.00 g HAc + 0.40 g (or 0.40 g Pd/C + 0.4 g Al2(SiO3)3 in entry 1) in 10.0 mL Tolu | Furfural and acetic acid | 66.4 | 96 |
| Bifunctional Pd/Al-SBA-15 | 5% Pd/Al-SBA-15 and 5% Pd/Al2(SiO3)3 | T = 150 °C, PH2 = 2.0 MPa, rpm = 800, time = 4 h, 9.60 g FAL + 6.00 g HAc + 10.0 mL toluene | Furfural and acetic acid | 70 | 97 |
| Pt with acidic supports | 5% Pt/HZSM-5 and 5% Pt/Al2(SiO3)3 | T = 150 °C, catalyst = 0.2 g, butyl aldehyde = 18 g, acetic acid = 15 g, time = 4 h, stirring speed = 750 rpm | Acetaldehyde and acetic acid | 91 | 98 |
4 Future prospects and conclusion
The increasing demand towards renewable and sustainable energy has *attracted the interest of researchers to develop sustainable biolubricants for automotive and machinery applications. Studies revealed that biolubricants exhibit a better price-performance ratio compared to conventional mineral oils, as they can adhere on metal surfaces for a longer time. This review demonstrates that biolubricants are potentially utilised as alternative lubricants for automobile applications such as engine oils, hydraulic and metal working fluids, and grease and chainsaw oils. Our review indicates that metal complex catalysts are potentially used to catalytically synthesise biolubricants as high Lewis catalytic activity of metal complex catalysts were observed in the reactions. For esterification and transesterification, tin complexes of Sn(II) and Sn(IV) catalysts were found to have enhanced the reaction significantly, as they exhibited Lewis acid character and the vacant 5d orbital was well coordinated with subtract molecules in the reaction mixture. Meanwhile, the bifunctional catalyst of 5% (w/w) Pt/Al2(SiO3)3 had the highest activity for ester formation via in situ hydrogenation–esterification reaction due to its large surface area, high pore volume and strong acidic nature, supplemented with 91% yield. In addition, research findings showed that the transition metal catalysts could maximise the selectivity of biolubricants and minimise the formation of undesirable products.To date, obtaining suitable and well-performed lubricants for specific engines operation is challenging in the present lubricant industry. Therefore, it is essential to develop new-generation lubricants for industries to meet the demand of lubricated automotives. Biodegradable and non-toxic lubricants can reduce environmental pollution by lowering carbon emission. In addition, the good lubricating properties, high load-carrying capacity, longer service life, and rapid biodegradability of biolubricants have enhanced the current field of interest.
Furthermore, the development of new lubricant additives, which give a better lubrication under extreme conditions, is another important challenge in the biolubricant field. The new transition metal complexes act as a homogeneous catalyst for upgrading biomass and non-edible oil based lubricants for automotive applications. Nevertheless, considerable works is yet to be accomplished in this field, especially in the development of a new and economic method to develop transition metal-complex catalysts. Moreover, the application of potential metal complex catalysts by upgrading biomass in ester-based lubricants is necessary. The development of effective catalysts for various types of biolubricants are vital and have been recognised to perform better than conventional petroleum oils in modern automotive applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support from Universiti Malaya (Grand Challenge (Innovative Technology (ITRC) (GC001B-14AET)), RU Geran (ST014-2017) and Postgraduate Research Grant (PPP, Project number: PG250-2016A).References
- R. Garcés Mancheño, E. Martínez-Force and J. J. Salas, Grasas Aceites, 2011, 62, 21–28 CrossRef.
- J. Marth, United Bio Lube, 2007.
- E. Wang, X. Ma, S. Tang, R. Yan, Y. Wang, W. W. Riley and M. J. Reaney, Biomass Bioenergy, 2014, 66, 371–378 CrossRef CAS.
