Open Access Article
Open Access ArticleDimeric cinchona ammonium salts with benzophenone linkers: enantioselective phase transfer catalysts for the synthesis of α-amino acids†
Seunga Woo,
Yong-Gyun Kim,
Baegeun Lim,
Jiin Oh,
Yeonji Lee,
Hyeri Gwon and
Keepyung Nahm *
*
School of Chemistry and Biochemistry, Yeungnam University, 280 Daehak-Ro, Gyeongsan, Gyeongbuk 38541, Republic of Korea. E-mail: kpnahm@yu.ac.kr
First published on 9th January 2018
Abstract
Chiral phase transfer catalysts of dimeric cinchona ammonium salts linked with a benzophenone bridge showed high enantioselectivity in the α-alkylation of a glycinate ester under mild industry-applicable conditions: 0.5 mol% PTC and near equivalents of alkyl halide. A dual function of the dimeric quinuclidiniums was proposed for the high efficiency.
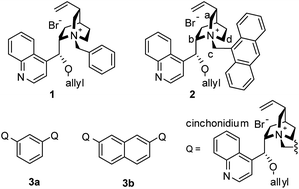
Since cinchona alkaloids were transformed to asymmetric quaternary quinuclidinium salts with benzyl halides and were used as a phase-transfer catalyst (PTC) (1) by Dolling1 and O'Donnell,2 cinchona alkaloids have been widely utilized as chiral templates for phase-transfer catalysis.3 (Scheme 1) These organic PTCs can be easily prepared from natural and low cost chiral cinchona alkaloids in a few synthetic steps and they are stable and facile under normal reaction conditions in water. Later N-9-anthracenylmethyl quinuclidinium salt PTC (2) was introduced and showed high enantioselectivity for the alkylation of the protected glycine tert-butyl ester.4 Quinuclidinium PTCs with each pseudo-enantiomeric cinchona alkaloids, such as (−)-cinchonidine and (+)-cinchonine, show enantiomeric selectivity each other, and have been successfully applied in various asymmetric organic synthesis including α-alkyl glycine derivatives synthesis.3
Enantioselectivity in the alkylation of glycine esters has been probed by several experimental trials. Crystallographic study of the p-nitrophenoxide salt of PTC 2 showed that N-anthracenylmethy moiety of 2 is located in staggered position between the Cc and the Cd,4a which provides more hindered spaces around the Cb–Cc–Cd face (F4) around the ammonium.5 The Ca–Cb–Cd face (F2) is blocked by O-allyl group and the Ca–Cc–Cd face (F3) are covered by bicyclic ring, but the Ca–Cb–Cc face (F1) is less hindered. Therefore, anionic glycinate derivatives could approach toward the F1. Enolates of glycine esters would form tight ionic complexes with the ammonium nitrogen on the F1 face of PTC 2, and the alkylation with alkyl halides could follow along the direction of less hindered side of the si/re-face of the enolates.4a
NOE correlations study of PTC 2 with borohydride ion6 indicated the borohydride occupies the F1 face of PTC 2. Computational simulation7 also described the stable transition states where an enolate locates on the F1 face.
Dimeric cinchona-derived PTCs linked by either benzene or naphthalene ligand have been introduced by Park et al.8a–c Among ortho, meta and para-connected PTCs, the meta-disubstituted phenyl PTC 3a or 2,7-disubstituted naphthyl PTC 3b showed highly improved catalytic effects compared to monomeric PTC 2, such as lower dosage of catalyst (1–5 mol%) and high enantioselectivity. Role of additional quinuclidinium was thought to be a steric blocker which could increase the stereoselectivity of the enolate complex on the F1 face.
Other dimeric cinchona alkaloid PTCs were also developed with various linkers, such as 9,10-dimethylanthracenyl,9 biphenyl, alkenyl,10 macrocyclic amine and calixarene,11 and their enantioselectivities were equal or lower than those of monomeric PTCs. Some dimeric PTCs were converted to ionic polymers by replacing bromides to a disufonate anion without loss of reactivity and enantioselectivity.12
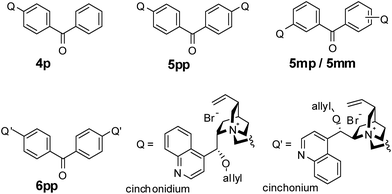
Alkylation of tert-butyl glycinate ester was performed with 1–10 mol% of PTC 1–3 and excess 5 equivalents of alkyl halide at 0 to −78 °C. These catalytic conditions are still to be improved for practical application; low mol% of PTC, near equimolar amount of alkyl halides and ambient temperature. Hence we investigated dimeric PTCs with various linkers for facile catalytic condition. Here we introduce new dimeric cinchona PTCs with a benzophenone linker and their application in asymmetric alkylation of glycine derivatives.
