Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Low temperature CO oxidation catalysed by flower-like Ni–Co–O: how physicochemical properties influence catalytic performance†
Yunan Yia,
Pan Zhanga,
Zuzeng Qina,
Chuxuan Yua,
Wei Lia,
Qiuju Qina,
Bin Li *ac,
Minguang Fana,
Xin Lianga and
Lihui Dong*ab
*ac,
Minguang Fana,
Xin Lianga and
Lihui Dong*ab
aGuangxi Key Laboratory of Petrochemical Resource Processing and Process Intensification Technology, School of Chemistry and Chemical Engineering, Guangxi University, Nanning 530004, P. R. China. E-mail: binli@gxu.edu.cn; donglihui2005@126.com
bJiangsu Key Laboratory of Vehicle Emissions Control, Centre of Modern Analysis, Nanjing University, Nanjing 210093, P. R. China
cGuangxi Colleges and Universities Key Laboratory of Applied Chemistry Technology and Resource Development, Guangxi University, Nanning, 530004, P. R. China
First published on 12th February 2018
Abstract
In this work, mesoporous Ni–Co composite oxides were synthesized by a facile liquid-precipitation method without the addition of surfactant, and their ability to catalyse a low temperature CO oxidation reaction was investigated. To explore the effect of the synergetic interaction between Ni and Co on the physicochemical properties and catalytic performance of these catalysts, the as-prepared samples were characterized using XRF, XRD, LRS, N2-physisorption (BET), SEM, TEM, XPS, H2-TPR, O2-TPD and in situ DRIFTS characterization techniques. The results are as follows: (1) the doping of cobalt can reduces the size of NiO, thus massive amorphous NiO have formed and highly dispersed on the catalyst surface, resulting in the formation of abundant surface Ni2+ ions; (2) Ni2+ ions partially substitute Co3+ ions to form a Ni–Co spinel solid solution, generating an abundance of surface oxygen vacancies, which are vital for CO oxidation; (3) the Ni0.8Co0.2 catalyst exhibits the highest catalytic activity and a satisfactory stability for CO oxidation, whereas a larger cobalt content results in a decrease in activity, suggesting that the amorphous NiO phase is the dominant active phase instead of Co3O4 for CO oxidation; (4) the introduction of Co can alter the morphology of catalyst from plate-like to flower-like and then to dense granules. This morphological variation is related to the textural properties and catalytic performance of the catalysts. Lastly, a possible mechanism for CO oxidation reaction is tentatively proposed.
1. Introduction
Carbon monoxide is a major atmospheric pollutant. Excessive use of fossil fuel and motor vehicles produces large quantities of exhaust emission, which has resulted in an increase in the level of carbon monoxide (CO) in the atmosphere. CO poses serious threats to humanity, in the form of air pollution and global warming. Catalytic systems play an important role in controlling the elimination of carbon monoxide.1 Catalysts containing precious metals (such as Pt, Rh, Pd and Au) are useful in CO oxidation.2,3 However, the high cost, low stability, high pollution and scarcity of precious metals limit their use in applications. It is thus imperative to develop a low cost, higher stability and environmentally friendly alternative.4,5 Transition metal oxides (such as CuO, CeO2, MnOx and CoOx) have received considerable attention as heterogeneous catalysts due in large part to their ability to support effective surface redox reactivity as well as their relative affordability.6Nickel oxide is an earth-abundant transition metal oxide with superior redox property, electrochemical performance and gas sensing property. It is used in many applications such as metallurgy and catalysis and has been used to construct electrodes and gas sensors. Ni-based catalysts are commonly studied for their potential ability to catalyse the dry-reforming reaction on an industrial scale.7 In addition, researchers have studied NiO catalysts with various morphologies for CO oxidation, and found that ring-like8 and flower-like9 NiO demonstrated high activity. NiO–CeO2 has recently demonstrated catalytic activity in the CO oxidation and the CO + NO model reactions, due to its high activity and durability. Tang et al.10 synthesized mesoporous NiO–CeO2 catalysts by a KIT-6-templating method, and demonstrated that interfacial NiO is the primary active species for CO oxidation. Cheng et al.11 combined in situ DRIFTS and MS techniques to explore the reaction mechanism of NO removal by CO over a NiO–CeO2 catalyst.
As a promising alternative to precious metals, cobalt oxide – particularly Co3O4, a representative spinel structure transition metal oxide, has been extensively studied and shows very high activity for CO oxidation at low temperatures. Researchers have assigned the effectiveness of Co3O4 in low-temperature CO catalytic oxidation to the fact that Co3O4 is the most active transition metal oxide for CO oxidation, as well as its possession of a unique Co3+/Co2+ redox couple.2 For example, Wang et al.12 obtained Co3O4 via a controlled liquid precipitation process without the use of any surfactant or oxidant and found that it exhibited very high activity for CO oxidation at room temperature or even at −78 °C. Zhang et al.13 used a dispersion–precipitation method to synthesize nanosized Co3O4 particles with a high activity (and stability) for the catalytic oxidation of carbon monoxide and propane. Moreover, it was deduced that the oxygen species contributes significantly to the enhanced catalytic activity. However, the low temperature CO oxidation activity of Co3O4 is inhibited by the presence of water, hydrocarbons and NO. Furthermore, their activity was found to decrease during steady-state CO oxidation although in the absence of inhibitors.14 It is one of the main problems that limits its practical application. Thus, the need to develop a stable and efficient catalyst is urgent. One way to overcome these deficiencies may be the formation of binary metal oxides. Benjamin Faure et al.15 report that CoxMn3−xO4 spinel oxide catalysts exhibited an outstanding activity for CO and propane oxidation at mild temperatures, which correlates with the high surface area and cobalt concentration of the catalyst. The Co/CeO2 (ref. 16 and 17) and Co3O4–CeO2 (ref. 18) catalysts also demonstrate high CO conversion and reasonable stability for the catalytic reaction of CO preferential oxidation and CO oxidation, respectively.
Furthermore, Ni and Co have similar electronic configurations, which likely results in a Ni–Co composite oxides able to demonstrate a synergistic catalytic effect. Ni–Co materials obtained by different synthetic methods have demonstrated modified catalytic performance and stability in various fields including CO and CO2 methanation,19,20 propane oxidation,21 reforming reactions22,23 and as an electrode material.24 Yu et al.19 have revealed that the synergetic effect between Ni and Co over bimetallic catalysts can reduce nickel size to enhance the metal particle dispersion and accelerate the activation of adsorbed CO, thereby improving the catalytic activity and coke resistance. Zhang et al.25 synthesized a Ni–Co bimetallic catalyst via a co-precipitation method and found that the Ni–Co bimetallic catalyst demonstrated superior performance in terms of activity and stability compared to other Ni–Me (Me = Fe, Cu and Mn) bimetallic oxides for the carbon dioxide reforming of methane. The superior catalytic performance was attributed to the synergetic effect, good metal dispersion, high metallic surface area, formation of different types of solid solutions, and a strong-metal-support-interaction. In addition, numerous researchers have recognized that the Ni–Co binary oxide shows a strong adsorption capacity for CO. It was inferred that Ni–Co composite oxides could be potential catalysts for CO oxidation at low temperatures. The use of Ni–Co composite oxides for low temperature CO catalytic oxidation has only been reported as follows. Liang et al.26 prepared a series of Ni–Co bimetal hydroxides nanosheets for CO oxidation and proposed a reaction analysis to explain the synergetic effect in the Ni–Co bimetal oxides system. The synergetic interaction between Ni and Co affecting the catalytic physicochemical properties and activity taking into account the diverse morphologies of the bimetallic oxide catalysts and the catalytic mechanism is worth further elucidation. In addition, the Ni–Co materials reported previous have ordinary morphologies and low specific surface areas, and their methods of preparation are complex.
In the present work, a series of Ni–Co composite oxides with diverse morphologies were prepared via a facile liquid-precipitation method, which is cost-effective and low polluting. The flower-like catalyst exhibits high CO conversion at low temperature, and excellent stability, and therefore has much potential to be used practically. The prepared powder catalysts were characterized with XRF, XRD, LRS, N2-physisorption, SEM, TEM, XPS, H2-TPR, O2-TPD, in situ DRIFTS and CO oxidation. This study focuses on: (1) investigating the effects of Co doping on textural properties, morphology, chemical composition, redox properties and catalytic performance of NiO; (2) studying the surface structure and structure–activity correlation of the Ni–Co catalysts for low temperature CO oxidation; (3) and analysing the interaction of CO or/and O2 over typical samples by in situ DRIFTS, to reveal a possible reaction mechanism for CO oxidation.