- J. C. Ssempebwa and D. O. Carpenter, J. Hazard. Mater., 2009, 161, 835–841 CrossRef CAS PubMed.
- R. Gao, B. Cao, Y. Hu, Z. Feng, D. Wang, W. Hu, J. Chen, Z. Jie, H. Qiu and K. Xu, N. Engl. J. Med., 2013, 368, 1888–1897 CrossRef CAS PubMed.
- P. Nagendramma and S. Kaul, Renewable Sustainable Energy Rev., 2012, 16, 764–774 CrossRef CAS.
- J. H. Urbanus, R. C. Lobo and A. J. Riley, Appl. Occup. Environ. Hyg., 2003, 18, 815–817 CrossRef CAS PubMed.
- L. Wie, X. Li, L. Yanxun, L. Tingliang and L. Guoji, Chem. Eng. Technol., 2013, 36, 559–566 CrossRef CAS.
- M. Stöcker, Angew. Chem., Int. Ed., 2008, 47, 9200–9211 CrossRef PubMed.
- P. Gallezot, Chem. Soc. Rev., 2012, 41, 1538–1558 RSC.
- A. Kumar and S. Sharma, Ind. Crops Prod., 2008, 28, 1–10 CrossRef CAS.
- J. Salimon, N. Salih and E. Yousif, Eur. J. Lipid Sci. Technol., 2010, 112, 519–530 CAS.
- C.-Y. Yang, Z. Fang, B. Li and Y.-f. Long, Renewable Sustainable Energy Rev., 2012, 16, 2178–2190 CrossRef CAS.
- A. Refaat, Int. J. Environ. Sci. Technol., 2010, 7, 183–213 CrossRef CAS.
- R. Kumar, R. Kumar, K. Mahiya and P. Mathur, Transition Met. Chem., 2015, 40, 189–195 CrossRef CAS.
- X. Wang, N. Xing, Z. D. Yan, Z. N. Wang, J. X. Wang, F. Y. Bai and Y. H. Xing, Transition Met. Chem., 2015, 40, 217–225 CrossRef CAS.
- A. J. Hallett, T. M. O'Brien, E. Carter, B. M. Kariuki, D. M. Murphy and B. D. Ward, Inorg. Chim. Acta, 2016, 441, 86–94 CrossRef CAS.
- M. Kotwal, A. Kumar and S. Darbha, J. Mol. Catal. A: Chem., 2013, 377, 65–73 CrossRef CAS.
- G. Lingg and A. Gosalia, in Technische Akademie Esslingen International Tribology Colloquium Proceedings, 2018, p. 16 Search PubMed.
- K. Company, 15th Edition Energy/Petroleum Practice, 2017, http://www.Klinegroup.com Search PubMed.
- A. Vasishth, P. Kuchhal and G. Anand, in Conference Papers in Science, Hindawi Publishing Corporation, 2014, vol. 2014 Search PubMed.
- L. R. Rudnick, Lubricant Additives: Chemistry and Applications, CRC Press, 2009 Search PubMed.
- W. Murphy, D. Blain, A. Galiano-Roth and P. Galvin, J. Synth. Lubr., 2002, 18, 301–325 CrossRef CAS.
- L. R. Rudnick, Synthetics, Mineral Oils, and Bio-based Lubricants: Chemistry and Technology, CRC press, 2013 Search PubMed.
- L. M. Soon, Universiti Malaysia Pahang, 2014.
- E. K. Heikal, M. Elmelawy, S. A. Khalil and N. Elbasuny, Egypt. J. Pet., 2017, 26, 53–59 CrossRef.
- H. Mobarak, E. N. Mohamad, H. Masjuki, M. Kalam, K. Al Mahmud, M. Habibullah and A. Ashraful, Renewable Sustainable Energy Rev., 2014, 33, 34–43 CrossRef CAS.
- A. Willing, Chemosphere, 2001, 43, 89–98 CrossRef CAS PubMed.