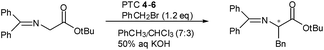
Monomeric PTC 4p, N-(4-benzoylbenzyl)-O(9)-allylcinchonidium bromide, which has a benzoyl benzyl at N(1), was synthesized from 4-bromomethyl-benzophenone and (−)-cinchonidine. (Scheme 2) Dimeric cinchona-based quarternay ammonium salts (PTC 5–6) were synthesized from meta/para-di(bromomethyl) benzophenone13 and two equivalent cinchona alkaloids. Coupling reaction of the di(bromomethyl)benzophenone and (−)-cinchonidine or (+)-cinchonine in EtOH/DMF/CHCl3 (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)8 for 5 h at 100 °C and the O(9)-allylation with allyl bromide gave the dimeric quarternary salts, bis(4-(O(9)-allyl-cinchonidium-N-methyl)phenyl) methanone dibromide (5) and bis(4-(O(9)-allyl-cinchonium-N-methyl)phenyl) methanone dibromide (6), respectively, in good yields.‡
2)8 for 5 h at 100 °C and the O(9)-allylation with allyl bromide gave the dimeric quarternary salts, bis(4-(O(9)-allyl-cinchonidium-N-methyl)phenyl) methanone dibromide (5) and bis(4-(O(9)-allyl-cinchonium-N-methyl)phenyl) methanone dibromide (6), respectively, in good yields.‡
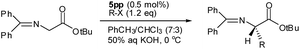
Enantioselective PTC 4–6 system was applied in the alkylation of N-(diphenylmethylene)glycine tert-butyl ester (7) to the α-alkylated glycinate (8) under the condition of 0.5–1.0 mol% catalysts and 1.2 equivalent alkyl halides. We also explored the variation of enantioselectivity depending on the various positions of dimeric cinchonidium at benzophenone; 5pp, 5mp and 5mm.
Monomeric PTC 4p showed enantioselectivity of 87% ee (S) at 25 °C (Table 1, entry 1) which is slightly higher than 81% ee of N-benzyl PTC 1.2 Geometric difference between PTC 1 and 4p is the extra p-benzoyl substituent on N-benzyl. Apparently the p-benzoyl moiety gives no big enhancement in enantioselectivity of 4p.
| Entry | PTC | mol% | Temp (°C) | Time (h) | Yieldb (%) | % eec (config)d |
|---|---|---|---|---|---|---|
a Benzylation of 7 (0.1 mmol) was carried out with 1.2 equivalents of benzyl bromide and 50% aqueous KOH (0.25 mL) in toluene/chloroform (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 0.75 mL) under nitrogen atmosphere, unless otherwise stated.b Yields of isolated product.c Enantiopurity of 8 was determined by HPLC analysis using a column with a chiral stationary phase (DAICEL Chiralcel OD) with hexane/isopropanol as the solvent.d The absolute configuration was determined by comparison of the HPLC retention time with that of an authentic sample, which was synthesized independently by reported procedures.2,4,8e 5.0 equivalents of benzyl bromide.f With the same conditions expect the increased amount of 7 (1.0 mmol). 3, 0.75 mL) under nitrogen atmosphere, unless otherwise stated.b Yields of isolated product.c Enantiopurity of 8 was determined by HPLC analysis using a column with a chiral stationary phase (DAICEL Chiralcel OD) with hexane/isopropanol as the solvent.d The absolute configuration was determined by comparison of the HPLC retention time with that of an authentic sample, which was synthesized independently by reported procedures.2,4,8e 5.0 equivalents of benzyl bromide.f With the same conditions expect the increased amount of 7 (1.0 mmol). |
||||||
| 1e | 4p | 5.0 | 20 | 1.5 | 89 | 87 (S) |
| 2 | 5pp | 1.0 | 20 | 2 | 92 | 97 (S) |
| 3 | 5pp | 1.0 | 0 | 3 | 95 | 98 (S) |
| 4 | 5pp | 0.5 | 0 | 4 | 95 | 98 (S) |
| 5 | 5pp | 0.25 | 0 | 8 | 94 | 97 (S) |
| 6 | 5pp | 0.5 | −20 | 6 | 95 | 99 (S) |
