Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Study on the growth of Al-doped ZnO thin films with (11![[2 with combining macron]](https://www.rsc.org/images/entities/h2_char_0032_0304.gif) 0) and (0002) preferential orientations and their thermoelectric characteristics
0) and (0002) preferential orientations and their thermoelectric characteristics
Jing-Ting Luoa,
Ao-Jie Quana,
Zhuang-Hao Zhenga,
Guang-Xing Lianga,
Fu Li a,
Ai-Hua Zhong*a,
Hong-Li Mab,
Xiang-Hua Zhangb and
Ping Fan
a,
Ai-Hua Zhong*a,
Hong-Li Mab,
Xiang-Hua Zhangb and
Ping Fan *a
*a
aInstitute of Thin Film Physics and Applications, Shenzhen Key Laboratory of Advanced Thin Films and Applications, College of Physics and Energy, Shenzhen University, 518060, China. E-mail: fanping308@126.com; zhongah@szu.edu.cn
bLaboratory of Glasses and Ceramics, Institute of Chemical Science UMR CNRS 6226, University of Rennes 1, Rennes 35042, France
First published on 6th February 2018
Abstract
In this work, using a conventional magnetron sputtering system, Al-doped ZnO (AZO) films with (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) and (0002) preferential orientations were grown on r-sapphire and a-sapphire substrates, respectively. The effect of substrate and deposition temperature on the growth of AZO films and their preferential orientations were investigated. The crystallographic characteristics of AZO films were characterized by X-ray diffraction (XRD). The surface morphology of AZO films was studied by scanning electron microscopy (SEM) and atomic force microscopy (AFM). It is found that the lattice mismatch between AZO and substrate determines the growth of AZO films and their preferential orientations. The thermoelectric properties are strongly dependent on the crystal grain shape and the grain boundaries induced by the preferred orientation. The highly connected and elongated grains lead to high thermoelectric properties. The in-plane anisotropy performances of thermoelectric characteristics were found in the (11
0) and (0002) preferential orientations were grown on r-sapphire and a-sapphire substrates, respectively. The effect of substrate and deposition temperature on the growth of AZO films and their preferential orientations were investigated. The crystallographic characteristics of AZO films were characterized by X-ray diffraction (XRD). The surface morphology of AZO films was studied by scanning electron microscopy (SEM) and atomic force microscopy (AFM). It is found that the lattice mismatch between AZO and substrate determines the growth of AZO films and their preferential orientations. The thermoelectric properties are strongly dependent on the crystal grain shape and the grain boundaries induced by the preferred orientation. The highly connected and elongated grains lead to high thermoelectric properties. The in-plane anisotropy performances of thermoelectric characteristics were found in the (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) preferential oriented ZnO films. The in-plane power factor of the (11
0) preferential oriented ZnO films. The in-plane power factor of the (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) preferential oriented ZnO films in the [0001] direction was more than 1.5 × 10−3 W m−1 K−2 at 573 K, which is larger than that of the (0002) preferential oriented ZnO films.
0) preferential oriented ZnO films in the [0001] direction was more than 1.5 × 10−3 W m−1 K−2 at 573 K, which is larger than that of the (0002) preferential oriented ZnO films.
1. Introduction
With the increase in the energy crisis and environmental pollution, sustainable energy technology has attracted more and more attention.1–3 Thermoelectric generators can convert heat directly into electricity without using steam and mechanical processes, and have become a hot research field throughout the world.4,5 Thermoelectric application requires thermoelectric materials possessing a large power factor (PF = σS2, where σ is the electrical conductivity, S is the Seebeck coefficient) and low thermal conductivity (κ).6 ZnO has attracted much attention as an alternative for n-type thermoelectric oxide materials due to its high PF, high chemical and thermal stability at elevated temperatures, non-toxicity and low-cost production from abundant raw materials.7–11 As for thermoelectric application, the electrical resistivity of undoped ZnO materials is too high.12 Fortunately, its electrical resistivity can be significantly reduced via doping. Therefore, various dopants were introduced into ZnO to improve its thermoelectric properties.3,8,13–15 Among these works, it is found that Al-doped ZnO (AZO) is one of the best thermoelectric materials for high-temperature thermoelectric application.12,16 Thermoelectric materials in low-dimensional are promising to possess better thermoelectric performance than their bulk materials.1,17,18 A promising approach to achieve the low-dimensional thermoelectric materials is to deposit thin films, which are suitable for small-scale thermoelectric applications.19 This is why so many research group focused on the investigation on the growth of high quality ZnO thin films for thermoelectric applications. ZnO thin film, a versatile multifunctional material,20–22 which has been widely used in solar cells,23 surface acoustic waves,24–27 dilute magnetic semiconductors,28 resistive memory,29 etc. Generally, ZnO is known to readily exhibit (0002)-preferred with the c-axis perpendicular to the substrate due to the lower surface free energy for the (0002) plane on single-crystalline substrates and even on amorphous substrates such as glass, and polymer substrate18,30_. Unusual, some researchers reported theoretical and experimental works concerning other crystalline planes such as (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0)- and (1000)-oriented ZnO films31–36 and ZnO films with different crystalline orientations possess different electronic, optical and acoustic properties. The thermoelectric anisotropic properties between ab-plane and along the c-axis of ZnO based films or ceramics with the c-axis perpendicular to the substrate has been reported. However, to our knowledge, the thermoelectric properties of (11