2. Experimental
2.1 Catalyst preparation
The Ni–Co composite oxides and the NiO and Co3O4 were prepared by the liquid-precipitation method without any surfactant. Briefly, an appropriate amount of Ni(NO3)2·6H2O and CO(NO3)2·6H2O were dissolved in deionized water to obtain 2 mol L−1 Ni(NO3)2 and CO(NO3)2 aqueous solutions. The two aqueous solutions were mixed with constant stirring at an ambient temperature to obtain mixed aqueous solutions of different Ni/Co molar ratios (theoretical ratios Ni/Co = 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 95
1, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 9
1, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 8
1, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 7
2, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Subsequently, excess diluted ammonia was added to these aqueous solutions dropwise (also with vigorous stirring) until the pH reached ∼10, and suspensions were obtained. After further stirring for 4 h and the samples were left to mature for 18 h at room temperature, before the products were collected by centrifugation. The products were washed consecutively with deionized water and absolute alcohol three times, in sequence, before being dried at 80 °C for 12 h. The obtained presoma were grinded fully and calcined in a muffle furnace at 400 °C for 4 h. For simplification, the samples are denoted as Ni1−xCox, for instance, the sample with a theoretical ratio Ni/Co = 99
3). Subsequently, excess diluted ammonia was added to these aqueous solutions dropwise (also with vigorous stirring) until the pH reached ∼10, and suspensions were obtained. After further stirring for 4 h and the samples were left to mature for 18 h at room temperature, before the products were collected by centrifugation. The products were washed consecutively with deionized water and absolute alcohol three times, in sequence, before being dried at 80 °C for 12 h. The obtained presoma were grinded fully and calcined in a muffle furnace at 400 °C for 4 h. For simplification, the samples are denoted as Ni1−xCox, for instance, the sample with a theoretical ratio Ni/Co = 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is denoted as Ni0.99Co0.1. For comparison, pure NiO, Co3O4 and other Ni0.8M0.2 (where M = Mn, Fe, Zn, Cr) oxides were prepared using the same procedure.
1 is denoted as Ni0.99Co0.1. For comparison, pure NiO, Co3O4 and other Ni0.8M0.2 (where M = Mn, Fe, Zn, Cr) oxides were prepared using the same procedure.
2.2 Characterization of catalysts
The bulk chemical compositions of samples were analysed by X-ray fluorescence (XRF), which was performed on an ARL ADVAT'X IntellipowerTM3600 X-ray fluorescence spectrometer.Power X-ray diffraction (XRD) patterns were obtained with a X'Pert PRO diffractometer (PANalytical, Netherlands) using Cu/Kα radiation (λ = 1.54060 Å). The scanning voltage and current were set to 40 kV and 40 mA. The scanning range of 2θ is 10° to 80°, with a scan rate of 8° min−1.
Laser Raman spectrometer (LRS) was carried out with a Renishaw InVia Reflex Raman spectrometer using an Ar+ laser beam. Raman spectra were obtained under an excitation wavelength of 532 nm and a laser power of 5 mW.
N2 adsorption–desorption isotherms at 77 K were obtained with a Micrometrics TriStar II 3020 analyser, and the specific surface area and pore distribution were expressed by Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods from the nitrogen sorption isotherm, respectively.
Scanning electron microscopy (SEM) measurements were performed with a HITACHI S-3400N electron microscope (Hitachi Company, Japan) at 20 kV. Samples for FESEM were suspended in ethanol and dispersed by ultrasonic, and then dropped onto an aluminium sheet.
Transmission electron microscopy (TEM) images were taken on a Tecnai G2 F20 S-TWIN instrument (FEI Company, America) at an acceleration voltage of 200 kV.
X-ray photoelectron spectroscopy (XPS) was performed on an ESCALAB 250Xi multifunctional imaging electron spectrometer (Thermo Fisher Company, America) using monochromatic Al Kα radiation (hν = 1486.6 eV) and operating at a power level of 150 W. The electron binding energy was calibrated based on C 1s (284.8 eV). The sample irradiation area and detecting depth were 2 mm × 1 mm and 2–5 nm, respectively. In addition, the peaks have been fitted by the CasaXPS.
H2-TPR was performed by a FINESORB-3010 automated chemisorption apparatus (Finetec Corporation). The sample (15 mg) was heated from room temperature to 110 °C under a N2 flow of 50 mL min−1 (it was kept under these conditions for 1 h prior to the analysis), before being cooled to room temperature in a N2 atmosphere and switched to a stream of mixture of H2–Ar (7% H2 by volume) at 10 mL min−1 for 30 min. Later, the temperature was increased from room temperature to 600 °C (10 °C min−1). The H2 consumption was continuously analyzed using a thermal conductivity detector (TCD).
O2-TPD was performed by a FINESORB-3010 automated chemisorption apparatus from Finetec Corporation. Firstly, the sample (100 mg) was heated from room temperature to 200 °C in a He flow of 30 mL min−1, which was maintained for 100 min, before the sample was cooled to room temperature (still in an He atmosphere), and then the sample was exposed to a stream of pure O2 (10 mL min−1) for 30 min. The sample was then exposed to a He flow for 30 min to clear residual oxygen. After that, it was heated from room temperature to 700 °C in He atmosphere at 10 °C min−1. The O2 consumption was continuously analysed using TCD.
In situ Diffusion Reflectance Infrared Fourier Transform (In situ DRIFTS) spectra were collected using a Nicolet iS50 FT-IR spectrometer equipped with a MCT detector set at a resolution of 4 cm−1 with 32 scans. The catalyst powders were placed in the sample pool and pre-treated with purified N2 at 300 °C for 1 h to eliminate any impurities, before being cooled to room temperature while the background spectra of catalysts at diverse target temperatures were collected. Subsequently, the catalyst was exposed to a stream of CO–N2 (2% CO by volume) or/and dry air (21% volume O2 and 79% volume N2) at a rate of 10.4 and 8.2 mL min−1, respectively, for 40 min (until saturation has been reached). The DRIFTS spectra of CO and CO + O2 were collected at the target temperature from 50 to 150 °C at a heating rate of 5 °C min−1. Finally, the results were obtained by removing the corresponding background reference.
2.3 Catalytic activity measurements
The activities of the catalysts for CO oxidation were measured under stationary conditions with a feed stream of 1.6% CO, 20.8% O2 and 77.6% N2. The 50 mg sample (40–60 mesh) was loaded into a quartz tube and pre-treated at 100 °C under a high purity N2 flow for 1 h to eliminate impurities. The sample was then cooled to room temperature before turning on the mixture gas. The reaction was carried out under different temperatures (ranging from room temperature to 160 °C) with a space velocity of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL h−1 gcat−1. A gas chromatographer (GC7890II, Shanghai Techcomp) equipped with a TCD was used to analyse the outlet gases. The following formula was used to calculate the CO conversion:
000 mL h−1 gcat−1. A gas chromatographer (GC7890II, Shanghai Techcomp) equipped with a TCD was used to analyse the outlet gases. The following formula was used to calculate the CO conversion:| CO conversion (%) = ([CO]in − [CO]out) × 100% |
3. Results and discussion
3.1 Catalytic performance of the as prepared catalysts
Catalytic oxidation of CO was conducted to estimate the catalytic performance of the as-prepared catalysts. Fig. 1(a) depicts the activities of Ni–Co, pure NiO and Co3O4 samples towards CO oxidation. The pure NiO sample displayed very poor CO conversion (less than 10%), and this hardly improved despite an increase in the temperature. The pure Co3O4 sample also displayed poor CO conversion at low temperatures, but this was observed to increase sharply when the temperature rose above 120 °C. For the Ni–Co composite oxides, all samples show a remarkably increased CO conversion compared with the NiO and Co3O4 samples, with the complete conversion occurring at temperatures ranging from 120 °C to 150 °C. Moreover, CO conversion increases sharply with temperatures above 50 °C. Interestingly, in comparing Ni0.99Co0.01 with NiO, despite the minor difference in the content of the two samples, the CO conversion improved greatly. The Ni0.8Co0.2 sample demonstrated the highest activity, with T50 at ∼80 °C and T100 at ∼120 °C. Further increasing the Co content causes the activity to decrease. The activity of the samples follows this order: Ni0.8Co0.2 > Ni0.7Co0.3 > Ni0.9Co0.1 > Ni0.95Co0.05 > Ni0.99Co0.01 > Co3O4 > NiO. The above results demonstrate that doping cobalt into nickel oxide can greatly improve the catalytic activity whilst the Ni/Co ratio influences the catalytic activity. In general, the CO conversions were observed to be different between the first and second runs. For example, the CO conversions results for the two runs of the Ni0.8Co0.2 sample are shown in the ESI.† As shown in Fig. S1,† the CO conversion of the second run is typically higher than that of the first run for temperatures less than 100 °C.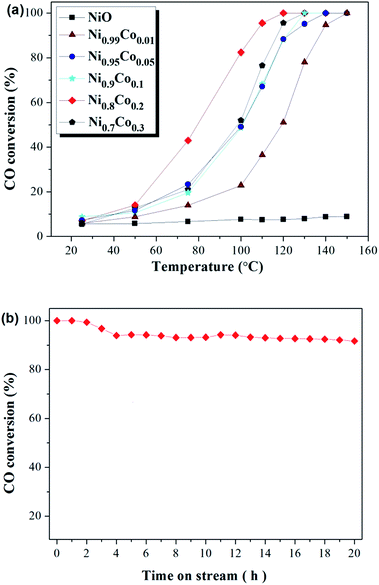 | ||
| Fig. 1 CO conversion (%) over (a) as prepared samples and (b) Ni0.8Co0.2 sample at 120 °C for 20 h. Feed stream composition: 1.6% CO, 20.8% O2 and 77.6% N2 by volume. | ||
In addition, catalytic stability is a crucial factor for the heterogeneous catalysis. In order to explore the long-term catalytic stability of the catalysts, the activity of the catalysts was examined at 120 °C over 20 h. The stability of the Ni0.8Co0.2 catalyst and the corresponding results are shown in Fig. 1(b). It is clear that the powder catalysts and feed gas were able to reach reaction equilibrium after a primary period of 4 h. Furthermore, the Ni0.8Co0.2 catalyst was found to maintain a high CO conversion, exceeding 90% after 20 h under the reaction conditions. The result demonstrates that the Ni0.8Co0.2 catalyst has excellent long-term catalytic stability for CO oxidation. In addition, the activity of Ni0.8Co0.2 is higher than that of the other Ni0.8M0.2 (M = Mn, Fe, Zn, Cr, Co) composite oxides, with the results for this experiment shown in the ESI (Fig. S2†).