| 7f | 5pp | 0.5 | 0 | 6 | 95 | 98 (S) |
| 8 | 5mp | 2.0 | 20 | 1.5 | 89 | 89 (S) |
| 9 | 5mm | 2.0 | 20 | 1.5 | 84 | 71 (S) |
| 10 | 6pp | 0.5 | 20 | 3 | 90 | 94 (R) |
| 11 | 6pp | 0.5 | 0 | 6 | 92 | 95 (R) |
| 12 | 6pp | 0.5 | −20 | 8 | 93 | 98 (R) |
When the dimeric PTC of bis(4-(O(9)-allyl-cinchonidium-N-methyl)phenyl)methanone dibromide (5pp) was applied in the benzylation, it showed big improvement of both enatioselectivity and catalytic condition.§ PTC 5pp (1.0 mol%) showed enantioselectivity of 97% ee (S) with 1.2 equivalents of benzyl bromide at 20 °C. (entry 2) At lower reaction temperature (0 °C: entry 3), its enantioselectivity increased to 98% ee. When 0.5 mol% of 5pp was applied at 0 and –20 °C, the product showed 98% and 99% ee. (entries 4 and 6) And with 0.25 mol% of 5pp, the enantioselectivity went down to 97% ee. Therefore, the practical catalytic condition for the benzylation with 5pp would be 0.5 mol% of PTC and 1.2 equivalents of benzyl bromide at 0 °C.
The isomeric PTC 5mp and 5mm showed lower % ee; 5mp showed 89% ee (S) in benzylation (entry 8) and 5mm showed 71% ee (entry 9). These enatioselectivity values are similar or lower than that of the monomeric 4p. There was no enhanced catalytic effect by two cinchonidiums at meta/para and meta/meta position of 5mp and 5mm.
Enantioselectivity of PTC 5 was varied in the order of 5pp > 5mp > 5mm depending on the position at benzophenone ring, which is different from those observed at PTC 3a (meta > para > ortho position).8 To deduce the enantioselectivity of PTC 5pp, one may consider a distance factor between dimeric cinchonidiums. The distance between two benzyl positions of the bridge benzophenone of PTC 5pp was calculated to be ∼10.4 Å at B3LYP/6-31G(d) level (see, ESI†), which is longer than those of PTC 3a and 3b (∼5.1 Å and ∼7.5 Å, respectively). And those of PTC 5pm and 5mm were 8.7 Å and 8.3 Å, respectively. The distance between two quarternary ammoniums could not be correlated with the enantioselectivity, and it would not be a main controlling factor for enantioselectivity.
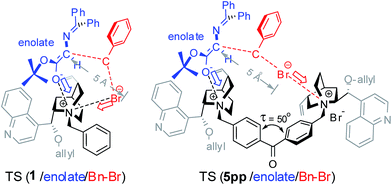
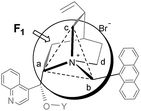
At the transition state for α-alkylation of glyciante 7 with benzyl bromide, there will be SN2-type bond formation/cleavage between the enolate carbon and the benzyl carbon and bromide, which occurs on the F1 face of the PTC.7b (Fig. 1) It is expected that the anionic oxygen of the enolate from 7 will form an ionic complex with the PTC ammonium at the transition state, and as the benzyl bromide approaches to the enolate of 7 in SN2 pattern, the leaving bromide will be attracted also by the ammonium.14 An estimated distance between enolate and the leaving bromide will be ∼5.0 Å.
At the monomeric PTC 1/2, both the enolate and bromide will be attracted by the same quinuclidinium. On the other hand, dimeric PTC 5 is expected to anchor the enolate of gylcinate 7 on one ammonium and attract the leaving bromide with another ammonium in a distance (Br–N(+)) of ∼5.0 Å at TS. (Fig. 1) Two phenyl rings of the benzophenone bridge of PTC 5 are not laid on a plane but twisted around the carbonyl (τ = 50°)15 and these two twisted cinchonidiums of PTC 5pp would provide a stabilized transition structure for the benzylation within ∼10 Å distance by dual functions of two quinuclidiniums. However, more crowded TSs will be formed in 5mp and 5mm because of the short ammonium distance, therefore their TSs will resemble to that of monomeric PTC 4p.