0)- and (1000)-oriented ZnO films31–36 and ZnO films with different crystalline orientations possess different electronic, optical and acoustic properties. The thermoelectric anisotropic properties between ab-plane and along the c-axis of ZnO based films or ceramics with the c-axis perpendicular to the substrate has been reported. However, to our knowledge, the thermoelectric properties of (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0)-oriented ZnO films have been rarely reported and their thermoelectric characteristics of ZnO films with different crystalline orientations have not been reported in detail yet. Generally, specific deposition techniques are used to deposit ZnO films with good quality, for example pulsed laser deposition technique.37,38 In this work, we use a simple magnetron sputtering technology to deposit AZO films. The growth of AZO thin films with (11
0)-oriented ZnO films have been rarely reported and their thermoelectric characteristics of ZnO films with different crystalline orientations have not been reported in detail yet. Generally, specific deposition techniques are used to deposit ZnO films with good quality, for example pulsed laser deposition technique.37,38 In this work, we use a simple magnetron sputtering technology to deposit AZO films. The growth of AZO thin films with (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) and (0002) preferential orientations and their thermoelectric characteristics of ZnO films with different grain shape and size was investigated. In general, the sapphire is the most commonly used substrate for growing ZnO films with different preferred-orientations. Furthermore, sapphire has low thermal conductivity, which is good for thermoelectric application. Therefore, r-sapphire and a-sapphire substrates were used to grow AZO films with (11
0) and (0002) preferential orientations and their thermoelectric characteristics of ZnO films with different grain shape and size was investigated. In general, the sapphire is the most commonly used substrate for growing ZnO films with different preferred-orientations. Furthermore, sapphire has low thermal conductivity, which is good for thermoelectric application. Therefore, r-sapphire and a-sapphire substrates were used to grow AZO films with (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) and (0002) preferential orientations respectively. It is found that AZO films deposited on r-sapphire and a-sapphire substrates exhibit different grain size and orientation which, in turn, leads to the difference in thermoelectric performance.
0) and (0002) preferential orientations respectively. It is found that AZO films deposited on r-sapphire and a-sapphire substrates exhibit different grain size and orientation which, in turn, leads to the difference in thermoelectric performance.
2. Experimental details
AZO thin films were deposited by radio frequency magnetron sputtering using a ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al (with 2 at% Al) ceramic target (99.995% pure). The single crystalline r-sapphire and a-sapphire substrates was used. The substrate was cut into rectangular pieces and ultrasonically cleaned in acetone, alcohol and deionized water for 8 minutes, respectively. The sputtering chamber was pumped to a base pressure of 1.0 × 10−4 Pa, and then a mixture of highly pure argon (99.999%) and oxygen (99.999%) gases was introduced into the chamber. The working pressure was kept at 0.5 Pa with 40 sccm of Ar and 5 sccm of O2. Prior to deposition, a 15 min pre-sputtering process was performed to remove native oxides and contaminants on the surfaces of the ZnO
Al (with 2 at% Al) ceramic target (99.995% pure). The single crystalline r-sapphire and a-sapphire substrates was used. The substrate was cut into rectangular pieces and ultrasonically cleaned in acetone, alcohol and deionized water for 8 minutes, respectively. The sputtering chamber was pumped to a base pressure of 1.0 × 10−4 Pa, and then a mixture of highly pure argon (99.999%) and oxygen (99.999%) gases was introduced into the chamber. The working pressure was kept at 0.5 Pa with 40 sccm of Ar and 5 sccm of O2. Prior to deposition, a 15 min pre-sputtering process was performed to remove native oxides and contaminants on the surfaces of the ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al target. The distance between the target and substrates was fixed at 70 mm. The sputtering power was kept at 100 watts and the deposition rate was about 20 nm min−1. According to our previous work, the stress of the thin films would be minimum when the thickness of AZO films is around 600 nm. Therefore, the deposition time was fixed at 30 minutes. After deposition, the thicknesses of the AZO films were measured using a stylus profilometer and all the ZnO films have similar thickness (∼600 nm).
Al target. The distance between the target and substrates was fixed at 70 mm. The sputtering power was kept at 100 watts and the deposition rate was about 20 nm min−1. According to our previous work, the stress of the thin films would be minimum when the thickness of AZO films is around 600 nm. Therefore, the deposition time was fixed at 30 minutes. After deposition, the thicknesses of the AZO films were measured using a stylus profilometer and all the ZnO films have similar thickness (∼600 nm).
The structure of the prepared AZO films was studied by X-ray diffraction (XRD) using Cu-Kα (λα = 0.15406 nm) radiation with the conventional θ–2θ mode. XRD Phi-scans was employed to investigate the epitaxial relationship between ZnO films and the substrate diffraction. The surface morphology of the deposited AZO films were examined by scanning electron microscope (SEM) and atomic force microscopy (AFM). The room temperature carrier concentrations and mobility of rectangular AZO thin film samples along the length and width direction, respectively, were evaluated using Hall measurement system in a four-probe van der Pauw configuration. The electrical conductivity of the AZO films were measured by the home-made four-point-probe technique. The four point measurements are co-linear and the conductivities of all the rectangular samples were measured along length and width, respectively. Seebeck coefficient was measured with the temperature gradient method (ΔT = 20 K) using a SDFP-1 measurement system and the Seebeck coefficient of all the rectangular samples were measured along length and width, respectively. All the measurement temperature was changed from room temperature to 573 K.