3.2 XRF results and textural properties analysis (XRD, LRS, N2-physisorption, SEM and TEM)
In order to determine the bulk chemical composition of the Ni–Co samples, XRF was conducted, and the results are shown in Table 1. It can be seen that the actual proportion of Co species is larger than the theoretical ratio for all samples, which may be caused by the loss of nickel during preparation.| Sample | Atomic ratio Co/(Ni + Co)a (%) | BET surface area (m2 g−1) | Total pore volume (cm3 g−1) | Average pore diameter/(nm) | Crystallite sizeb (nm) |
|---|---|---|---|---|---|
| a Calculated by XRF results.b Determined by the Scherrer equation with the (111) diffraction peak of face-centred cubic phase for NiO, Ni0.99Co0.01, Ni0.95Co0.05 and Ni0.9Co0.1 samples, and with the (311) diffraction peak of spinel phase for Co3O4, Ni0.8Co0.2 and Ni0.7 Co0.3 samples. | |||||
| NiO | — | 112 | 0.443 | 15.891 | 11.4 |
| Ni0.99Co0.01 | 3.41 | 117 | 0.369 | 12.620 | 10.9 |
| Ni0.95Co0.05 | 16.29 | 135 | 0.552 | 16.582 | 8.2 |
| Ni0.9Co0.1 | 29.56 | 119 | 0.562 | 18.840 | 3.6 |
| Ni0.8Co0.2 | 43.76 | 111 | 0.551 | 19.796 | 2.9 |
| Ni0.7Co0.3 | 55.06 | 83 | 0.403 | 19.448 | 6.0 |
| Co3O4 | — | 61 | 0.180 | 11.782 | 18.6 |
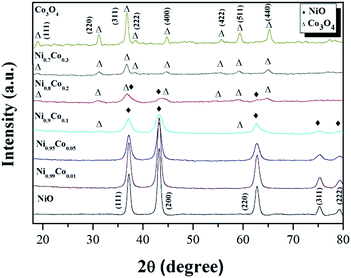
The XRD patterns of the Ni–Co composite oxides and the reference samples NiO and Co3O4 are shown in Fig. 2. From the reference patterns, the diffraction peaks at 37.2°, 43.3°, 62.9°, 75.4° and 79.4° are assigned to crystallographic planes (111), (200), (220), (311) and (222), respectively, of the face-centered cubic structure of NiO (JCPDS# 47-1049). The diffraction peaks at 19.0°, 31.3°, 36.8°, 38.8°, 44.8°, 55.7°, 59.4° and 65.2° are assigned to crystallographic planes (111), (220), (311), (222), (400), (422), (511) and (440), respectively, of the cubic spinel structure of Co3O4 (JCPDS# 42-1467). After the Co doping and when the Co content reaches 16%, only the diffraction peaks of the NiO phase were observed, indicating that no crystallized cobalt species were isolated from the NiO. Some possible reasons for the absence of cobalt diffraction peaks: (1) Co ions are incorporated into the nickel lattice; (2) Co particles are very small and highly dispersed, and therefore difficult to detect with XRD.10,27 However, the first argument can be ruled out since no shift of the diffraction peaks is observed, which would be expected with a modification of the NiO crystal lattice by incorporation of Co. By increasing the Co content, the diffraction peaks ascribed to the NiO phase become wider and weaker which is likely due to a gradual decrease in the crystallite size of the nickel oxide,28 as is also suggested by the calculated crystallite sizes presented in Table 1. When the Co content reaches 29%, diffraction peaks from the Co3O4 phase appear and the NiO phase peaks disappear. It indicates that Co3O4 crystals were formed and coexist with the NiO phase in the catalyst. Since the NiO reflections appear weaker and broader when the Co content is increased, this may indicate that the NiO loses its crystalline character. For the Ni0.8Co0.2 sample, only the diffraction peaks of spinel Co3O4 phase can be observed, suggesting that only small amounts of crystalline NiO is present in the catalyst. As mentioned before, the introduction of Co into NiO can effectively reduce the size of NiO, resulting in high dispersion of the metal oxide particles. We, therefore, deduce that amorphous NiO are formed and highly dispersed on the Co3O4 surface. Furthermore, it is worth noting that there is a negative shift for the diffraction peaks of the Co3O4 phase in x = 0.2 and 0.3 samples, compared with those of pure Co3O4, suggesting that Ni2+ could partially substitute Co3+ and move into the Co3O4 crystal lattice, forming the Ni–Co spinel structure.25,29–31 The octahedral Co3+ is coordinated to 6 O atoms; when it is substituted by Ni2+, oxygen vacancies form to compensate for the loss of positive charges, thereby retaining an overall neutrality of charges.31
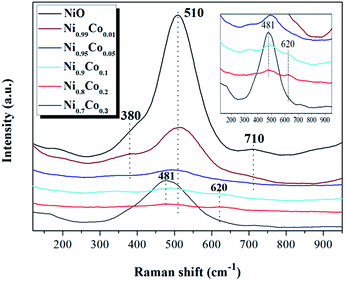
LRS was carried out to further investigate the influence of cobalt incorporation on the interior properties and surface structure of the Ni–Co samples. As shown in Fig. 3, the Raman spectra of pure NiO exhibits a main band at 510 cm−1 and a small band at 710 cm−1, corresponding to Ni–O stretching vibrational modes, and a shoulder peak at 380 cm−1, which is indicative of the non-stoichiometry of NiO.32–34 Compared with NiO, the intensity of the peaks from the Ni–Co samples become weaker and shift to lower frequencies, indicating that a strong interaction occurred between NiO and Co3O4 during preparation. Furthermore, when x ≥ 0.1, a broad peak is detected at 620 cm−1 which can be ascribed to surface oxygen vacancies, also related to the Frenkel defect-induced mode (D mode).35 It is interesting to note that the intensity of the peak at 481 cm−1 for the Ni0.7Co0.3 sample suddenly increased. According to the XRD results, this may be due to increased crystallinity of Co3O4. The band at 481 cm−1 can be assigned to vibrations of the spinel Co3O4.36
The textural information is summarized in Table 1. Relative to the data of pure NiO, the specific surface area, total pore volume and average pore diameter are all larger for the Ni–Co samples (with a few exceptions), which implies that the properties of the catalyst can be significantly modified with an appropriate amount of cobalt doping. Interestingly, the data exhibits a “Bell shape” as the cobalt content increases. The specific surface area increases from 112 m2 g−1 for the pure NiO sample to 135 m2 g−1 for the Ni0.95Co0.05 sample, likely due to a reduction in the size of the crystallites. As the cobalt content continues to increase, the crystal phase forms for Co3O4, and the specific surface area declines to 83 m2 g−1 for the Ni0.7Co0.3 sample, as supported by XRD results. Co3O4 possesses the smallest specific surface area (61 m2 g−1). Moreover, despite the Ni0.95Co0.05 sample having the largest specific surface area, it does not demonstrate optimal activity, suggesting that surface area is not a primary factor influencing catalytic behaviour. The Ni0.8Co0.2 sample possesses the largest average pore diameter, a relatively high specific surface area and total pore volume, and a unique mesoporous structure, and overall these contribute to enabling the compound to demonstrate the most effective catalytic activity. Moreover, from the N2-physisorption analysis, it can be seen that the Ni–Co samples are mesoporous (2–50 nm) structure, and their adsorption capacities are higher than NiO. The N2 adsorption–desorption isotherms (Fig. S3†) and corresponding analyses are presented in the ESI.†
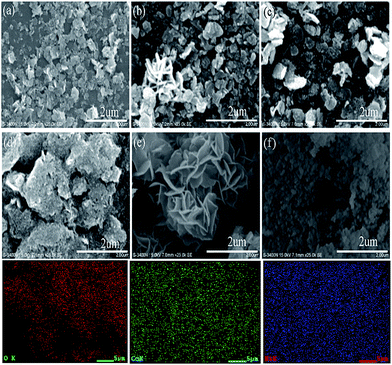
SEM analysis was employed to observe the morphology of the NiO and Ni–Co samples. As depicted in Fig. 4, the chemical composition of the catalyst influences the morphology. For 0 ≤ x ≤ 0.05, the samples are uniformly small and plate-like with sizes between 300 to 500 nm. For x = 0.1, the sample remains plate-like but the plate sizes are larger and irregular. Interestingly, when x = 0.2, the catalyst morphology changed to “flower-like”, whereas, it became dense granules with a mean size of 70 nm for values of x up to 0.3. This variation of catalyst morphology may be associated with the sudden drop in specific surface area for the Ni0.9Co0.1 and Ni0.7Co0.3 samples. The unique flower-like morphology of the Ni0.8Co0.2 sample likely leads to distinctive textural properties, enabling it to exhibit excellent catalytic activity in the CO oxidation reaction. Elemental mapping analysis of SEM provides an intuitionistic elemental distribution of the Ni0.8Co0.2 sample, and proves the uniform distribution of Ni, Co and O in the sample. This result clearly indicates that the Ni species is highly dispersed, although there is an enrichment of Ni on the surface, which is consistent with the XRD and XPS results.