- S. Z. Erhan and S. Asadauskas, Ind. Crops Prod., 2000, 11, 277–282 CrossRef CAS.
- I. Kralova and J. Sjöblom, J. Dispersion Sci. Technol., 2010, 31, 409–425 CrossRef CAS.
- H. Wagner, R. Luther and T. Mang, Appl. Catal., A, 2001, 221, 429–442 CrossRef CAS.
- P. Nagendramma and S. Kaul, J. Synth. Lubr., 2008, 25, 131–136 CrossRef CAS.
- P. S. Chauhan and D. Chhibber, Int. Res. J. Humanit., Eng. Pharm. Sci., 2013, 2, 299–305 Search PubMed.
- P. Bondioli, L. Della Bella and A. Manglaviti, Ol., Corps Gras, Lipides, 2003, 10, 150–154 CrossRef CAS.
- A. Adhvaryu, Z. Liu and S. Erhan, Ind. Crops Prod., 2005, 21, 113–119 CrossRef CAS.
- H. A. Mahmud, N. Salih and J. Salimon, Mal. J. Anal. Sci., 2015, 19, 97–105 Search PubMed.
- P. M. Mortensen, J.-D. Grunwaldt, P. A. Jensen, K. Knudsen and A. D. Jensen, Appl. Catal., A, 2011, 407, 1–19 CrossRef CAS.
- A. Suhane, A. Rehman and H. Khaira, Int. J. Eng. Res. Ind. Appl., 2012, 2, 1330–1335 Search PubMed.
- L. R. Rudnick and W. J. Bartz, Chem. Ind., 2006, 111, 331 CAS.
- A. K. Jaina and A. Suhanea, Int. J. Curr. Eng. Technol., 2013, 3, 179–183 Search PubMed.
- S. Bilal, M. Nuhu and S. Kasim, J. Chem. Eng. Mater. Sci., 2013, 4, 72–79 CrossRef CAS.
- G. Perin, G. Álvaro, E. Westphal, L. Viana, R. Jacob, E. Lenardão and M. D'Oca, Fuel, 2008, 87, 2838–2841 CrossRef CAS.
- N. Scarlat, J.-F. Dallemand, F. Monforti-Ferrario and V. Nita, Environ. Dev., 2015, 15, 3–34 CrossRef.
- J. Petran, L. Pedisic and M. Orlovic, Goriva Maziva, 2008, 47, 471 Search PubMed.
- D. Ahmed, S. Kasolang, R. Dwyer-Joyce, K. Sainan and N. N. Roselina, J. Tribol., 2014, 3, 1–10 Search PubMed.
- P. D. Stevens, G. Li, J. Fan, M. Yen and Y. Gao, Chem. Commun., 2005, 4435–4437 RSC.
- D. J. Cole-Hamilton, Science, 2003, 299, 1702–1706 CrossRef CAS PubMed.
- W. H. Mahmoud, G. G. Mohamed and M. M. I. El-Dessouky, Int. J. Electrochem. Sci., 2014, 9, 1415–1438 CAS.
- H. Ji, B. Wang, X. Zhang and T. Tan, RSC Adv., 2015, 5, 100443–100451 RSC.
- J.-Y. Park, Z.-M. Wang, D.-K. Kim and J.-S. Lee, Renewable Energy, 2010, 35, 614–618 CrossRef CAS.
- A. De Oliveira, I. Jorge, P. Suarez, N. D. S. Basso and S. Einloft, Polym. Bull., 2000, 45, 341–344 CrossRef CAS.
- P. San Kong, M. K. Aroua, W. M. A. W. Daud, H. V. Lee, P. Cognet and Y. Pérès, RSC Adv., 2016, 6, 68885–68905 RSC.
- H. A. Noor and J. Salimon, J. Chem., 2011, 8, S33–S40 Search PubMed.
- L. C. Abiney, S. C. G. Neves and M. J. Da Silva, Energies, 2008, 1, 79–92 CrossRef.