3. Results and discussion
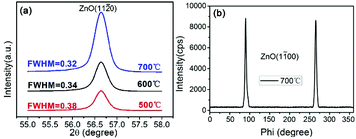
The crystal structures of all the deposited AZO films were investigated by XRD. Fig. 1(a) shows the XRD patterns of AZO films deposited on r-plane sapphire substrates with the substrate temperatures of 500 °C, 600 °C and 700 °C. The reflections observed corresponding to ZnO plane centered at 56.6°, with two additional substrate reflections arising from the sapphire (2![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 04) and (3
04) and (3![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) 06) plane. No other peaks can be seen in the patterns under the detection limit of XRD, indicating that the deposited ZnO films have preferred (11
06) plane. No other peaks can be seen in the patterns under the detection limit of XRD, indicating that the deposited ZnO films have preferred (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) orientation with c-axis parallel to the substrate. From these reflections, it is suggested that the out of plane epitaxial relationship between ZnO films and the sapphire substrates is (11
0) orientation with c-axis parallel to the substrate. From these reflections, it is suggested that the out of plane epitaxial relationship between ZnO films and the sapphire substrates is (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0)‖(2
0)‖(2![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 04). And the epitaxial relationship does not change with the increase of substrate temperature. However, the peak intensity of ZnO (11
04). And the epitaxial relationship does not change with the increase of substrate temperature. However, the peak intensity of ZnO (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) increases and the full-width at half-maximum (FWHM) value of ZnO (11
0) increases and the full-width at half-maximum (FWHM) value of ZnO (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) decreases a little with the increase of substrate temperature, indicating that ZnO film has relative stronger (11
0) decreases a little with the increase of substrate temperature, indicating that ZnO film has relative stronger (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) texture and better crystalline at the elevated temperature.
0) texture and better crystalline at the elevated temperature.
Azimuthal scans (phi-scans) were performed to investigate the in-plane epitaxial relationship between ZnO film and r-sapphire substrate. Since ZnO films deposited at the substrate temperature of 700 °C has the best crystalline, Fig. 1(b) shows the phi-scans through off normal ZnO (1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 00) reflections of AZO films deposited at 700 °C. In the range of 0–360°, only two diffraction peaks can be seen in the pattern and they are spacing at ∼180°, indicating that ZnO films in a-plane shows two-fold symmetry. Based on the phi-scans of r-sapphire, we conclude that the in-plane epitaxial relationship between ZnO film and substrate is [0001]ZnO‖[
00) reflections of AZO films deposited at 700 °C. In the range of 0–360°, only two diffraction peaks can be seen in the pattern and they are spacing at ∼180°, indicating that ZnO films in a-plane shows two-fold symmetry. Based on the phi-scans of r-sapphire, we conclude that the in-plane epitaxial relationship between ZnO film and substrate is [0001]ZnO‖[![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 101]Al2O3 and [
101]Al2O3 and [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 100]ZnO‖[
100]ZnO‖[![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 20]Al2O3, which is coincident to the previous literatures.34,39
20]Al2O3, which is coincident to the previous literatures.34,39
Fig. 2 illustrates the in-plane epitaxial relationship between ZnO film and the sapphire substrate. The calculated lattice mismatch along the two different directions in-plane is marked in the diagram. Along the c-axis of ZnO (ZnO [0001] direction), the in-plane lattice parameters of ZnO [0001] and sapphire [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 101] are 5.2069 and 5.1272 Å, respectively,34 and the mismatch of ZnO thin film in-plane along [0001] direction is 1.55% ((5.2069−5.1272)/5.1272). For the direction along ZnO [
101] are 5.2069 and 5.1272 Å, respectively,34 and the mismatch of ZnO thin film in-plane along [0001] direction is 1.55% ((5.2069−5.1272)/5.1272). For the direction along ZnO [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 100], the lattice parameters of ZnO [
100], the lattice parameters of ZnO [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 100] and sapphire [
100] and sapphire [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 20] are 5.629 and 4.756 Å respectively,34 and the mismatch of ZnO thin film in-plane along [
20] are 5.629 and 4.756 Å respectively,34 and the mismatch of ZnO thin film in-plane along [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 100] direction is 18.36% ((5.629−4.756)/4.756). It can be seen that ZnO thin film has structure anisotropy in-plane and the mismatch along the [0001] direction is much smaller than along [
100] direction is 18.36% ((5.629−4.756)/4.756). It can be seen that ZnO thin film has structure anisotropy in-plane and the mismatch along the [0001] direction is much smaller than along [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 100] direction. The mismatch along [
100] direction. The mismatch along [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 100] direction can be made up by the two alternate domains of ZnO thin films, namely five ZnO (1
100] direction can be made up by the two alternate domains of ZnO thin films, namely five ZnO (1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 00) planes matches with six sapphire (11
00) planes matches with six sapphire (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) planes, as well as six ZnO (1
0) planes, as well as six ZnO (1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 00) planes matches with seven sapphire (11
00) planes matches with seven sapphire (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) planes.