TEM and HRTEM images of the Ni0.8Co0.2 sample are shown in Fig. 5. It is made up of agglomerated particles with irregular shapes and sizes. The HRTEM images reveal the crystalline nature of the sample. Both bulk Co3O4 crystallites and tiny NiO crystallites can be observed from Fig. 6(d), as evidenced by the interplanar spacings of 0.461, 0.286, 0.243 and 0.238, 0.211 nm that correspond to the (111), (220), (311) planes of Co3O4 and the (111), (200) planes of NiO, respectively, which conforms to the XRD results. In addition, it can be noted that there is an amorphous phase marked by red circle in Fig. 6(d), which is highly likely to be amorphous NiO.
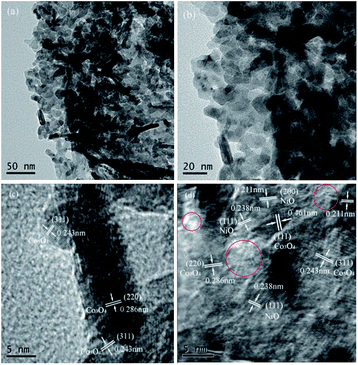 | ||
| Fig. 5 The morphology and microstructure of TEM (a, b) and HRTEM (c, d) images of Ni0.8Co0.2 sample. | ||
3.3 Surface chemical compositions analysis (XPS results)
XPS measurements were performed to explore the surface composition and elemental valence configuration of several selected samples, and the results are displayed in Fig. 6. The surface composition of these samples as calculated by XPS data are summarized in Table 2. Moreover, the binding energy values calibrated by C 1s (284.8 eV) allow for some error associated with charging effects during XPS analysis.| Samples | Atomic concentration and atomic ratio by XPS | |||||||
|---|---|---|---|---|---|---|---|---|
| Atomic concentration (at%) | Atomic ratio (at%) | Ni/Co atomic ratio | ||||||
| C | Ni | Co | O | Co3+/(Co3+ + Co2+) | O′′/(O′′ + O′) | Bulk | Surface | |
| NiO | 6.37 | 50.21 | — | 43.42 | — | 24.45 | — | — |
| Ni0.99Co0.01 | 12.03 | 43.18 | 0.4 | 44.79 | 57.49 | 35.66 | 28.57 | 107.95 |
| Ni0.95Co0.05 | 12.75 | 38.78 | 3.9 | 44.53 | 63.03 | 33.02 | 5.13 | 9.94 |
| Ni0.8Co0.2 | 15.84 | 29.53 | 8.53 | 46.1 | 72.36 | 56.91 | 1.29 | 3.46 |
| Ni0.7Co0.3 | 17.40 | 21.47 | 12.69 | 48.44 | 62.67 | 60.96 | 0.82 | 1.69 |
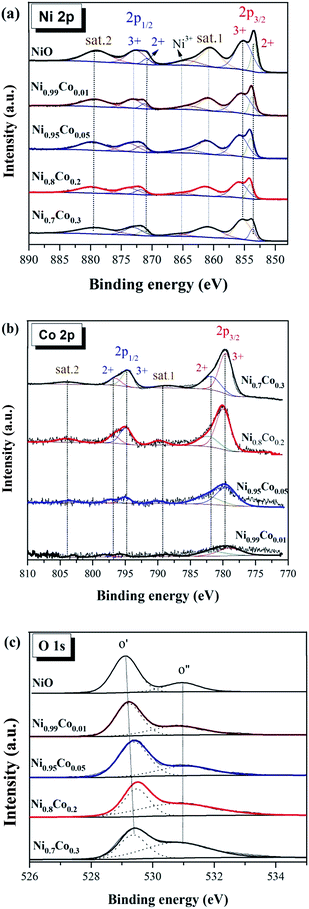
Fig. 6(a) shows the XPS spectra of Ni 2p. The spectra from all as-prepared samples are similar and consist of two spin–orbit doublets and two shake-up satellites (denoted as sat.1 and sat.2). The first doublet at ∼853.8 and ∼871.5 eV and the second doublet at ∼855.8 and ∼873.2 eV are assigned to Ni2+ and Ni3+, respectively.23,31,33,37 The two intensive shake-up satellites (∼861.0 and ∼879.6 eV) are usually observed for paramagnetic Ni2+, and arise from charge transfer multi-electron transitions.38,39 In addition, the small peak at ∼865.0 eV is assigned to the shake-up satellite of Ni3+.33,40 It is clear that the proportion of this shake-up satellite for Ni0.8Co0.2 is smaller than that of other samples, suggesting the diminution of Ni3+ in the Ni0.8Co0.2 sample. This reveals that an intimate electronic transfer between nickel and cobalt may have occurred, which can be expressed as: Ni3+ + Co2+ → Ni2+ + Co3+. In general, the XPS results of Ni 2p suggest the formation of a defective NiO structure on the catalyst's surface, and the satellite peaks indicate that Ni2+ is the major component.
