Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fe-catalyzed esterification of amides via C–N bond activation†
Xiuling Chen *,
Siying Hu,
Rongxing Chen,
Jian Wang,
Minghu Wu,
Haibin Guo and
Shaofa Sun*
*,
Siying Hu,
Rongxing Chen,
Jian Wang,
Minghu Wu,
Haibin Guo and
Shaofa Sun*
Hubei University Of Science and Technology, China
First published on 25th January 2018
Abstract
An efficient Fe-catalyzed esterification of primary, secondary, and tertiary amides with various alcohols for the preparation of esters was performed. The esterification process was accomplished with FeCl3·6H2O, which is a stable, inexpensive, environmentally friendly catalyst with high functional group tolerance.
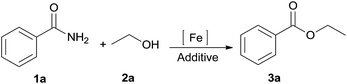
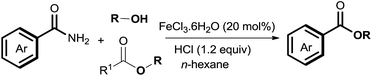
The amide bond is not only employed as an important natural peptide skeleton in biological systems, but also as a versatile functional group in organic transformations.1,2 Among amide transformations,3,4 transamidation and esterification of amides have attracted widespread attention from organic chemists.5–11 In contrast to transamidation of amides, esterification of amides is relatively difficult because of the low nucleophilicity of alcohols compared with amines.12 To overcome this shortcoming, various processes for the esterification of amides have been developed, such as using an activating agent,6 employing twisted amides,7 and forming intramolecular assisted groups.8 For increasing the synthetic flexibility of esterification of amides, new catalytic systems Zn(OTf)2 and Sc(OTf)3 were developed by Mashima and Williams for the esterification of amides.9 Shimizu et al. reported CeO2 catalyzed esterification of amides.10 Off late, progress of the significant Ni-catalyzed esterification of amides has gained precedence.11 Although this approach produces satisfactory yield, the drawbacks such as the use of an expensive catalyst, generation of reagent waste, high temperature and the limited scope of the substrate still persists. Herein, under mild reaction conditions, we report a novel and efficient Fe-catalyzed esterification of amides for the synthesis of esters (eqn (1)). Compared to conventional methods, this esterification procedure is distinguished by using a stable, inexpensive, environment-friendly catalyst, i.e., FeCl3·6H2O with a low toxicity solvent. The catalytic system has wide functional group compatibility. The reactions of several primary, secondary, and tertiary amides with various alcohols have been well tolerated in this process. Moreover, esters were also as effective as esterification reagents for the esterification of amides by the acyl–acyl exchange process. In the course of our present study, a Co-catalyzed amide C–N cleavage to form esters was reported by Danoun et al.13 However, an additional additive (Bipy) and Mn (3 equiv.) were needed and the scope of the reaction was limited to only tertiary amides; primary or secondary amides were not compatible with this catalytic oxidation system.
 | (1) |
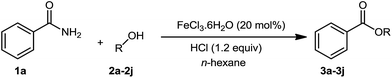
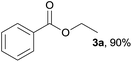
We initially selected benzamide 1a and ethanol 2a as model substrates to screen the reaction conditions, and the results are summarized in Table 1. First, in the presence of FeCl2, ethyl benzoate 3a was afforded in 31% yield (Table 1, entry 1). Next, our catalyst-screening results showed that various iron complexes such as FeCl3, FeBr3, FeSO4·7H2O, FeCl3·6H2O, and Fe(NO3)3·9H2O could catalyze the reaction (Table 1, entries 2–6), and FeCl3·6H2O was the best catalyst for this reaction as ethyl benzoate 3a was afforded in 35% yield (Table 1, entry 6). Other metal complexes such as CuCl2, PdCl2, and NiCl2·6H2O did not improve the efficiency of this transformation (Table 1, entries 7–9). In the absence of an iron complex, this reaction could not work and the product was not detected (Table 1, entry 10). Next, in the presence of FeCl3·6H2O, the influence of additives on the reaction was investigated (Table 1, entries 11–18). Among the tested additives, the stronger bidentate ligands such as 1,10-phenanthroline or 2,2-bipyridine did not show any efficiency, while hydrochloric acid gave the best reactive activity as ethyl benzoate 3a was obtained in 91% yield (Table 1, entry 18). In general, H2SO4 or HNO3 was used as a catalyst for esterification; however, they did not efficiently promote the reaction under the present system (Table 1, entries 16–17). In the absence of FeCl3·6H2O, the product 3a was afforded in 35% yield (Table 1, entry 19). For the solvents investigated (Table 1, entries 20–23), n-hexane was the best choice (Table 1, entry 18).