Dimeric PTC of bis(4-(O(9)-allyl-cinchonium-N-methyl)phenyl) methanone dibromide (6pp) derived from (+)-cinchonine is a pseudo enantiomer of PTC 5pp. PTC 6pp showed also high enantioselectivity of 94% ee (R) in the benzylation at room temperature. (entry 10) Its selectivity increased to 95–98% ee at lower temperature (entries 11 and 12).
The alkylation with selected alkyl halides with 0.5 mol% of PTC 5pp were summarized in Table 2. The results showed that 5pp has high enantioselectivity of 93–97% ee with allyl bromide derivatives and 93–98% ee with benzyl bromide derivatives. Alkyl iodides were also alkylated in high % ee (Table 2, entries 7 and 8), but tert-butyl bromoacetate showed low 85% ee at the same condition because of the known background reaction.16 (entry 9) PTC 5pp has been proved to be an advanced phase transfer catalyst for the synthesis of various α-amino acids under mild catalytic conditions.
| Entry | R–X | Time (h) | Yieldb (%) | % ee (config)c |
|---|---|---|---|---|
a Alkylation of 7 (0.1 mmol) was carried out with 0.5 mol% of 5pp, 1.2 equivalents of R-X and 50% aqueous KOH (0.25 mL) in toluene/chloroform (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 0.75 mL) at 0 °C under nitrogen atmosphere, unless otherwise noted.b Yields of isolated product.c Enantiopurity of 8 was determined by HPLC analysis using a column with a chiral stationary phase (DAICEL Chiralcel OD) with hexane/isopropanol as the solvent. The absolute configuration was determined by comparison of the HPLC retention time with that of an authentic sample, which was synthesized independently by reported procedures.2,4,8d R–I (5.0 eq.) and CsOH·H2O (5.0 eq.) was uses as base.e With 1.0 mol% of the catalyst.f N-Benzoyl derivative. 3, 0.75 mL) at 0 °C under nitrogen atmosphere, unless otherwise noted.b Yields of isolated product.c Enantiopurity of 8 was determined by HPLC analysis using a column with a chiral stationary phase (DAICEL Chiralcel OD) with hexane/isopropanol as the solvent. The absolute configuration was determined by comparison of the HPLC retention time with that of an authentic sample, which was synthesized independently by reported procedures.2,4,8d R–I (5.0 eq.) and CsOH·H2O (5.0 eq.) was uses as base.e With 1.0 mol% of the catalyst.f N-Benzoyl derivative. |
||||
| 1 | PhCH2–Br | 4 | 95 | 98 (S) |
| 2 |  |
11 | 89 | 97 (S) |
| 3 | 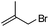 |
12 | 86 | 97 (S) |
| 4 | 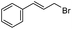 |
3 | 88 | 93 (S) |
| 5 | 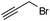 |
9 | 69 | 95 (S) |
| 6 | 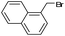 |
9 | 93 | 94 (S) |
| 7d | CH3CH2I | 6 | 92 | 95 (S) |
| 8d,e | CH3I | 4 | 64 | 93 (S) |
| 9 | 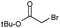 |
5 | 51 | 85f (S) |
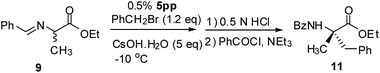
α,α-Dialkylation of aldimine Schiff base of amino acid17 was examined with 5pp. Aldimine Schiff base of D,L-alanine ethyl ester 9, benzyl bromide (1.2 eq.) and CsOH·H2O (5 eq.) with PTC 5pp (1.0 mol%) in toluene/CHCl3 (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) at −10 °C for 4 hour, then the acid work-up gave ethyl 2-amino-2-methyl-3-phenylpropionate 10 in 94% yield, which was benzoylated to N-benzoyl α,α-dialkylated product 11 and analysed with chiral HPLC (92% ee (S)).
3) at −10 °C for 4 hour, then the acid work-up gave ethyl 2-amino-2-methyl-3-phenylpropionate 10 in 94% yield, which was benzoylated to N-benzoyl α,α-dialkylated product 11 and analysed with chiral HPLC (92% ee (S)).