Fig. 6(b) shows the XPS spectrum of Co 2p. Four main peaks can be seen at ∼780, ∼795, ∼782 and ∼797 eV, and are assigned to Co3+ 2P3/2, Co3+ 2p1/2, Co2+ 2p3/2 and Co2+ 2p1/2, respectively, with an energy difference of the spin orbit split of 15 eV. Thus, the Co atom in these samples has two valence states (octahedral Co3+ and tetrahedral Co2+), indicating the formation of Co3O4;41 this is in line with the XRD results. The relative percentage content of Co and Co3+ is presented in Table 3. According to the literature,2,6,42,43 the surface Co3+ ions present a favourable site for CO adsorption and oxidation. However, Ni0.7Co0.3 possesses the highest surface Co3+ content, although its activity declines as compared to Ni0.8Co0.2. This indicates that Co species are not the dominant active species for the CO oxidation reaction.
| Samples | H2-TPR results | |||||||
|---|---|---|---|---|---|---|---|---|
| Peak (ε) area | Peak (α) area | Peak (β) area | Peak (γ) area | Total peak area | Theoretical H2 consumption (mmol g−1) | Actual H2 consumption (mmol g−1) | T/A | |
| Ni0.99Co0.01 | 390.75 | — | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300.36 300.36 |
1553.20 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 244.31 244.31 |
13.45 | 13.50 | 1.00 |
| Ni0.95Co0.05 | 471.93 | 830.32 | 9050.53 | 3419.55 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 772.33 772.33 |
13.92 | 13.05 | 1.07 |
| Ni0.9Co0.1 | 727.86 | 1556.82 | 6952.89 | 4385.28 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 622.85 622.85 |
14.23 | 12.91 | 1.10 |
| Ni0.8Co0.2 | 768.94 | 1724.64 | 6240.31 | 5901.31 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 635.2 635.2 |
14.85 | 13.78 | 1.08 |
| Ni0.7 Co0.3 | 801.24 | 2907.85 | 4820.18 | 7342.01 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 871.28 871.28 |
15.16 | 15.04 | 1.01 |
| NiO | — | — | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 579.17 579.17 |
— | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 579.17 579.17 |
13.39 | 12.82 | 1.04 |
| Co3O4 (0.01 g) | — | 2402.55 | — | 8659.63 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 062.18 062.18 |
16.61 | 15.93 | 1.04 |
The high-resolution spectrum of O 1 s of these samples in Fig. 6(c) is fitted with two peaks: the main peak O′ at ∼529.2 eV, which is ascribed to the characteristic lattice oxygen bonding to the metal cations, and the shoulder peak O′′ with the higher binding energy at ∼530.8 eV, which is attributed to the chemisorbed oxygen.44 It reveals that oxygen vacancies exist on the sample's surface, and the O is adsorbed onto the surface in the form of O2− or O− ions,45 also demonstrated by the LRS results. The ratio of the chemisorbed oxygen is quantified based on the area ratio of O′′/(O′′ + O′) for these samples (Table 2) and follows the order: NiO < Ni0.99Co0.01 < Ni0.95Co0.05 < Ni0.8Co0.2 < Ni0.7Co0.3. The NiO sample has the lowest chemisorbed oxygen content (24.45%), whereas the chemisorbed oxygen content of the Ni0.8Co0.2 sample (56.91%) is much larger than that of NiO. It indicates that the introduction of cobalt facilitates the formation of oxygen vacancies on the catalyst surface. Moreover, the surface Ni/Co atomic ratios of the Ni–Co samples are higher than the corresponding bulk values, suggesting that there is an enrichment of Ni species on the catalyst surface.
3.4 Redox behavior and desorption analysis (H2-TPR and O2-TPD)
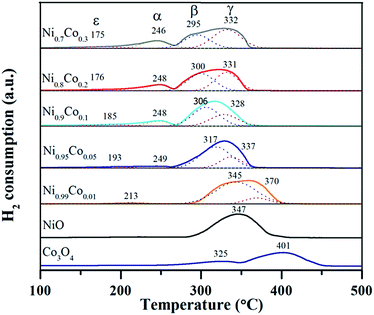
H2-TPR characterization was performed to explore the reducibility of the samples and the interaction between NiO and Co3O4 on the Ni–Co catalysts, as shown in Fig. 7. The Co3O4 sample exhibited two reduction peaks at 325 and 405 °C, which correspond to the well-defined two-step reduction of Co3+ to Co2+ and Co2+ to Co0.13,28,46 For the NiO sample, only one broad peak at 347 °C was observed. As reported,5,10,47 the reduction of pure NiO particles usually takes place at around 350 °C. Accordingly, this broad peak is ascribed to the reduction of the NiO particles. When it comes to the Ni–Co samples, the former small peak (ε) with the lowest reduction temperature (175–213 °C) belongs to the reduction of surface oxygen species adsorbed on oxygen vacancies.13,33 The position of peak (ε) shifts slightly to a lower temperature as the Co content increases, indicating that the surface oxygen species on the Ni–Co samples is similar. The peaks (α) and (γ) at ∼246 °C and ∼332 °C, respectively, are assigned to the two-step reduction of the Co ions. The peak (β) in the temperature range from 295 to 345 °C is likely associated with the reduction of the NiO particles. Tang et al.35 assigned the peak around 300 °C to the reduction of well-dispersed NiO interacting strongly with the Ni–Ce solid solution. According to this point and combined with the XRD and TEM results, we infer that the peak (β) at lower temperature (∼300 °C) can be attributed to the highly dispersed amorphous NiO phase interacting strongly with the Ni–Co spinel, which supports the existence of amorphous NiO on the catalyst. In addition, peak (α) cannot be observed from the spectra of the Ni0.99Co0.01 sample, which is possibly due to the fact that the content of cobalt is too low and therefore the amount of Co3+ is extremely low. The position of peak (β) shifts to lower reduction temperatures gradually, in sync with an increase in Co content, which can be explained as follows. Firstly, there is an enhanced intense synergistic effect between NiO and Co3O4 through Ni3+ + Co2+ → Ni2+ + Co3+, which makes the nickel species easier to reduce. Secondly, as deduced from the XRD and TPR results, the introduction of Co turns the crystalline NiO into amorphous NiO and enhances the dispersion of NiO particles, which also contributes to the reduction of NiO particles. The positions of peak (α) and peak (γ) also shift towards lower reduction temperatures, which can be likened to pure Co3O4, likely due to the strong synergistic effect and the accumulation of Co3+ on the catalyst surface.Detailed data of the reduction peak areas are displayed in Table 3. By comparing the peak area, it is clear that the area of peak (α) and peak (γ) increase as the Co content increases. However, the peak (β) area shows the opposite variation tendency. This suggests that the Co3O4 increases as the NiO decreases, which is in agreement with the XRF and XRD results. The peak (ε) exhibits the same trend with peak (α) and peak (γ), implying that doping with cobalt facilitates the generation of surface oxygen species. This trend agrees with the proportion of surface oxygen species within the Ni–Co catalysts (higher Co doping results in larger amount of surface oxygen) from the XPS results. In addition, the H2 consumption was calculated and is listed for each catalyst in Table 3. It can be seen that the ratios of theoretical H2 consumption (denoted as T) to actual H2 consumption (denoted as A) are slightly larger than 1.0, indicating that most of the H2 consumption come from the reduction of NiO. Furthermore, low valence Co2+ exists in the samples, also causing a decrease in the actual H2 consumption. This is in agreement with the XPS results.
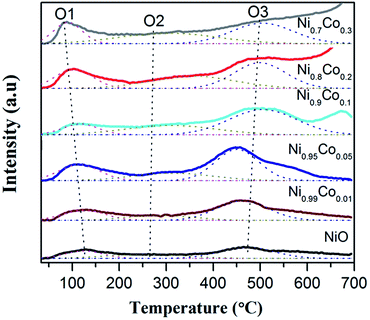
O2-TPD experiments were carried out to further investigate how the surface oxygen species possibly affects the redox chemistry of the catalysts. According to the literature,45 desorption peaks below 400 °C usually belong to superficial oxygen species and are weakly bound to the surface. From Fig. 8, it can be noted that each sample has three desorption peaks at about 100, 325 and 495 °C. The first intense peak (O1) is ascribed to physically adsorbed oxygen species faintly bound to the surface, which is easily desorbed, even in a low temperature range. The second broad weak peak (O2) is attributed to the O2− or O− species, formed by the oxygen adsorbed on the surface vacancies; this corresponds well with the XPS results.44,48 The peaks above 450 °C are associated with the surface lattice oxygen species,31 which have nothing to do with the reaction due to their relatively high desorption temperatures. The first two peaks should be further analysed since they may be closely related to the oxidation and redox reactions.