| Entry | Catalyst (20%) | Additive (40%) | Yieldb |
|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.1 mL), catalyst (0.04 mmol, 20 mol%), H2SO4 (concentrated, 0.24 mmol), HNO3 (concentrated, 0.24 mmol), HCl (0.24 mmol, 36–38%), n-hexane (1.0 mL), 80 °C, 14 h.b GC yield using hexadecane as internal standard.c DMF was used as a solvent.d 1,4-Dioxane was used as solvent.e Toluene was used as solvent.f CH3CN was used as solvent. | |||
| 1 | FeCl2 | — | 31 |
| 2 | FeCl3 | — | 31 |
| 3 | FeBr3 | — | 25 |
| 4 | FeSO4·7H2O | — | 20 |
| 5 | Fe(NO3)3·9H2O | — | 20 |
| 6 | FeCl3·6H2O | — | 35 |
| 7 | CuCl2 | — | Trace |
| 8 | PdCl2 | — | Trace |
| 9 | Ni2Cl·6H2O | — | Trace |
| 10 | — | — | — |
| 12 | FeCl3·6H2O | Pyridine | Trace |
| 13 | FeCl3·6H2O | 1,10-Phenanthroline | Trace |
| 14 | FeCl3·6H2O | Formic acid | Trace |
| 15 | FeCl3·6H2O | L-Proline | Trace |
| 16 | FeCl3·6H2O | H2SO4 | 22 |
| 17 | FeCl3·6H2O | HNO3 | 25 |
| 18 | FeCl3·6H2O | HCl | 91 |
| 19 | — | HCl | 35 |
| 20c | FeCl3·6H2O | HCl | Trace |
| 21d | FeCl3·6H2O | HCl | 36 |
| 22e | FeCl3·6H2O | HCl | 28 |
| 23f | FeCl3·6H2O | HCl | 40 |
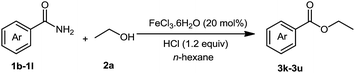
The substrate scope of esterification with alcohols was investigated under the optimized reaction conditions. As shown in Table 2, esterification of benzamide by primary alcohols, secondary alcohols, tertiary alcohols, and ring alcohols took place smoothly, and the corresponding esters were afforded in good to high yields. When primary alcohols 2a, 2b, 2c were used as substrates, affording the ester products 3a–3c in high yields, it should be noted that the reactivity of the alcoholysis of amides was independent of the alkyl chain length (Table 2, entries 1–3). Secondary alcohols 2d and tertiary alcohols 2e also worked effectively as the substrates, producing the esters in 81–91% yield (Table 2, entries 4–5). Treating 1a with cyclohexanol 2f could afford the ester 3f in 80% yield (Table 2, entry 6). Alcoholysis products 3g and 3h were obtained from corresponding alcohols (Table 2, entries 7–8). Substrate alcohol having trifluoromethyl also participated in this catalytic reaction to form the ester 3i in high yield (Table 2, entry 9). Moreover, when ethane-1,2-diol was treated with 1a, only one product 3j was obtained (Table 2, entry 10).