- N. Ieda, K. Mantri, Y. Miyata, A. Ozaki, K. Komura and Y. Sugi, Ind. Eng. Chem. Res., 2008, 47, 8631–8638 CrossRef CAS.
- V. S. Shivankar, L. V. Gavali, S. P. Yadav and N. V. Thakkar, IOSR J. Appl. Chem., 2013, 3, 31–39 CrossRef.
- V. S. Shivankar and L. V. Gavali, Chem. Sci. Trans., 2013, 2, 1204–1213 Search PubMed.
- M. R. Meneghetti and S. M. P. Meneghetti, Catal. Sci. Technol., 2015, 5, 765–771 CAS.
- M. Zendehdel, F. Zamani and H. Khanmohamadi, Microporous Mesoporous Mater., 2016, 225, 552–563 CrossRef CAS.
- E. Zangrando, M. Islam, M. A.-A. A. Islam, M. Sheikh, M. Tarafder, R. Miyatake, R. Zahan and M. Hossain, Inorg. Chim. Acta, 2015, 427, 278–284 CrossRef CAS.
- L. M. Payawan Jr, J. A. Damasco and K. Sy Piecco, Philipp. J. Sci., 2010, 139, 105–116 Search PubMed.
- J. Oh, S. Yang, C. Kim, I. Choi, J. H. Kim and H. Lee, Appl. Catal., A, 2013, 455, 164–171 CrossRef CAS.
- K. Saravanan, B. Tyagi and H. Bajaj, Catal. Sci. Technol., 2012, 2, 2512–2520 CAS.
- F. Adam, K. M. Hello and S.-J. Chai, Chem. Eng. Res. Des., 2012, 90, 633–642 CrossRef CAS.
- T. Maki, K. Ishihara and H. Yamamoto, Org. Lett., 2005, 7, 5047–5050 CrossRef CAS PubMed.
- K. Y. Nandiwale, S. K. Yadava and V. V. Bokade, J. Energy Chem., 2014, 23, 535–541 CrossRef.
- H.-B. Sun, R. Hua and Y. Yin, Molecules, 2006, 11, 263–271 CrossRef CAS PubMed.
- X. Hao, A. Yoshida and J. Nishikido, Tetrahedron Lett., 2004, 45, 781–785 CrossRef CAS.
- F. R. Abreu, D. G. Lima, E. H. Hamú, C. Wolf and P. A. Suarez, J. Mol. Catal. A: Chem., 2004, 209, 29–33 CrossRef CAS.
- F. R. Abreu, D. G. Lima, E. H. Hamu, S. Einloft, J. C. Rubim and P. A. Suarez, J. Am. Oil Chem. Soc., 2003, 80, 601–604 CrossRef CAS.
- T. M. Serra, D. R. De Mendonca, J. P. Da Silva, M. R. Meneghetti and S. M. P. Meneghetti, Fuel, 2011, 90, 2203–2206 CrossRef CAS.
- D. A. Ferreira, M. R. Meneghetti, S. M. Meneghetti and C. R. Wolf, Appl. Catal., A, 2007, 317, 58–61 CrossRef CAS.
- D. R. de Mendonça, J. P. da Silva, R. M. de Almeida, C. R. Wolf, M. R. Meneghetti and S. M. Meneghetti, Appl. Catal., A, 2009, 365, 105–109 CrossRef.
- M. Di Serio, R. Tesser, M. Dimiccoli, F. Cammarota, M. Nastasi and E. Santacesaria, J. Mol. Catal. A: Chem., 2005, 239, 111–115 CrossRef CAS.
- M. Di Serio, B. Apicella, G. Grieco, P. Iengo, L. Fiocca, R. Po and E. Santacesaria, J. Mol. Catal. A: Chem., 1998, 130, 233–240 CrossRef CAS.