0) planes.
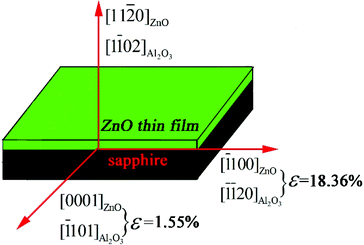 | ||
| Fig. 2 The schematic illustration of the in-plane epitaxial relationship between ZnO film and r-sapphire substrate. | ||
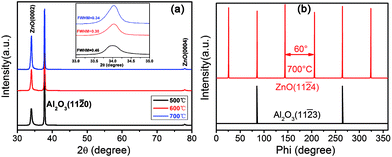
The XRD patterns of the AZO thin films grown on a-sapphire at 500 °C, 600 °C and 700 °C showed in Fig. 3(a). The two diffraction peaks centered at ∼34.4° and ∼72.5° are characteristic of the wurtzite structure of ZnO, corresponding to the reflections of ZnO (0002) and (0004) planes, respectively.33 There is another peak centered at ∼37.7° arising from the (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) planes of the a-sapphire substrate. No other peaks exhibited in the patterns under the detection limit of XRD, indicating that the deposited ZnO films have preferred (0002) orientation with c-axis perpendicular to the substrate. The peak intensity of ZnO (0002) increases and the FWHM value of ZnO (0002) decreases from 0.46° to 0.34° with the substrate temperature increase from 500 °C to 700 °C, indicating that ZnO film has better crystalline with the increase of substrate temperature. In addition, the microstructure and crystalline preferred orientation do not change with increasing in substrate temperature. Therefore, we chose ZnO films with better crystallinity deposited at 700 °C to investigate the epitaxial relationship between ZnO film and sapphire using Phi-scans. Fig. 3(b) shows the phi-scans through off normal ZnO (11
0) planes of the a-sapphire substrate. No other peaks exhibited in the patterns under the detection limit of XRD, indicating that the deposited ZnO films have preferred (0002) orientation with c-axis perpendicular to the substrate. The peak intensity of ZnO (0002) increases and the FWHM value of ZnO (0002) decreases from 0.46° to 0.34° with the substrate temperature increase from 500 °C to 700 °C, indicating that ZnO film has better crystalline with the increase of substrate temperature. In addition, the microstructure and crystalline preferred orientation do not change with increasing in substrate temperature. Therefore, we chose ZnO films with better crystallinity deposited at 700 °C to investigate the epitaxial relationship between ZnO film and sapphire using Phi-scans. Fig. 3(b) shows the phi-scans through off normal ZnO (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 4) reflections of ZnO films deposited at 700 °C together with the phi-scan through a-sapphire (11
4) reflections of ZnO films deposited at 700 °C together with the phi-scan through a-sapphire (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 3) plane. The figure shows six-fold symmetry with about 60° intervals, indicating a good hexagonal symmetry. Two peak positions of ZnO (11
3) plane. The figure shows six-fold symmetry with about 60° intervals, indicating a good hexagonal symmetry. Two peak positions of ZnO (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 4) match good with that of sapphire substrate with (11
4) match good with that of sapphire substrate with (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 3) reflections, yielding an in-plane epitaxial relationship of [
3) reflections, yielding an in-plane epitaxial relationship of [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 100]ZnO‖[
100]ZnO‖[![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 100]Al2O3.
100]Al2O3.
It is reported that the different substrate caused different grain size and shape of AZO thin films,15 which result in different thermoelectric performance. The grain size of AZO films can be calculated using Scherrer equation:
D = kλ/(β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) θ)
| (1) |
| AZO films | FWHM (°) | D (nm) |
|---|---|---|
| AZO/r-sapphire at 500 °C | 0.38 | 24.9 |
| AZO/r-sapphire at 600 °C | 0.34 | 27.8 |
| AZO/r-sapphire at 700 °C | 0.32 | 29.5 |
| AZO/a-sapphire at 500 °C | 0.46 | 17.9 |
| AZO/a-sapphire at 600 °C | 0.38 | 21.6 |
| AZO/a-sapphire at 700 °C | 0.34 | 24.2 |
Furthermore, SEM and AFM were used to characterize the surface morphology of AZO films. Fig. 4(a)–(c) show the top-view SEM images of AZO films deposited on a-sapphire at 500 °C, 600 °C and 700 °C, respectively. Fig. 4(a)–(c) show nearly rounded grains morphology of AZO deposited on a-sapphire and AZO films deposited at 700 °C has denser and uniform grains than that deposited at 500 °C and 600 °C. Fig. 4(d)–(f) show the top-view SEM images of AZO films deposited on r-sapphire at 500 °C, 600 °C and 700 °C, respectively. It shows elongated grains morphology commonly observed on ZnO film with (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) preferred orientation.40,41 The elongated direction is parallel to [0001] usually seen on ZnO film with (11
0) preferred orientation.40,41 The elongated direction is parallel to [0001] usually seen on ZnO film with (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) preferred orientation.42
0) preferred orientation.42
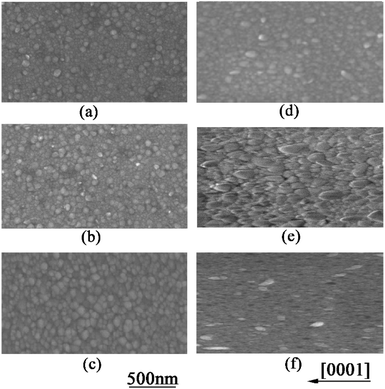 | ||
| Fig. 4 SEM image of AZO thin film deposited on a-sapphire at 500, 600, and 700 °C (a)–(c), and on r-sapphire at 500, 600 and 700 °C (d)–(f). | ||
We also used AFM to confirm the influence of substrate on the grain shape of AZO films. Fig. 5 (a)–(c) show the AFM images of AZO films deposited on a-sapphire at 500, 600 and 700 °C, which (d)–(f) show the AFM images of AZO films deposited on r-sapphire at 500, 600 and 700 °C, respectively. AZO films deposited on a-sapphire exhibits rounded and uniform grains as shown in Fig. 5(a)–(c) and they seem isotropic in all directions. AZO films deposited on r-sapphire show the elongated grains with different sizes as shown in Fig. 5(d)–(f). AZO films show stretched morphology in [0001] direction, illustrating anisotropic in-plane ZnO thin film. It is suggested that the stretch direction is along [0001] direction since the required growth momentum along [0001] direction is minimum in AZO (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) plane. With the increase of substrate temperature, the stretched morphology is more and more obvious. This is because the higher temperature provide more sufficient energy for the growth of AZO grains. The thin film morphology as revealed by AFM showed good agreement with SEM results. Therefore, one can conclude that substrate strongly affects the grain shape and size of AZO films.