In conclusion, we have provided the novel dimeric cinchona-based PTCs with a benzophenone bridge. The p,p′-linked PTC 5pp and 6pp showed high enantioselectivity (93–99% ee) in the alkylation of a glycine ester under mild catalytic conditions of 0.5 mol% PTC and near stoichiometric alkyl halide (1.2 equivalents) at 0–20 °C. Dialkylation under similar conditions gave high % ee with the aldimine Schiff base of alanine. Their efficiency and enantioselectivity were explained by dual functions dimeric cinchonidiums; one as an alkylating site and another as a receptor for a leaving anion. Novel PTCs 5pp and 6pp would be applied in the synthesis of natural and non-natural chiral α-amino acids and their derivatives. Applications to other asymmetric phase-transfer catalytic reactions with 5pp are under investigation.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (2013R1A1A2066041).Notes and references
- U.-H. Dolling, P. Davis and E. J. J. Grabowski, J. Am. Chem. Soc., 1984, 106, 446 CrossRef CAS.
- M. J. O'Donnell, W. D. Benett and S. Wu, J. Am. Chem. Soc., 1989, 111, 2353 CrossRef.
- For reviews, see: (a) M. J. O'Donnell, Acc. Chem. Res., 2004, 37, 506 CrossRef PubMed; (b) B. Lygo and B. I. Andrews, Acc. Chem. Res., 2004, 37, 518 CrossRef CAS PubMed; (c) S.-s. Jew and H.-g. Park, Chem. Commun., 2009, 7090 RSC; (d) S. Shirakawa and K. Maruoka, Angew. Chem., Int. Ed., 2013, 52, 4312 CrossRef CAS PubMed; (e) K. Brak and E. N. Jacobsen, Angew. Chem., Int. Ed., 2013, 52, 534 CrossRef CAS PubMed.
- (a) E. J. Corey, F. Xu and M. C. Noe, J. Am. Chem. Soc., 1997, 119, 12414 CrossRef CAS; (b) B. Lygo and P. G. Wainwright, Tetrahedron Lett., 1997, 38, 8595 CrossRef CAS.
- When the priority of substituents around ammonium is in the order of Ca > Cb > Cc > Cd, one can assume a tetrahedral structure with four carbons and four faces of the tetra-alkyl ammonium; F1 (Ca–Cb–Cc), F2 (Ca–Cb–Cd), F3 (Ca–Cc–Cd) and F4 (Cb–Cc–Cd)
 .
. - C. Hofstetter, P. S. Wilkinson and T. C. Pochapsky, J. Org. Chem., 1999, 64, 8794 CrossRef CAS PubMed.
- (a) C. E. Cannizzaro and K. N. Houk, J. Am. Chem. Soc., 2002, 124, 7163 CrossRef CAS PubMed; (b) T. C. Cook, M. B. Andrus and D. H. Ess, Org. Lett., 2012, 14, 5836 CrossRef CAS PubMed.
- (a) S.-s. Jew, B.-S. Jeong, M.-S. Yoo, H. Huh and H.-g. Park, Chem. Commun., 2001, 1244 RSC; (b) H.-g. Park, B.-S. Jeong, M.-S. Yoo, J.-H. Lee, M.-k. Park, J.-J. Lee, M.-J. Kim and S.-s. Jew, Angew. Chem., Int. Ed., 2002, 41, 3036 CrossRef CAS; (c) J.-H. Lee, M.-S. Yoo, J.-H. Jung, S.-s. Jew, H.-g. Park and B.-S. Jeong, Tetrahedron, 2007, 63, 7906 CrossRef CAS; (d) N. Baba, J. Oda and M. Kawaguchi, Agric. Biol. Chem., 1986, 50, 3113 CAS.
- R. Chinchilla, P. Mazón and C. Nájera, Tetrahedron: Asymmetry, 2002, 13, 927 CrossRef CAS.
- J.-H. Lee, M.-S. Yoo, J.-H. Jung, S.-S. Jew, H.-G. Park and B.-S. Jeong, Tetrahedron, 2007, 63, 7906 CrossRef CAS.
- S. Bozkurt, M. Durmaz, M. Yilmaz and A. Sirit, Tetrahedron: Asymmetry, 2008, 19, 618 CrossRef CAS.