| Entry | Alcohol | Products | Yieldb |
|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.1 mL), 2b–2j (0.24 mmol), FeCl3·6H2O (0.04 mmol, 20 mol%), HCl (0.24 mmol, 36–38%), n-hexane (1.0 mL), 80 °C, 14 h.b Isolated yield. | |||
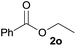
| 1 | Ethanol 2a | 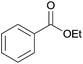 |
3a, 85% |
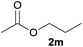
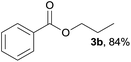
| 2 | n-Propanol 2b | 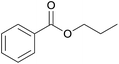 |
3b, 88% |
| 3 | n-Octanol 2c | 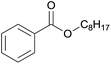 |
3c, 82% |
| 4 | i-Propanol 2d | 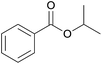 |
3d, 91% |
| 5 | t-Butyl alcohol 2e | 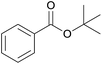 |
3e, 81% |
| 6 | Cyclohexanol 2f | 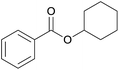 |
3f, 80% |
| 7 | Phenethanol 2g | 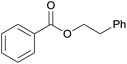 |
3g, 83% |
| 8 | Phenethanol 2h | 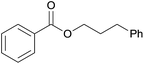 |
3h, 81% |
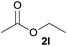
| 9 | Trifluoroethanol 2i | 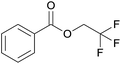 |
3i, 81% |
| 10 | Ethane-1,2-diol 2j | 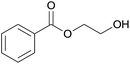 |
3j, 85% |
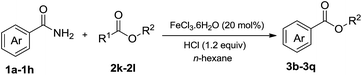
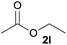
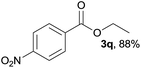
We then examined the generality of amides and the results are compiled in Table 3. Under the optimized conditions, both electron-rich and electron-deficient group substituted benzamide could undergo C–N bond cleavage and esterification by ethanol to afford the corresponding ester products in high yields (Table 3, entries 1–7). Various functional groups such as alkoxy, hydroxyl, amino, halide, and nitro remained intact during the reaction. 2-Naphthamide also served as an efficient substrate and could react with 2a, furnishing the ester 3r in 86% yield. Interestingly, heteroaryl substituted esters 3s and 3t were obtained from the reaction of 2-thiophenecarboxamide and 3-indoleacetamide with 2a using the present reaction conditions, indicating that this method will be an efficient approach to introduce an aromatic heterocycle into functional molecules. Substrates containing alkynes such as cinnamamide (1l) could also react with ethanol smoothly to give an esterification product 3u with 88% yield. The reaction was not sensitive to the steric environment of the amide moiety; when meta- and ortho-substituted amides 1m and 1n were subjected to the reaction system, the corresponding ester products were obtained in high yields.
| Entry | Amides | Products and yieldb |
|---|---|---|
| a Reaction conditions: 1b–1n (0.2 mmol), 2a (0.1 mL), FeCl3·6H2O (0.04 mmol, 20 mol%), HCl (0.24 mmol, 36–38%), n-hexane (1.0 mL), 80 °C, 14 h.b Isolated yield. | ||
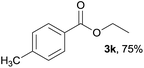
| 1 | 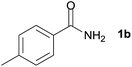 |
3k, 86% |
| 2 | 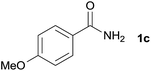 |
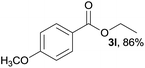
3l, 85% |
| 3 | 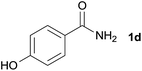 |
3m, 78% |
| 4 | 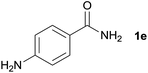 |
3n, 75% |
| 5 | 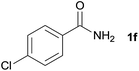 |
3o, 86% |
| 6 | 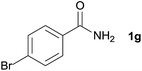 |
3p, 84% |
| 7 | 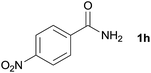 |
3q, 90% |
| 8 | 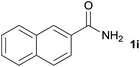 |
3r, 86% |
| 9 | 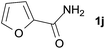 |
3s, 84% |
| 10 | 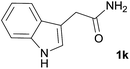 |
3t, 55% |
| 11 | 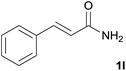 |
3u, 88% |
| 12 | 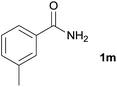 |
3v, 85% |
| 13 | 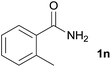 |
3w, 81% |
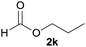
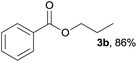
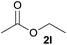
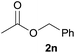
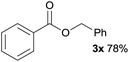
In addition to alcohols, esters were also used as substrates for esterification of amides through the acyl–acyl exchange process under the optimal reaction conditions.14 As shown in Table 4, in the current catalytic system, formate, acetate and benzoate also served as efficient substrates and could react with amides, furnishing the corresponding esters in high yield (Table 4, entries 1–7).