0) plane. With the increase of substrate temperature, the stretched morphology is more and more obvious. This is because the higher temperature provide more sufficient energy for the growth of AZO grains. The thin film morphology as revealed by AFM showed good agreement with SEM results. Therefore, one can conclude that substrate strongly affects the grain shape and size of AZO films.
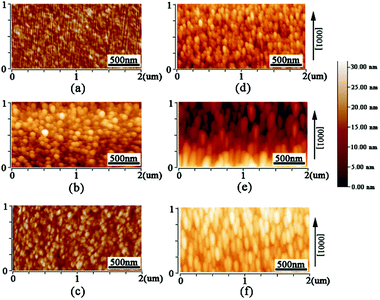 | ||
| Fig. 5 AFM images of AZO films deposited on a-sapphire at 500, 600 and 700 °C (a)–(c) and AZO films deposited on r-sapphire at 500, 600 and 700 °C (d)–(f). | ||
The electrical conductivity σ of AZO thin films with different orientation depends on the testing temperature is shown in Fig. 6(a). The σ values of all the films decrease slightly with the increase of measuring temperature, indicating the metallic electrical conductivity behaviour. The carrier concentrations of AZO films shown in Table 2 are around 22–24 × 1020 cm−3, which are similar to that of metal conductor. The prepared AZO films are n-type semiconductor and the carrier concentration is so high that the Fermi energy is approaching or entering the conductive band. Therefore, AZO films become n-type degenerate semiconductor. It is well known that the degenerate semiconductor exhibit metallic electrical conductivity behavior. Similarly, the deposited AZO thin films exhibit metallic electrical conductivity behavior. The conductivity of AZO (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) thin films along [0001] is larger than all the other films and AZO (11
0) thin films along [0001] is larger than all the other films and AZO (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) thin films show anisotropic electrical conductivity in plane along [0001] and [
0) thin films show anisotropic electrical conductivity in plane along [0001] and [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 100] direction. All the AZO films deposited at 700 °C shows similar carrier concentrations regardless of preferred orientation, however, AZO films deposited at 700 °C shows larger carrier concentrations than that of AZO films deposited at 500 °C as shown in Table 2. AZO (11
100] direction. All the AZO films deposited at 700 °C shows similar carrier concentrations regardless of preferred orientation, however, AZO films deposited at 700 °C shows larger carrier concentrations than that of AZO films deposited at 500 °C as shown in Table 2. AZO (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) thin film along [0001] direction possesses large elongated grains parallel to the substrate, which can be used as the efficient carrier pathway. Furthermore, it has less grain boundaries along [0001] direction, and the electron can transport easier in AZO (11
0) thin film along [0001] direction possesses large elongated grains parallel to the substrate, which can be used as the efficient carrier pathway. Furthermore, it has less grain boundaries along [0001] direction, and the electron can transport easier in AZO (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) thin films along [0001] direction. Therefore, AZO (11
0) thin films along [0001] direction. Therefore, AZO (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) thin films along [0001] direction exhibits largest carrier mobility as shown in Table 2, which results in high conductivity. AZO (0002) thin films have columnar structure with large grains perpendicular to the substrate, making more grain boundaries in ab plane. The larger amount of grain boundaries in-plane induces stronger scattering of electrons. Therefore, the carrier mobility as shown in Table 2 in AZO (0002) thin films are smaller than that of AZO (11
0) thin films along [0001] direction exhibits largest carrier mobility as shown in Table 2, which results in high conductivity. AZO (0002) thin films have columnar structure with large grains perpendicular to the substrate, making more grain boundaries in ab plane. The larger amount of grain boundaries in-plane induces stronger scattering of electrons. Therefore, the carrier mobility as shown in Table 2 in AZO (0002) thin films are smaller than that of AZO (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) thin films along [0001] direction and the conductivity of AZO (0002) thin films is smaller. However, with the increase of substrate temperature, the carrier concentration increases and the crystalline improves with denser and uniform grain (see Fig. 4(b) and (c)), which makes the electron transport easier. That is why the conductivity of AZO (0002) thin film deposited at 700 °C is larger than that deposited at 500 °C. It is reported that AZO (11
0) thin films along [0001] direction and the conductivity of AZO (0002) thin films is smaller. However, with the increase of substrate temperature, the carrier concentration increases and the crystalline improves with denser and uniform grain (see Fig. 4(b) and (c)), which makes the electron transport easier. That is why the conductivity of AZO (0002) thin film deposited at 700 °C is larger than that deposited at 500 °C. It is reported that AZO (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) thin film along [
0) thin film along [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 100] direction is the least stable and tends to form long groove, which make the electrons transport more difficult.35 Therefore, although it has similar carrier concentration, the carrier mobility is smaller as shown in Table 2. That is why the AZO (11
100] direction is the least stable and tends to form long groove, which make the electrons transport more difficult.35 Therefore, although it has similar carrier concentration, the carrier mobility is smaller as shown in Table 2. That is why the AZO (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) thin film along [
0) thin film along [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 100] direction has the lowest conductivity.