- (a) S. Itsuno, D. K. Paul, M. A. Salam and N. Haraguchi, J. Am. Chem. Soc., 2010, 132, 2864–2865 CrossRef CAS PubMed; (b) M. M. Parvez, N. Haraguchi and S. Itsuno, Macromolecules, 2014, 47, 1922 CrossRef CAS.
- M. F. Geer, M. D. Walla, K. M. Solntsev, C. A. Strassert and L. S. Shimizu, J. Org. Chem., 2013, 78, 5568 CrossRef CAS PubMed.
- (a) K. Nahm and S. Lee, Bull. Korean Chem. Soc., 2012, 33, 2711 CrossRef CAS; (b) E. F. Martins and J. R. Pliego Jr, ACS Catal., 2013, 3, 613 CrossRef.
- (a) E. B. Fleischer, N. Sung and S. Hawkinson, J. Phys. Chem., 1968, 72, 4311 CrossRef CAS; (b) R. Hoffmann and J. R. Swenson, J. Phys. Chem., 1970, 74, 415 CrossRef CAS.
- B. Lygo, J. Crosby, T. R. Lowdon, J. A. Peterson and P. G. Wainwright, Tetrahedron, 2001, 57, 2403 CrossRef CAS.
- (a) M. J. O'Donnell and S. Wu, Tetrahedron: Asymmetry, 1992, 3, 591 CrossRef; (b) B. Lygo, J. Crosby and J. A. Perterson, Tetrahedron Lett., 1999, 40, 8671 CrossRef CAS; (c) S. s. Jew, B.-S. Jeong, J.-H. Lee, M.-S. Yoo, Y.-J. Lee, B.-s. Park, M. G. Kim and H.-g. Park, J. Org. Chem., 2003, 68, 4514 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra12499f |
| ‡ For the detailed synthesis of 5pp, 6pp and their precursors, see ESI.† |
§ General alkylation procedure: Benzyl bromide (14.5 μL, 0.122 mmol) was added to a solution of N-(diphenylmethylene) glycine tert-butyl ester (7, 30 mg, 0.102 mmol) and the catalyst 5pp (50 μL, 1 × 10−2 M in CH2Cl2, 0.5 mol%, the diluting solvent was evoporated) in toluene/chloroform (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 0.75 mL). The solution was then cooled (0 °C), purged with nitrogen (10 min), and aqueous 50% KOH (0.25 mL, 22 equiv.) was added. The suspension was stirred at 0 °C until the starting material had been consumed (3 h). The reaction mixture was diluted with diethyl ether (20 mL), washed with saturated NaBr aqueous solution (3 × 5 mL). Organic layer was dried over MgSO4, filtered, and concentrated. Purification by flash column chromatography on silica gel (hexanes/EtOAc 98 3, 0.75 mL). The solution was then cooled (0 °C), purged with nitrogen (10 min), and aqueous 50% KOH (0.25 mL, 22 equiv.) was added. The suspension was stirred at 0 °C until the starting material had been consumed (3 h). The reaction mixture was diluted with diethyl ether (20 mL), washed with saturated NaBr aqueous solution (3 × 5 mL). Organic layer was dried over MgSO4, filtered, and concentrated. Purification by flash column chromatography on silica gel (hexanes/EtOAc 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) afforded the desired product 8 (37 mg, 95% yield) as a colourless oil. Enantioselectivity was determined by chiral HPLC analysis (DAICEL Chiralcel OD, hexane/2-propanol (100 2) afforded the desired product 8 (37 mg, 95% yield) as a colourless oil. Enantioselectivity was determined by chiral HPLC analysis (DAICEL Chiralcel OD, hexane/2-propanol (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0), flow rate 0.5 mL min−1, 25 °C, λ = 254 nm, retention times: R (minor) 12.5 min, S (major) 20.5 min, 98% ee). The absolute configuration was confirmed by comparison of the HPLC retention time with the authentic sample synthesized by the reported procedure.2,4,8 1.0), flow rate 0.5 mL min−1, 25 °C, λ = 254 nm, retention times: R (minor) 12.5 min, S (major) 20.5 min, 98% ee). The absolute configuration was confirmed by comparison of the HPLC retention time with the authentic sample synthesized by the reported procedure.2,4,8 |
| This journal is © The Royal Society of Chemistry 2018 |