The oxygen-supplying ability depends on the number of oxygen-supplying centers and the activity.49 Data for the former two peaks is displayed in Table 4. We can see that the area of peak O1 is enhanced compared with pure NiO with an addition of Co. It is interesting to note that the area of peak O1 is sequentially consistent with the specific surface area, indicating that a large specific surface area is beneficial to the physical adsorption of oxygen.45 It is also clear that the amount of adsorbed O2− or O− species for Ni–Co oxides increases remarkably compared with pure NiO.
| Samples | O2-TPD results | |
|---|---|---|
| Peak (O1) area | Peak (O2) area | |
| Ni0.99Co0.01 | 1129.85 | 897.93 |
| Ni0.95Co0.05 | 1674.72 | 1573.05 |
| Ni0.9Co0.1 | 998.87 | 2253.28 |
| Ni0.8Co0.2 | 1636.10 | 2480.52 |
| Ni0.7Co0.3 | 1539.24 | 2702.76 |
| NiO | 752.85 | 846.18 |
3.5 CO and/or O2 interaction with NiO and Ni0.8Co0.2 samples (in situ DRIFTS results)
In order to gain more of an understanding about the nature of how the CO oxidation reaction occurs on the surface of the catalyst, the interactions between the reactants on the samples and the changes of the surface adsorbed species need to be examined. Therefore, the CO and/or O2 adsorption in situ DRIFTS spectra of the NiO and Ni0.8Co0.2 samples were recorded under simulated reaction conditions with temperatures ranging from 50 to 150 °C.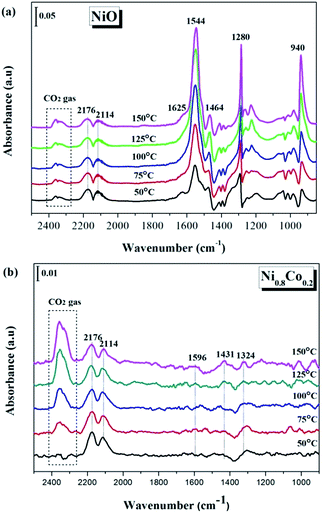 | ||
| Fig. 9 In situ DRIFTS spectra of (a) NiO and (b) Ni0.8Co0.2 samples under CO stream at different temperature. | ||
The in situ DRIFTS spectra of CO adsorption on the Ni0.8Co0.2 sample are shown in Fig. 9b. Peaks belonging to carbonate and formate species can also be detected at low temperatures; these peaks increase in intensity with a further rise in temperature. The bands ascribed to gaseous CO and gaseous CO2 are also observed at similar wavenumbers to the bands observed for the NiO sample (Fig. 9a). Moreover, the peak intensity of gaseous CO gradually reduces whilst the peak intensity of gaseous CO2 increases as the temperature increases, which is most likely a result of the catalyst reduction,45 suggesting that the Ni0.8Co0.2 sample is much easier to be reduced compared to the NiO sample.
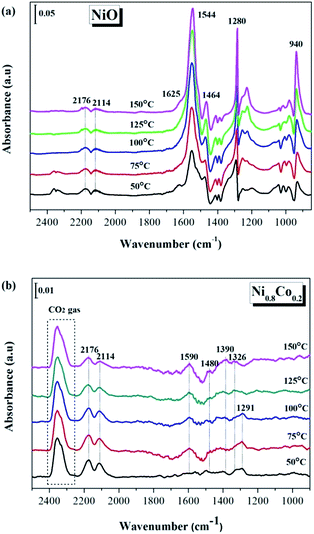 | ||
| Fig. 10 In situ DRIFTS spectra of (a) NiO and (b) Ni0.8Co0.2 samples under CO + O2 stream at different temperature. | ||
It is worth noting that for the NiO sample a large number of carbonate and carboxylate species are generated during the CO or/and O2 adsorption processes, while the Ni0.8Co0.2 sample shows the opposite behaviour. It is generally accepted that monometallic Ni catalysts are prone to carbon formation, which usually causes catalyst deactivation.24,53 Therefore, the following can be concluded: in the case of the NiO sample, the CO molecules were oxidized by surface active O species, initially forming a large number of carbonate and carboxylate species. These were deposited on the NiO surface but only a few of each species was converted to CO2 at low temperatures; therefore it was these that covered the active sites on the surface. On the contrary, the Ni0.8Co0.2 sample possesses many surface oxygen vacancies and therefore only a few carbonate species were generated on the surface under the simulated reaction conditions, indicating that CO molecules can rapidly be oxidized by surface-active O to CO2 gas. The oxygen vacancies therefore need to be fully exposed to support an efficient and stable CO oxidation reaction. Ren et al.31 have also reported that doping of Ni in Co3O4 reduces the formation of stable carbonates on the catalyst surface, which promotes the desorption of CO2 during the oxidation of propane.
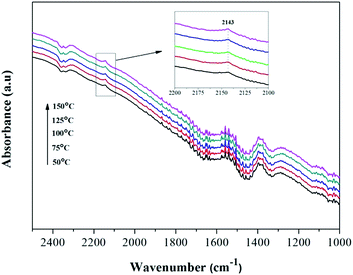
Amorphous NiO has been recognized as being active for CO oxidation with CO molecules being adsorbed by Ni2+ to form of Ni2+–(CO) and Ni2+–(CO)2.9 However, the FT-IR signals of these complexes are typically too weak to be detected or they overlap with the signal for gaseous CO. To understand whether surface–adsorbed CO exists on the Ni0.8Co0.2 catalyst surface under reaction conditions, the reference of gaseous CO was measured in the DRIFTS cell and the contribution subtracted (the results are shown in Fig. 11). It is interesting to notice the appearance of a small peak at 2143 cm−1. Solsona et al.33 assigned a band at 2148 cm−1 to adsorbed CO on pure NiO. Hence, we inferred this small peak should be attributed to surface–absorbed CO on the surface Ni species. The peak intensity increases as the temperature increases, indicating that higher temperatures are favourable to the adsorption of CO. Furthermore, the single CO adsorption in situ DRIFTS spectra of Ni0.8Co0.2 and NiO was compared after subtracting the contribution of gaseous CO. Fig. S4† shows that the intensity of the adsorbed CO band of Ni0.8Co0.2 is stronger than that of NiO, indicating that Ni0.8Co0.2 has a stronger CO adsorption.
3.6 The possible mechanism for CO oxidation reaction over Ni–Co–O catalysts
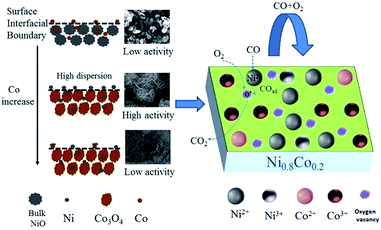
As far as catalyst development is concerned, it is critical to explore the structure–activity correlation of catalysts. To the best of our knowledge, little has been reported about the reaction mechanism of CO oxidation catalysed by nickel–cobalt catalysts. Based on the information from previous characterization, especially the CO or/and O2 adsorption in situ DRIFTS results, a mechanism for CO oxidation reaction is tentatively proposed. Considering the activity test results, cobalt doping is required for the most effective catalytic activity. Combining the results of XRD and H2-TPR, it can be concluded that a low cobalt content does not result in a high dispersion of the NiO particles, leading to a low activity. On the other hand, a large cobalt content can reduce the surface NiO concentration and the specific surface area, resulting in a decrease of activity. Therefore, it can be deduced that highly dispersed surface amorphous NiO is the dominant active species, similar to the study performed by Tang et al.10,35 By comparing the in situ DRIFTS results of NiO and Ni0.2Co0.8 samples, it was deduced that a high concentration of oxygen vacancies play an important role in the CO + O2 reaction, which is supported by Raman, XPS and O2-TPD results. Mahammadunnisa et al.54 fabricated NiO/Ce1−xNixO2−δ catalysts for CO oxidation and observed that an enrichment of oxygen vacancies on the surface of the catalyst can promote the activation of oxygen species on surface and accelerate the reaction.Based on the above results, when the surface–adsorbed CO reacts with activated O over the Ni–Co samples, it does so according to a Langmuir–Hinshelwood (L–H) mechanism. As depicted in Fig. 12 and taking the Ni0.8Co0.2 sample as an example, O2 molecules preferentially adsorb on the catalyst surface in an oxygen-enriched atmosphere, forming surface-active oxygen species (such as O2− or O−), which occupy surface vacancies. CO molecules are adsorbed on the surface NiO (amorphous) to form Ni2+–CO species, and the adsorbed CO then reacts with the active oxygen species on nearby surface oxygen vacancies and is transformed into gaseous CO2. Finally, the surface oxygen vacancies are regenerated by gaseous O2, completing the catalytic cycle.55
4. Conclusions
In this work, a series of Ni–Co composited oxide catalysts with different Ni/Co ratios were synthesised by a facile liquid-precipitation method and tested for their ability to catalyse the CO oxidation reaction. Based on the above characterization, results and discussion, several major conclusions were formulated:(1) The doping of Co species to form binary composite oxides can effectively enhance the redox properties and catalytic activity of nickel oxide. The synergetic effect between Ni and Co leads to a significant decrease in the size of the NiO, resulting in the formation of highly dispersed amorphous NiO on the catalyst surface, which strongly reacts with Co3O4. The highly dispersed amorphous NiO is presumed to be the dominant active species for the CO oxidation. The direction of the redox equilibrium, expressed as Ni3+ + Co2+ → Ni2+ + Co3+, translates as the Ni–Co oxides being more easily reduced than pure NiO.
(2) The surface oxygen vacancies play an important role in the reaction atmosphere. For the Ni0.8Co0.2 sample, the combination of a high concentration of surface oxygen vacancies and the regeneration of oxygen vacancies leads to excellent catalytic activity and stability in the CO oxidation reaction.