100] direction has the lowest conductivity.
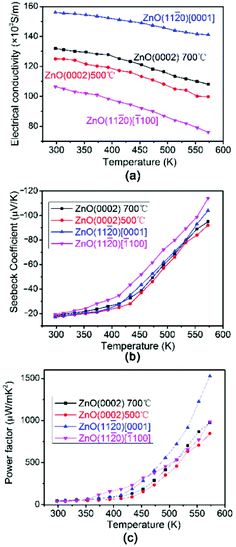 | ||
| Fig. 6 The thermoelectric properties of AZO thin films with different orientation as a function of the temperature. (a) Electrical conductivity (b) Seebeck coefficient and (c) power factor. | ||
| AZO films | Carrier concentration (×1020 cm−3) | Mobility (cm2 V−1 s−1) |
|---|---|---|
| AZO (0002) 700 °C | 23.4 | 15.6 |
| AZO (0002) 500 °C | 22.8 | 15.1 |
AZO (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0)[0001] 0)[0001] |
23.7 | 18.2 |
AZO (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0)[ 0)[![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 100] 100] |
23.1 | 12.3 |
In short, we believe that the different grain shapes and sizes induced by the preferred orientation results in different conductivities. The substrate effects on the conductivity of AZO thin films were also reported by Saini et al.43 They found that AZO thin films deposited on Al2O3 substrate exhibited c-axis oriented and larger electrical conductivity than deposited on SrTiO3 and fused silica.
Fig. 6(b) shows the testing temperature dependence of the Seebeck coefficient of AZO (0002) thin films deposited at different temperature and AZO (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) thin films along different direction. As can be seen from the figure, the negative S indicates that all of the AZO films are n-type semiconductor thermoelectric materials. This is expected since Al dissolved in the ZnO crystal lattice acting as donor, which will create extra electrons. In general, Seebeck coefficient S can be expressed as following:19
0) thin films along different direction. As can be seen from the figure, the negative S indicates that all of the AZO films are n-type semiconductor thermoelectric materials. This is expected since Al dissolved in the ZnO crystal lattice acting as donor, which will create extra electrons. In general, Seebeck coefficient S can be expressed as following:19
 | (2) |
![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) thin films along [
0) thin films along [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 100] direction have smaller carrier concentration and mobility, the absolute value of S of AZO (11
100] direction have smaller carrier concentration and mobility, the absolute value of S of AZO (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) thin films along [
0) thin films along [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 100] direction is larger than others. The S of other samples does not exhibit too much difference as shown in Fig. 6(b).
100] direction is larger than others. The S of other samples does not exhibit too much difference as shown in Fig. 6(b).
From the values of Seebeck coefficient and electrical conductivity, we have calculated the PF of thin films depends on the testing temperature as shown in Fig. 6(c). The PF of all the AZO films increases with increasing temperature. The power factor of (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) preferential oriented AZO film in [0001] direction is more than 1.5 × 10−3 W m−1 K−2 at 573 K because of the large electrical conductivity, which is larger than all the other preferential oriented ZnO films. The PF in our work is comparable to the previously reported values.12,44,45 The PF of AZO (0002) thin film deposited at 700 °C is slightly larger than that of AZO (0002) thin film deposited at 500 °C, indicating that PF depends slightly on orientation degree in AZO (0002) thin films. Saini et al.43 reported that AZO thin film deposited on fused silica substrate showed as large as −200 μV K−1, however, the small σ limited its PF. Fortunately, the formation of the seed layer lead to the significant decrease of thermal conductivity, which improved the thermoelectric figure.
0) preferential oriented AZO film in [0001] direction is more than 1.5 × 10−3 W m−1 K−2 at 573 K because of the large electrical conductivity, which is larger than all the other preferential oriented ZnO films. The PF in our work is comparable to the previously reported values.12,44,45 The PF of AZO (0002) thin film deposited at 700 °C is slightly larger than that of AZO (0002) thin film deposited at 500 °C, indicating that PF depends slightly on orientation degree in AZO (0002) thin films. Saini et al.43 reported that AZO thin film deposited on fused silica substrate showed as large as −200 μV K−1, however, the small σ limited its PF. Fortunately, the formation of the seed layer lead to the significant decrease of thermal conductivity, which improved the thermoelectric figure.