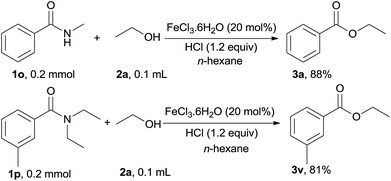
The substrate scope of this iron-catalyzed esterification of secondary and tertiary amides with alcohols was investigated. Remarkably, when a secondary amide, viz., N-methylbenzamide (1o) and a tertiary amide, viz., N,N-diethylbenzamide (1p) were used as substrates, the corresponding esters 3a and 3v were afforded in high yield (Scheme 1).
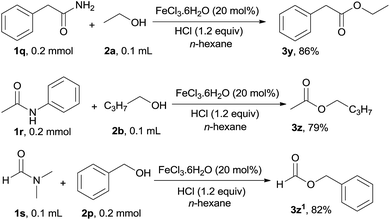
In addition to aromatic primary, secondary and tertiary amides, the esterification of aliphatic primary, secondary and tertiary amides was also investigated under the optimal reaction conditions, the corresponding esters were afforded in good to excellent yield and the results are shown in Scheme 2. 2-Phenylacetamide (1q) participated in this catalytic reaction to form the ester 3y in 86% yield. N-Acetylaniline 1r and N,N-dimethylformamide 1s were also applicable to this catalytic system, and transformed into the corresponding esters 3z and 3z1 in 79% and 82% yield respectively.
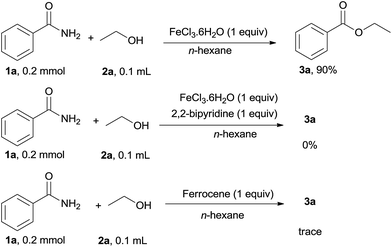
To demonstrate the effect of iron salt in the current catalytic system, several control experiments were carried out as shown in Scheme 3. When 1 equivalent FeCl3·6H2O was used as the catalyst in the absence of HCl, 3a was obtained in 90% yield. This result indicated that the catalytic cycle of the iron salt was hindered in the present reaction conditions. When the stronger bidentate ligands such as 2,2-bipyridine was used, 3a was not obtained and trace amounts of 3a were detected on using ferrocene as the catalyst, indicating that the iron salt catalyst showed low efficiency in the presence of the stronger ligand. Thus, it was deduced that free Fe(II or III) was effective for this reaction.
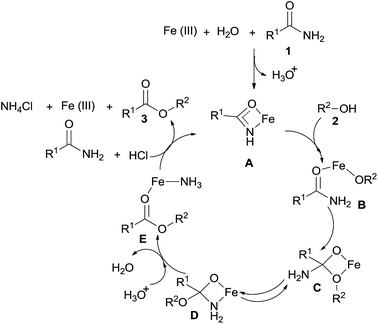
According to the reported literature5d and our experimental results, the catalytic reaction pathways for the Fe-catalyzed esterification of amides by alcohols are proposed as shown in Scheme 4. The first step is the generation of the amidate complex A, which can be formed from free Fe(III) and amide 1. The complex A reacts with alcohol 2 to produce an unstable intermediate B. The interaction between the alcohol oxygen and the carbonyl results in cyclic intermediate C, which is in equilibrium with its isomer D. Intermediate E can also be produced from D via C–N bond cleavage. Through the reaction of HCl and a new molecule amide, intermediate E produces the desired ester 3, ammonium chloride (colorless crystal, which was detected after the reaction), and the amidate complex A.