(3) As the amount of cobalt increases, the morphology of the catalyst changes from plate-like to flower-like, and, eventually, to dense granules. The Ni0.8Co0.2 shows a novel flower-like morphology and demonstrates the best catalytic performance.
(4) O2 molecules can be translated into activating O species (O2− or O−) through absorption by the surface oxygen vacancies. The surface-adsorbed CO reacts with the activating O species to produce CO2 via a classic L–H reaction mechanism.
(5) The Ni–Co composite oxide exhibits higher catalytic activity than other Ni-based composite oxide including Ni–Mn, Ni–Fe, Ni–Zn and Ni–Cr.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the National Nature Science Foundation of China (No. 21507014, 21663006, 21763003), the Key Project of Chinese National Programs for Fundamental Research and Development (973 program) (No. 2012CB21500203), and the Program for Science and Technology Development Plan of Nanning (No. 20163146).Notes and references
- M. V. Twigg, Progress and future challenges in controlling automotive exhaust gas emissions, Appl. Catal., B, 2007, 70, 2–15 CrossRef CAS.
- X. W. Xie, Y. Li, Z. Q. Liu, M. Haruta and W. J. Shen, Low-temperature oxidation of CO catalysed by Co3O4 nanorods, Nature, 2009, 458, 746–749 CrossRef CAS PubMed.
- G. Busca, E. Finocchio and V. S. Escribano, Infrared studies of CO oxidation by oxygen and by water over Pt/Al2O3 and Pd/Al2O3 catalysts, Appl. Catal., B, 2012, 113–114, 172–179 CrossRef CAS.
- Tarjomannejad, A. Niaei, A. Farzi, D. Salari and P. R. Zonouz, Catalytic Oxidation of CO Over LaMn1−xBxO3 (B = Cu, Fe) Perovskite-type Oxides, Catal. Lett., 2016, 146, 1544–1551 CrossRef CAS.
- H. L. Li, K. Yu, C. Wan, J. J. Zhu, X. Li, S. Tong and Y. X. Zhao, Comparison of the nickel addition patterns on the catalytic performances of LaCoO3 for low-temperature CO oxidation, Catal. Today, 2017, 281, 534–541 CrossRef CAS.
- S. C. Petitto, E. M. Marsh, G. A. Carson and M. A. Langell, Cobalt oxide surface chemistry: The interaction of CoO(1 0 0), Co3O4(1 1 0) and Co3O4(1 1 1) with oxygen and water, J. Mol. Catal. A: Chem., 2008, 281, 49–58 CrossRef CAS.
- C. Z. Wang, N. N. Sun, N. Zhao, W. Wei and Y. X. Zhao, Template-free preparation of bimetallic mesoporous Ni–Co–CaO–ZrO2 catalysts and their synergetic effect in dry reforming of methane, Catal. Today, 2017, 281, 268–275 CrossRef CAS.
- D. S. Wang, R. Xu, X. Wang and Y. D. Li, NiO nanorings and their unexpected catalytic property for CO oxidation, Nanotechnology, 2006, 17, 979–983 CrossRef CAS PubMed.
- B. Zhao, X. K. Ke, J. H. Bao, C. L. Wang, L. Dong, Y. W. Chen and H. L. Chen, Synthesis of Flower-Like NiO and Effects of Morphology on Its Catalytic Properties, J. Phys. Chem. C, 2009, 113, 14440–14447 CAS.
- C. J. Tang, J. C. Li, X. J. Yao, J. F. Sun, Y. Cao, L. Zhang, F. Gao, Y. Deng and L. Dong, Mesoporous NiO–CeO2 catalysts for CO oxidation: Nickel content effect and mechanism aspect, Appl. Catal., A, 2015, 494, 77–86 CrossRef CAS.
- X. Q. Cheng, A. M. Zhu, Y. Z. Zhang, Y. Wang, C. T. Au and C. Shi, A combined DRIFTS and MS study on reaction mechanism of NO reduction by CO over NiO/CeO2 catalyst, Appl. Catal., B, 2009, 90, 395–404 CrossRef CAS.
- Y. Z. Wang, Y. X. Zhao, C. G. Gao and D. S. Liu, Preparation and catalytic performance of Co3O4 catalysts for low-temperature CO oxidation, Catal. Lett., 2007, 116, 136–142 CrossRef CAS.
- W. D. Zhang, F. Wu, J. J. Li and Z. X. You, Dispersion–precipitation synthesis of highly active nanosized Co3O4 for catalytic oxidation of carbon monoxide and propane, Appl. Surf. Sci., 2017, 411, 136–143 CrossRef CAS.
- J. Jansson, A. E. C. Palmqvist, E. Fridell, M. Skoglundh, L. Österlund, P. Thormählen and V. Langer, On the Catalytic Activity of Co3O4 in Low-Temperature CO Oxidation, J. Catal., 2002, 211, 387–397 CrossRef CAS.
- B. Faure and P. Alphonse, Co–Mn-oxide spinel catalysts for CO and propane oxidation at mild temperature, Appl. Catal., B, 2016, 180, 715–725 CrossRef CAS.
- L. E. Gómez, I. S. Tiscornia, A. V. Boix and E. E. Miró, CO preferential oxidation on cordierite monoliths coated with Co/CeO2 catalysts, Int. J. Hydrogen Energy, 2012, 37, 14812–14819 CrossRef.
- M. P. Woods, P. Gawade, B. Tan and U. S. Ozkan, Preferential oxidation of carbon monoxide on Co/CeO2 nanoparticles, Appl. Catal., B, 2010, 97, 28–35 CrossRef CAS.
- R. S. Shang, Y. P. Duan, X. Y. Zhong, W. Xie, Y. Luo and L. H. Huang, Formic Acid Modified Co3O4–CeO2 Catalysts for CO Oxidation, Catalysts, 2016, 6, 48 CrossRef.
- Y. Yu, G. Q. Jin, Y. Y. Wang and X. Y. Guo, Synthesis of natural gas from CO methanation over SiC supported Ni–Co bimetallic catalysts, Catal. Commun., 2013, 31, 5–10 CrossRef CAS.
- R. Razzaq, H. W. Zhu, L. Jing, U. Muhammad, C. S. Li and S. J. Zhang, Catalytic Methanation of CO and CO2 in Coke Oven Gas over Ni−Co/ZrO2−CeO2, Ind. Eng. Chem. Res., 2013, 52, 2247–2256 CrossRef CAS.
- A. Maione, P. Ruiz and M. Devillers, Rationalization of the role played by bismuth and lanthanides in modified Ni–Co molybdates as catalysts for partial and total oxidation of propane, Catal. Today, 2004, 91–92, 121–125 CrossRef CAS.
- X. X. Zhao and G. X. Lu, Modulating and controlling active species dispersion over Ni–Co bimetallic catalysts for enhancement of hydrogen production of ethanol steam reforming, Int. J. Hydrogen Energy, 2016, 41, 3349–3362 CrossRef CAS.
- A. Tsoukalou, Q. Imtiaz, S. M. Kim, P. M. Abdala, S. Yoon and C. R. Müller, Dry-reforming of methane over bimetallic Ni–M/La2O3 (M = Co, Fe): The effect of the rate of La2O2CO3 formation and phase stability on the catalytic activity and stability, J. Catal., 2016, 343, 208–214 CrossRef CAS.
- Q. B. Zhang, B. T. Zhao, J. X. Wang, C. Qu, H. B. Sun, K. L. Zhang and M. L. Liu, High-performance hybrid supercapacitors based on self-supported 3D ultrathin porous quaternary Zn–Ni–Al–Co oxide nanosheets, Nano Energy, 2016, 28, 475–485 CrossRef.
- J. G. Zhang, H. Wang and A. K. Dalai, Development of stable bimetallic catalysts for carbon dioxide reforming of methane, J. Catal., 2007, 249, 300–310 CrossRef CAS.
- Y. Guo, X. Liang and B. H. Chen, Porous Ni–Co bimetal oxides nanosheets and catalytic properties for CO oxidation, J. Alloys Compd., 2013, 574, 181–187 CrossRef.
- X. M. Zhang, Y. Q. Deng, P. F. Tian, H. H. Shang, J. Xu and Y. F. Han, Dynamic active sites over binary oxide catalysts: In situ/operando spectroscopic study of low-temperature CO oxidation over MnOx–CeO2 catalysts, Appl. Catal., B, 2016, 191, 179–191 CrossRef CAS.
- D. B. Li, X. H. Liu, Q. H. Zhang, Y. Wang and H. L. Wan, Cobalt and Copper Composite Oxides as Efficient Catalysts for Preferential Oxidation of CO in H2-Rich Stream, Catal. Lett., 2009, 127, 377–385 CrossRef CAS.
