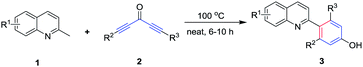Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Catalyst- and solvent-free approach to 2-arylated quinolines via [5 + 1] annulation of 2-methylquinolines with diynones†
Hai-Yuan Zhao‡
ab,
Fu-Song Wu‡b,
Li Yangc,
Ying Liang*a,
Xiao-Lin Caob,
Heng-Shan Wang b and
Ying-Ming Pan
b and
Ying-Ming Pan *b
*b
aSchool of Life and Environmental Sciences, Guilin University of Electronic Technology, Guilin, 541004, China. E-mail: yingl@aliyun.com
bState Key Laboratory for Chemistry and Molecular Engineering of Medicinal Resources, School of Chemistry and Pharmaceutical Sciences of Guangxi Normal University, Guilin 541004, China. E-mail: panym@mailbox.gxnu.edu.cn; Fax: +86-773-5803930
cGuangxi Key Laboratory of Special Non-wood Forest Cultivation and Utilization, Guangxi Zhuang Autonomous Region Forestry Research Institute, Nanning, 530002, China
First published on 25th January 2018
Abstract
A novel route for the synthesis of 2-arylated quinolines through a [5 + 1] annulation directly from 2-methylquinolines and diynones under catalyst-free and solvent-free conditions was disclosed. This synthetic process was atom-economic, with good tolerance of a broad range of functional groups, and with great practical worth.
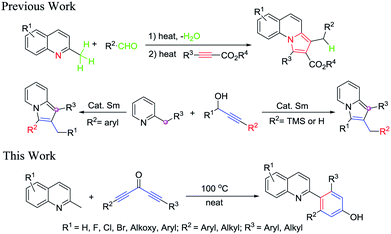
Nitrogen-containing heterocyclic compounds are ubiquitous in natural molecules and exhibit a wide array of biological activities.1 Among various N-heterocycles, quinoline nuclei are privileged scaffolds that occupy an important role in many medicinally relevant compounds.2 2-Arylated quinolines are found in many medicinal compounds including etoricoxib,3 rosuvastatin,4 and gleevec,5 as well as molecules designed for other purposes including P, N ligands, such as QUINAP.6 Because of their unique biological activity and wide application, the functionalized 2-arylated quinoline elicited considerable synthetic interest, and a variety of synthetic routes have been established.7 In addition, some classical synthetic methods such as Kumada,8 Suzuki,9 Negishi,10 or Stille9b are usually used to efficiently prepare these compounds, but these methods require the preparation of cross-coupling reagents such as Grignard reagents, boronic acids, organozincs, and organostannanes in advance and these cross-coupling reagents are unstable or toxic or can't be isolated as solids.11 More recently, much research has been directed toward the synthesis of 2-arylated quinolones and their derivatives via transition-metal-catalyzed C–H arylation12 and many other methods also have been developed by transitional-metal-catalyzed cross-couplings.13 These transition metals included Co, Cu or Pd. Moreover, 2-arylated quinolines synthesis via direct C–H arylation of quinolones with various aryl bromides, arylboronic acid, or arylzinc reagents catalyzed by transition metal catalyst, such as Rh, Fe, or Ni, have been investigated by Bergman,14 Maiti,15 and Tobisu.16 Ru17 or Mn18 also was used to catalyze indirect Friedländer synthesis to obtain 2-arylated quinolines that involved oxidative cyclization of 2-aminobenzyl alcohol with either ketones or alcohols. Although these processes were highly efficient and significance, none of these procedures could directly provide the final products with a low content of the heavy metal impurities which are strict restrictions in drugs and pharmaceuticals.19 Thus, an alternative, general, solvent-free, and environmentally sustainable procedure is urgently required for the quick synthesis of 2-arylated quinolines.
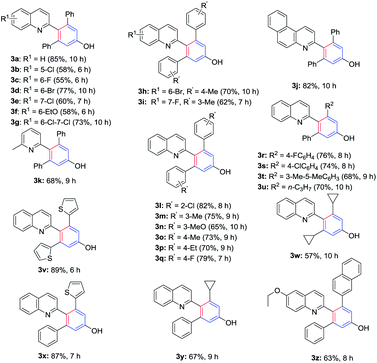
Recently, as our continuous study on the C(sp3)–H activation of 2-methylquinolines, which provided a facile synthetic approach to access substituted pyrrolo[1,2-a]quinolones (Scheme 1),20 we had found that the methyl of 2-methylquinolines has very high reactivity. Based on this, herein we reported the catalyst-free and solvent-free [5 + 1] annulation of 2-methylquinolines and diynones to access 4-(quinolin-2-yl)-phenols. To the best of our knowledge, there were none of the group that reported direct construction of six-member aromatic-ring at the methyl of 2-methylquinolines with diynones to give 4-(quinolin-2-yl)-phenols. The present novel construction protocol for 4-(quinolin-2-yl)-phenols had several significant advantages: (1) this chemistry provided a novel and simple strategy for the synthesis of highly valuable 4-(quinolin-2-yl)-phenols under very simple conditions; (2) according to the atom economy concept, this protocol was carried out under catalyst-free and solvent-free conditions, without the addition of any acid, base, or other reagents, which provided the final products without heavy metal impurities and improved its potential utility; (3) the method featured high functional group tolerance, high yields, and broad substrate scopes. Particularly, this route could directly introduce two different substituent groups on the newly formed of six-member aromatic-ring (see Table 2).