- C. Jäkel and R. Paciello, Chem. Rev., 2006, 106, 2912–2942 CrossRef PubMed.
- J. G. De Vries and L. Lefort, High-Throughput Experimentation and Ligand Libraries, Wiley Online Library, 2007 Search PubMed.
- T. Schaaf and H. Greven, Lipid Technol., 2010, 22, 31–35 CrossRef CAS.
- V. Pozdeev, S. Safronov, S. Levanova and E. Krasnykh, Russ. J. Appl. Chem., 2012, 85, 261–266 CrossRef CAS.
- T. Zell and D. Milstein, Acc. Chem. Res., 2015, 48, 1979–1994 CrossRef CAS PubMed.
- M. Alcon, A. Corma, M. Iglesias and F. Sánchez, J. Organomet. Chem., 2002, 655, 134–145 CrossRef CAS.
- S. Chakraborty, P. Bhattacharya, H. Dai and H. Guan, Acc. Chem. Res., 2015, 48, 1995–2003 CrossRef CAS PubMed.
- M. Amézquita-Valencia and A. Cabrera, J. Organomet. Chem., 2014, 768, 145–150 CrossRef.
- S. Chakraborty, H. Dai, P. Bhattacharya, N. T. Fairweather, M. S. Gibson, J. A. Krause and H. Guan, J. Am. Chem. Soc., 2014, 136, 7869–7872 CrossRef CAS PubMed.
- I. Karamé, M. Jahjah, A. Messaoudi, M. L. Tommasino and M. Lemaire, Tetrahedron: Asymmetry, 2004, 15, 1569–1581 CrossRef.
- R. N. Prabhu and R. Ramesh, J. Organomet. Chem., 2012, 718, 43–51 CrossRef CAS.
- N. Nikolaou, C. E. Papadopoulos, A. Lazaridou, A. Koutsoumba, A. Bouriazos and G. Papadogianakis, Catal. Commun., 2009, 10, 451–455 CrossRef CAS.
- S. Shimazu, K. Ro, T. Sento, N. Ichikuni and T. Uematsu, J. Mol. Catal. A: Chem., 1996, 107, 297–303 CrossRef CAS.
- W.-M. Xiong, M.-Z. Zhu, L. Deng, Y. Fu and Q.-X. Guo, Energy Fuels, 2009, 23, 2278–2283 CrossRef CAS.
- S. Zhang, Y. Yan, T. Li and Z. Ren, Bioresour. Technol., 2005, 96, 545–550 CrossRef CAS PubMed.
- B. Zhang, L. Lin, J. Zhuang, Y. Liu, L. Peng and L. Jiang, Molecules, 2010, 15, 5139–5152 CrossRef CAS PubMed.
- Y. Kuwahara, Y. Magatani and H. Yamashita, Catal. Today, 2015, 258, 262–269 CrossRef CAS.
- X. Yang, S. Chatterjee, Z. Zhang, X. Zhu and C. U. Pittman Jr, Ind. Eng. Chem. Res., 2010, 49, 2003–2013 CrossRef CAS.
- Y. Tang, S. Miao, B. H. Shanks and X. Zheng, Appl. Catal., A, 2010, 375, 310–317 CrossRef CAS.
- Y. Xu, L. Zhang, J. Chang, X. Zhang, L. Ma, T. Wang and Q. Zhang, Energy Convers. Manage., 2016, 108, 78–84 CrossRef CAS.
- W. Yu, Y. Tang, L. Mo, P. Chen, H. Lou and X. Zheng, Bioresour. Technol., 2011, 102, 8241–8246 CrossRef CAS PubMed.
- W. Yu, Y. Tang, L. Mo, P. Chen, H. Lou and X. Zheng, Catal. Commun., 2011, 13, 35–39 CrossRef CAS.
- Y. Tang, W. Yu, L. Mo, H. Lou and X. Zheng, Energy Fuels, 2008, 22, 3484–3488 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2018 |