Conclusions
In summary, we have discovered a simple, useful and general method for the esterification of amides using inexpensive and readily available iron salts as catalysts. The reaction shows high substrate tolerance as a wide range of aromatic or aliphatic primary, secondary and tertiary amides can be effectively used to produce corresponding esters in good to excellent yields. In addition to alcohol, esters were also used as substrates for esterification of amides through the acyl–acyl exchange process. The present findings not only provide a general and concise method for Fe-catalyzed amide C–N bond cleavage, but also open an avenue for the preparation of esters.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support by Natural Science Foundation of China (21603068) and research fund for the doctoral program (2016-19XB011 or KY11066) of Hubei University of Science and Technology are gratefully appreciated.Notes and references
- (a) N. Sewald and H. D. Jakubke, Peptides: Chemistry and Biology, Wiley-VCH: Weinheim, 2002 Search PubMed; (b) B. L. Bray, Nat. Rev. Drug Discovery, 2003, 2, 587 CrossRef CAS PubMed; (c) T. Cupido, J. Tulla-Puche, J. Spengler and F. Albericio, Curr. Opin. Drug Discovery Dev., 2007, 10, 768 CAS; (d) J. W. Bode, Curr. Opin. Drug Discovery Dev., 2006, 9, 765 CAS; (e) J. M. Humphrey and A. R. Chamberlin, Chem. Rev., 1997, 97, 2243 CrossRef CAS PubMed; (f) A. Greenberg, C. M. Breneman and J. F. Liebman, Amide Linkage: Selected Structural Aspects in Chemistry, Biochemistry, and Materials Science, Wiley-Interscience, New York, 2000 Search PubMed.
- (a) G. W. Wang, T. T. Yuan and D. D. Li, Angew. Chem., Int. Ed., 2011, 50, 1380 CrossRef CAS PubMed; (b) X. X. Zhang, W. T. Teo and P. W. H. Chan, J. Organomet. Chem., 2011, 696, 331 CrossRef CAS; (c) E. Valeur and M. Bradley, Chem. Soc. Rev., 2009, 38, 606 RSC.
- For reviews in C–N bond activation, see: (a) K. Ouyang, W. Hao, W.-X. Zhang and Z. Xi, Chem. Rev., 2015, 115, 12045 CrossRef CAS PubMed; (b) Q. Wang, Y. Su, L. Li and H. Huang, Chem. Soc. Rev., 2016, 45, 1257 RSC.
- For a review about amide reactivity see: (a) M. C. Whisler, S. MacNeil, V. Snieckus and P. Beak, Angew. Chem., Int. Ed., 2004, 43, 2206 CrossRef CAS PubMed; (b) V. Snieckus, Chem. Rev., 1990, 90, 879 CrossRef CAS; (c) V. Pace, W. Holzer and B. Olofsson, Adv. Synth. Catal., 2014, 356, 3697 CrossRef CAS.
- For transamidation of amides see: (a) T. B. Nguyen, J. Sorres, M. Q. Tran, L. Ermolenko and A. Al-Mourabit, Org. Lett., 2012, 14, 3202 CrossRef CAS PubMed; (b) N. A. Stephenson, J. Zhu, S. H. Gellman and S. S. Stahl, J. Am. Chem. Soc., 2009, 131, 10003 CrossRef CAS PubMed; (c) S. N. Rao, D. C. Mohan and S. Adimurthy, Org. Lett., 2013, 15, 1496 CrossRef CAS PubMed; (d) L. Becerra-Figueroa, A. Ojeda-Porras and D. Gamba-Sanchez, J. Org. Chem., 2014, 79, 4544 CrossRef CAS PubMed; (e) Y. Liu, S. Shi, M. Achtenhagen, R. Liu and M. Szostak, Org. Lett., 2017, 19, 1614 CrossRef CAS PubMed; (f) G. Meng, P. Lei and M. Szostak, Org. Lett., 2017, 19, 2158 CrossRef CAS PubMed.
- (a) S. Yamada, Angew. Chem., Int. Ed., 1995, 34, 1113 CrossRef CAS; (b) S. Yamada, J. Org. Chem., 1996, 61, 941 CrossRef CAS; (c) M. Hutchby, C. E. Houlden, M. F. Haddow, S. N. G. Tyler, G. C. Loyd-Jones and K. I. Booker-Milburn, Angew. Chem., Int. Ed., 2012, 51, 548 CrossRef CAS PubMed; (d) J. Aubé, Angew. Chem., Int. Ed., 2012, 51, 3063 CrossRef PubMed.