- J. G. Zhang, H. Wang and A. K. Dalai, Effects of metal content on activity and stability of Ni–Co bimetallic catalysts for CO2 reforming of CH4, Appl. Catal., A, 2008, 339, 121–129 CrossRef CAS.
- L. Wang, D. L. Li, M. Koike, H. Watanabe, Y. Xu, Y. Nakagawa and K. Tomishige, Catalytic performance and characterization of Ni–Co catalysts for the steam reforming of biomass tar to synthesis gas, Fuel, 2013, 112, 654–661 CrossRef CAS.
- Z. Ren, Z. L. Wu, W. Q. Song, W. Xiao, Y. B. Guo, J. Ding, S. L. Suib and P. X. Gao, Low temperature propane oxidation over Co3O4 based nano-array catalysts: Ni dopant effect, reaction mechanism and structural stability, Appl. Catal., B, 2016, 180, 150–160 CrossRef CAS.
- B. Savova, S. Loridant, D. Filkova and J. M. M. Millet, Ni–Nb–O catalysts for ethane oxidative dehydrogenation, Appl. Catal., A, 2010, 390, 148–157 CrossRef CAS.
- B. Solsona, P. Concepción, S. Hernández, B. Demicol and J. M. L. Nieto, Oxidative dehydrogenation of ethane over NiO–CeO2 mixed oxides catalysts, Catal. Today, 2012, 180, 51–58 CrossRef CAS.
- J. H. Li, C. C. Wang, C. J. Huang, Y. F. Sun, W. Z. Weng and H. L. Wan, Mesoporous nickel oxides as effective catalysts for oxidative dehydrogenation of propane to propene, Appl. Catal., A, 2010, 382, 99–105 CrossRef CAS.
- K. Tang, W. Liu, J. Li, J. X. Guo, J. C. Zhang, S. P. Wang, S. L. Niu and Y. Z. Yang, The Effect of Exposed Facets of Ceria to the Nickel Species in Nickel–Ceria Catalysts and Their Performance in a NO + CO Reaction, ACS Appl. Mater. Interfaces, 2015, 7, 26839–26849 CAS.
- J. Y. Luo, M. Meng, X. Li, X. G. Li, Y. Q. Zha, T. D. Hu, Y. N. Xie and J. Zhang, Mesoporous Co3O4–CeO2 and Pd/Co3O4–CeO2 catalysts: Synthesis, characterization and mechanistic study of their catalytic properties for low-temperature CO oxidation, J. Catal., 2008, 254, 310–324 CrossRef CAS.
- L. W. Mi, W. T. Wei, S. B. Huang, S. Z. Cui, W. X. Zhang, H. W. Hou and W. H. Chen, A nest-like Ni@Ni1.4Co1.6S2 electrode for flexible high-performance rolling supercapacitor device design, J. Mater. Chem. A, 2015, 3, 20973–20982 CAS.
- N. V. Kosova, E. T. Devyatkina and V. V. Kaichev, Mixed layered Ni–Mn–Co hydroxides: Crystal structure, electronic state of ions, and thermal decomposition, J. Power Sources, 2007, 174, 735–740 CrossRef CAS.
- M. A. Peck and M. A. Langell, Comparison of Nanoscaled and Bulk NiO Structural and Environmental characteristics by XRD, XAFS, and XPS, Chem. Mater., 2012, 24, 4483–4490 CrossRef CAS.
- P. Salagre, J. L. G. Fierro, F. Medina and J. E. Sueiras, Characterization of nickel species on several γ-alumina supported nickel samples, J. Mol. Catal. A: Chem., 1996, 106, 125–134 CrossRef CAS.
- C. P. Yuan, H. G. Wang, J. Q. Liu, Q. Wu, Q. Duan and Y. H. Li, Facile synthesis of Co3O4–CeO2 composite oxide nanotubes and their multifunctional applications for lithium ion batteries and CO oxidation, J. Colloid Interface Sci., 2017, 494, 274–281 CrossRef CAS PubMed.
- F. Grillo, M. M. Natile and A. Glisenti, Low temperature oxidation of carbon monoxide: the influence of water and oxygen on the reactivity of a Co3O4 powder surface, Appl. Catal., B, 2004, 48, 267–274 CrossRef CAS.
- P. Broqvist, I. Panas and H. Persson, A DFT Study on CO Oxidation over Co3O4, J. Catal., 2002, 210, 198–206 CrossRef CAS.
- C. S. Deng, Q. Q. Huang, X. Y. Zhu, Q. Hu, W. L. Su, J. N. Qian, L. H. Dong, B. Li, M. G. Fan and C. Y. Liang, The influence of Mn-doped CeO2 on the activity of CuO/CeO2 in CO oxidation and NO + CO model reaction, Appl. Surf. Sci., 2016, 389, 1033–1049 CrossRef CAS.
- J. H. Ma, G. Z. Jin, J. B. Gao, Y. Y. Li, L. H. Dong, M. N. Huang, Q. Q. Huang and B. Li, Catalytic effect of two-phase intergrowth and coexistence CuO-CeO2, J. Mater. Chem. A, 2015, 3, 24358–24370 CAS.
- F. Wang, X. Wang, D. P. Liu, J. M. Zhen, J. Q. Li, Y. H. Wang and H. J. Zhang, High-Performance ZnCo2O4@CeO2 Core@shell Microspheres for Catalytic CO Oxidation, ACS Appl. Mater. Interfaces, 2014, 6, 22216–22223 CAS.
- H. Mori, C. J. Wen, J. Otomo, K. Eguchi and H. Takahashi, Investigation of the interaction between NiO and yttria-stabilized zirconia (YSZ) in the NiO/YSZ composite by temperature-programmed reduction technique, Appl. Catal., A, 2003, 245, 79–85 CrossRef CAS.
- C. S. Deng, J. N. Qian, C. X. Yu, Y. N. Yi, P. Zhang, W. Li, L. H. Dong, B. Li and M. G. Fan, Influences of doping and thermal stability on the catalytic performance of CuO/Ce20M1Ox (M = Zr, Cr, Mn, Fe, Co, Sn) catalysts for NO reduction by CO, RSC Adv., 2016, 6, 113630–113647 RSC.
- L. H. Dong, Y. X. Tang, B. Li, L. Y. Zhou, F. Z. Gong, H. X. He, B. Z. Sun, C. J. Tang, F. Gao and L. Dong, Influence of molar ratio and calcination temperature on the properties of TixSn1−xO2 supporting copper oxide for CO oxidation, Appl. Catal., B, 2016, 180, 451–462 CrossRef CAS.
- C. S. Deng, B. Li, L. H. Dong, F. Y. Zhang, M. G. Fan, G. Z. Jin, J. B. Gao, L. W. Gao, F. Zhang and X. P. Zhou, NO reduction by CO over CuO supported on CeO2-doped TiO2: the effect of the amount of a few CeO2, Phys. Chem. Chem. Phys., 2015, 17, 16092–16109 RSC.
- L. J. Liu, Q. Yu, J. Zhu, H. Q. Wan, K. Q. Sun, B. Liu, H. Y. Zhu, F. Gao, L. Dong and Y. Chen, Effect of MnOx modification on the activity and adsorption of CuO/Ce0.67Zr0.33O2 catalyst for NO reduction, J. Colloid Interface Sci., 2010, 349, 246–255 CrossRef CAS PubMed.
- Y. X. Tang, L. H. Dong, C. S. Deng, M. N. Huang, B. Li and H. L. Zhang, In situ FT-IR Investigation of CO Oxidation on CuO/TiO2 Catalysts, Catal. Commun., 2016, 78, 33–36 CrossRef CAS.
- J. R. Rostrup-Nielsen, Coking on nickel catalysts for steam reforming of hydrocarbons, J. Catal., 1974, 33, 184–201 CrossRef.
- S. Mahammadunnisa, P. M. K. Reddy, N. Lingaiah and C. Subrahmanyam, NiO/Ce1−xNixO2−δ as an alternative to noble metal catalysts for CO oxidation, Catal. Sci. Technol., 2013, 3, 730 CAS.
- T. K. Liu, Y. Y. Yao, L. Q. Wei, Z. F. Shi, L. Y. Han, H. X. Yuan, B. Li, L. H. Dong, F. Wang and C. Z. Sun, Preparation and Evaluation of Copper–Manganese Oxide as a High-Efficiency Catalyst for CO Oxidation and NO Reduction by CO, J. Phys. Chem. C, 2017, 121, 12757–12770 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra12635b |
| This journal is © The Royal Society of Chemistry 2018 |