4. Conclusions
AZO thin films with (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) and (0002) preferential orientations were respectively grown on r-sapphire and a-sapphire substrates using a conventional magnetron sputtering method. AZO film shows relative stronger preferred orientation and better crystallinity with the increase of substrate temperature. The electrical conductivity is strongly depended on the crystal grain shape and the grain boundaries induced by preferred orientation. Due to the elongated grains and less grain boundaries, the electrical conductivity of (11
0) and (0002) preferential orientations were respectively grown on r-sapphire and a-sapphire substrates using a conventional magnetron sputtering method. AZO film shows relative stronger preferred orientation and better crystallinity with the increase of substrate temperature. The electrical conductivity is strongly depended on the crystal grain shape and the grain boundaries induced by preferred orientation. Due to the elongated grains and less grain boundaries, the electrical conductivity of (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) oriented ZnO films along [0001] direction is much larger than that of all other preferential oriented ZnO films. The in-plane PF of (11
0) oriented ZnO films along [0001] direction is much larger than that of all other preferential oriented ZnO films. The in-plane PF of (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) preferential oriented AZO films in [0001] direction was more than 1.5 × 10−3 W m−1 K−2 at 573 K, which are larger than that of all the other preferential oriented AZO films.
0) preferential oriented AZO films in [0001] direction was more than 1.5 × 10−3 W m−1 K−2 at 573 K, which are larger than that of all the other preferential oriented AZO films.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
J. T. Luo and Z. H. Zheng contributed equally. This work was supported by National Key Research and Development Program of China (Grant no. 2016YFB0402705), Shenzhen Key Lab Fund (ZDSYS20170228105421966), PhD Start-up Fund of Natural Science Foundation of Guangdong Province, China (2017A030310375).References
- P. Fan, Z.-H. Zheng, Z.-K. Cai, T.-B. Chen, P.-J. Liu, X.-M. Cai, D.-P. Zhang, G.-X. Liang and J.-T. Luo, Appl. Phys. Lett., 2013, 102, 033904 CrossRef.
- G.-X. Liang, P. Fan, J.-T. Luo, D. Gu and Z.-H. Zheng, Prog. Photovoltaics Res. Appl., 2015, 23, 1901 CrossRef CAS.
- P. Jood, R. J. Mehta, Y. Zhang, G. Peleckis, X. Wang, R. W. Siegel, T. Borca-Tasciuc, S. X. Dou and G. Ramanath, Nano Lett., 2011, 11, 4337 CrossRef CAS PubMed.
- J. Yang and F. R. Stabler, J. Electron. Mater., 2009, 38, 1245 CrossRef CAS.
- G. J. Snyder and E. S. Toberer, Nat. Mater., 2008, 7, 105 CrossRef CAS PubMed.
- Z.-H. Zheng, P. Fan, J.-T. Luo, G.-X. Liang and D.-P. Zhang, J. Electron. Mater., 2013, 42, 3421 CrossRef CAS.
- P. Fan, Y.-Z. Li, Z.-H. Zheng, Q.-Y. Lin, J.-T. Luo, G.-X. Liang, M.-Q. Zhang and M.-C. Chen, Appl. Surf. Sci., 2013, 284, 145 CrossRef CAS.
- K. P. Ong, D. J. Singh and P. Wu, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 115110 CrossRef.
- S. Harizanova, E. Zhecheva, R. Stoyanova, V. Valchev, and M. Khristov, Materials and Technologies for Energy Efficiency, Brown Walker Press, USA, 2015, pp. 254–258 Search PubMed.
- E. A. Mondarte, V. Copa, A. Tuico, C. J. Vergara, E. Estacio, A. Salvador and A. Somintac, Mater. Sci. Semicond. Process., 2016, 45, 27 CrossRef CAS.
- D.-B. Zhang, B.-P. Zhang, D.-S. Ye, Y.-C. Liu and S. Li, J. Alloys Compd., 2016, 656, 784 CrossRef CAS.
- N. Vogel-Schäuble, T. Jaeger, Y. E. Romanyuk, S. Populoh, C. Mix, G. Jakob and A. Weidenkaff, Phys. Status Solidi RRL, 2013, 7, 364 CrossRef.
- M. Ohtaki, K. Araki and K. Yamamoto, J. Electron. Mater., 2009, 38, 1234 CrossRef CAS.
- H. Yamaguchi, Y. Chonan, M. Oda, T. Komiyama, T. Aoyama and S. Sugiyama, J. Electron. Mater., 2011, 40, 723 CrossRef CAS.
- P. Mele, S. Saini, H. Honda, K. Matsumoto, K. Miyazaki, H. Hagino and A. Ichinose, Appl. Phys. Lett., 2013, 102, 253903 CrossRef.
- T. Tsubota, M. Ohtaki, K. Eguchi and H. Arai, J. Mater. Chem., 1997, 7, 85 RSC.