- (a) L. M. Berreau, M. M. Makowsak-Grzyska and A. M. Arif, Inorg. Chem., 2000, 39, 4390 CrossRef CAS; (b) S. Kawaguchi and K. Araki, Inorg. Chim. Acta, 2005, 358, 947 CrossRef CAS; (c) E. Szajna-Fuller, G. K. Lngle, R. W. Watkins, A. M. Arif and L. M. Berreau, Inorg. Chem., 2007, 46, 2353 CrossRef CAS PubMed; (d) M. C. Bçhmer and W. Bannwarth, Eur. J. Org. Chem., 2008, 4412 Search PubMed; (e) M. C. Bröhmer, S. Mundinger, S. Bräse and W. Bannawarth, Angew. Chem., Int. Ed., 2011, 50, 6175 CrossRef PubMed; (f) M. A. R. Raycroft, C. I. Maxwell, R. A. A. Oldham, A. S. Andrea, A. A. Neverov and R. S. Brown, Inorg. Chem., 2012, 51, 10325 CrossRef CAS PubMed; (g) M. Hutchby, C. E. Houlden, M. F. Haddow, S. N. G. Tyler, G. C. Lloyd-Jones and K. I. Booker-Milburn, Angew. Chem., Int. Ed., 2012, 51, 548 CrossRef CAS PubMed.
- (a) S. Yamada, Angew. Chem., Int. Ed., 1993, 32, 1083 CrossRef; (b) S. Yamada, Angew. Chem., Int. Ed., 1995, 34, 1113 CrossRef CAS; (c) S. Yamada, J. Org. Chem., 1996, 61, 941 CrossRef CAS; (d) S. Yamada, J. Org. Chem., 1996, 61, 5932 CrossRef CAS; (e) M. Hutchby, C. E. Houlden, M. F. Haddow, S. N. G. Tyler, G. C. Loyd-Jones and K. I. Booker-Milburn, Angew. Chem., Int. Ed., 2012, 51, 548 CrossRef CAS PubMed; (f) S. Yamada, J. Org. Chem., 1992, 57, 1591 CrossRef CAS; (g) M. Hutchby, C. E. Houlden, M. F. Haddow, S. N. G. Tyler, G. C. Lloyd-Jones and K. I. Booker-Milburn, Angew. Chem., Int. Ed., 2012, 51, 548 CrossRef CAS PubMed.
- (a) Y. Kita, Y. Nishii, T. Higuchi and K. Mashima, Angew. Chem., Int. Ed., 2012, 51, 5723 CrossRef CAS PubMed; (b) Y. Kita, Y. Nishii, A. Onoue and K. Mashima, Adv. Synth. Catal., 2013, 355, 3391 CrossRef CAS; (c) B. N. Atkinson and J. M. J. Williams, Tetrahedron Lett., 2014, 55, 6935 CrossRef CAS; (d) Y. Nishii, S. Akiyama, Y. Kita and K. Mashima, Synlett, 2015, 26, 1831 CrossRef CAS.
- S. M. A. H. Siddiki, A. S. Touchy, M. Tamura and K. Shimizu, RSC Adv., 2014, 4, 35803 RSC.
- (a) L. Hie, N. F. Fine Nathel, T. K. Shah, E. L. Baker, X. Hong, Y. F. Yang, P. Liu, K. N. Houk and N. K. Garg, Nature, 2015, 524, 79 CrossRef CAS PubMed; (b) L. Hie, E. L. Baker, S. M. Anthony, J. N. Desrosiers, C. Senanayake and N. K. Garg, Angew. Chem., Int. Ed., 2016, 55, 15129 CrossRef CAS PubMed; (c) T. Deguchi, H. L. Xin, H. Morimoto and T. Ohshima, ACS Catal., 2017, 7, 3157 CrossRef CAS.
- Based on Mayr's reactivity parameters, the difference of nucleophilicity between amines and alcohols is ∼105 orders of magnitude, for details, see: http://www.cup.lmu.de/oc/mayr/reaktionsdatenbank/, accessed Dec. 15, 2016.
- Y. Bourne-Branchu, C. Gosmini and G. Danoun, Chem.–Eur. J., 2017, 23, 10043 CrossRef CAS PubMed.
- Acyl–acyl exchange between amides and esters was reported by Bian and co-worker, however the scope of amide was limited to primary amides. Y. Bian and X. Qu, Org. Biomol. Chem., 2016, 14, 3869 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra12152k |
| This journal is © The Royal Society of Chemistry 2018 |