- P. Fan, Y. Zhang, Z.-H. Zheng, W.-F. Fan, J.-T. Luo, G.-X. Liang and D.-P. Zhang, J. Electron. Mater., 2015, 44, 630 CrossRef CAS.
- P. Fan, Z.-H. Zheng, Y.-Z. Li, Q.-Y. Lin, J.-T. Luo, G.-X. Liang, X.-M. Cai, D.-P. Zhang and F. Ye, Appl. Phys. Lett., 2015, 106, 073901 CrossRef.
- Y. Kinemuchi, M. Mikami, K. Kobayashi, K. Watari and Y. Hotta, J. Electron. Mater., 2009, 39, 2059 CrossRef.
- S. Pearton, D. Norton, K. Ip, Y. Heo and T. Steiner, Prog. Mater. Sci., 2005, 50, 293 CrossRef CAS.
- M. Snure and A. Tiwari, J. Appl. Phys., 2007, 101, 124912 CrossRef.
- A. Tiwari, M. Park, C. Jin, H. Wang, D. Kumar and J. Narayan, J. Mater. Res., 2002, 17, 2480 CrossRef CAS.
- D. H. Kim, J.-H. Park, T. I. Lee and J.-M. Myoung, Sol. Energy Mater. Sol. Cells, 2015, 150, 65 CrossRef.
- J. T. Luo, F. Pan, P. Fan, F. Zeng, D. P. Zhang, Z. H. Zheng and G. X. Liang, Appl. Phys. Lett., 2012, 101, 172909 CrossRef.
- J. T. Luo, P. Fan, F. Pan, F. Zeng, D. P. Zhang, Z. H. Zheng, G. X. Liang and X. M. Cai, Phys. Status Solidi RRL, 2012, 6, 436 CrossRef CAS.
- J. Luo, P. Luo, M. Xie, K. Du, B. Zhao, F. Pan, P. Fan, F. Zeng, D. Zhang, Z. Zheng and G. Liang, Biosens. Bioelectron., 2013, 49, 512 CrossRef CAS PubMed.
- J. Luo, M. Xie, P. Luo, B. Zhao, K. Du and P. Fan, Mater. Lett., 2014, 130, 14 CrossRef CAS.
- F. Pan, C. Song, X. Liu, Y. Yang and F. Zeng, Mater. Sci. Eng., 2008, 2, 1 Search PubMed.
- Y. C. Yang, F. Pan, Q. Liu, M. Liu and F. Zeng, Nano Lett., 2009, 9, 1636 CrossRef CAS PubMed.
- L. M. Zheng, Jpn. J. Appl. Phys., 2005, 44, 28 CrossRef.
- H. Liu, F. Zeng, Y. Lin, G. Wang and F. Pan, Appl. Phys. Lett., 2013, 102, 181908 CrossRef.
- X. Wang, Z. Tian, T. Yu, H. Tian, J. Zhang, S. Yuan, X. Zhang, Z. Li and Z. Zou, Nanotechnology, 2010, 21, 065703 CrossRef PubMed.
- H. Liu, S. Gao, F. Zeng, C. Song and F. Pan, Phys. Status Solidi RRL, 2013, 7, 587 CrossRef CAS.
- J.-M. Chauveau, P. Vennéguès, M. Laügt, C. Deparis, J. Zuniga-Perez and C. Morhain, J. Appl. Phys., 2008, 104, 073535 CrossRef.
- O. Dulub, L. A. Boatner and U. Diebold, Surf. Sci., 2002, 519, 201 CrossRef CAS.
- Y. Wu, L. Zhang, G. Xie, J. Ni and Y. Chen, Solid State Commun., 2008, 148, 247 CrossRef CAS.
- C. Jin, R. Narayan, A. Tiwari, H. Zhou, A. Kvit and J. Narayan, Mater. Sci. Eng., C, 2005, 117, 348 CrossRef.
- S. Saini, P. Mele, H. Honda, K. Matsumoto, K. Miyazaki, L. M. Luna and P. Hopkins, J. Electron. Mater., 2015, 44, 1547 CrossRef CAS.
- P. Pant, J. D. Budai and J. Narayan, Acta Mater., 2010, 58, 1097 CrossRef CAS.
- P. Ding, X. Pan, J. Huang, B. Lu, H. Zhang, W. Chen and Z. Ye, Mater. Lett., 2012, 71, 18 CrossRef CAS.
- P. Pant, J. D. Budai, R. Aggarwal, R. J. Narayan and J. Narayan, Acta Mater., 2009, 57, 4426 CrossRef CAS.
- H. Wang, C. Chen, Z. Gong, J. Zhang, M. Gaevski, M. Su, J. Yang and M. A. Khan, Appl. Phys. Lett., 2004, 84, 499 CrossRef CAS.
- S. Saini, P. Mele, H. Honda, T. Suzuki, K. Matsumoto, K. Miyazaki, A. Ichinose, L. M. Luna, R. Carlini and A. Tiwari, Thin Solid Films, 2016, 605, 289 CrossRef CAS.
- O. J. Gregory and M. Amani, J. Electrochem. Soc., 2011, 158, 15 CrossRef.
- T. Tsubota, M. Ohtaki, K. Eguchi and H. Arai, J. Mater. Chem., 1997, 7, 85 RSC.
| This journal is © The Royal Society of Chemistry 2018 |