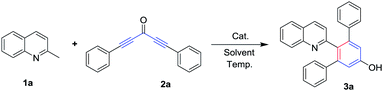
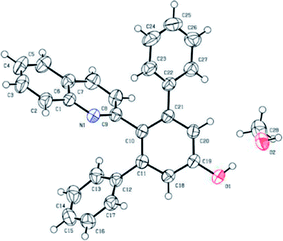
To examine the feasibility of our proposed protocol, 2-methylquinoline (1a) and 1,5-diphenylpenta-1,4-diyn-3-one (2a) were chosen as the model substrates and diverse reaction conditions were screened as shown in Table 1. Initially, treatment of 1a (0.6 mmol) with 2a (0.5 mmol) in chlorobenzene (PhCl) at 120 °C for 10 hours led to the arylation product 2′-(quinolin-2-yl)-[1,1′:3′,1′′-terphenyl]-5′-ol (3a) in 65% yield (Table 1, entry 1). The structure of 3a was confirmed by its 1H and 13C NMR spectra, mass spectra, and single-crystal X-ray diffraction analysis (Fig. 1).21 To improve the efficiency, we used Sm(OTf)3 as catalyst, but the result provided 3a in less than 60% (Table 1, entry 2). And then, when we used base (Cs2CO3, Et3N) or acid (AcOH) as additives, no desired products was isolated (Table 1, entries 4–6) because of 2a degradation in the presence of acid or base. Subsequently, we used other solvents such as toluene, DMF, DMSO, and 1,4-dioxane in place of PhCl and these reactions were completed in the absence of additives, providing the yields of 3a in less than 65% (Table 1, entries 7–10). Gratifyingly, when the reaction was carried out in the absence of solvent, the yield of corresponding product 3a was increased to 73% (Table 1, entry 11). Subsequently, we carefully adjusted the reaction temperature (Table 1, entries 12–14) and the desired product 3a was obtained in the best yield (85%) when the reaction was performed at 100 °C. The reaction time extended to 15 hours, but the yield of 3a was not increased (Table 1, entry 15).
| Entry | Cat. | Solvent | Temp. (°C) | Yieldb (%) |
|---|---|---|---|---|
| a Reactions conditions: 1a (0.6 mmol), 2a (0.5 mmol), catalyst (10 mol% to 2a), solvent (1 mL), sealed tube, 10 h.b Isolated yield of pure product based on 2a.c Cs2CO3 as base was added to the reaction.d 1a (1.5 mmol), 2a (0.5 mmol), sealed tube, 10 h.e Reaction time was 15 h. | ||||
| 1 | — | PhCl | 120 | 65 |
| 2 | Sm(OTf)3 | PhCl | 120 | 55 |
| 3c | Sm(OTf)3 | PhCl | 120 | 0 |
| 4 | Cs2CO3 | PhCl | 120 | 0 |
| 5 | Et3N | PhCl | 120 | 0 |
| 6 | AcOH | PhCl | 120 | 0 |
| 7 | — | Toluene | 120 | 35 |
| 8 | — | DMF | 120 | 63 |
| 9 | — | DMSO | 120 | 45 |
| 10 | — | 1,4-Dioxane | 120 | Trace |
| 11d | — | — | 120 | 73 |
| 12d | — | — | 110 | 75 |
| 13d | — | — | 100 | 85 |
| 14d | — | — | 90 | 60 |
| 15d,e | — | — | 100 | 86 |
With the optimized conditions in hand, a series of diynones and 2-methylquinolines were subjected to the reaction to investigate the scope and the results were shown in Table 2. The 2-methylquinoline ring has been substituted with electron-rich or electron-deficient groups R1 whereas R2, R3 in the diynones included alkyl and aryl moieties. All reactions proceeded smoothly to afford the corresponding 4-(quinolin-2-yl)phenols/4-(pyridin-2-yl)phenols in moderate to high yields (55–89%). Desired products 3b–3f were obtained in moderate to good yields (55–77%) with an electron-rich group (–EtO) or electron-deficient substituent (–F, –Cl, –Br) at C-5, C-6, or C-7 of 2-methylquinolines. The reaction of 6,7-dichloro-2-methylquinoline and diynones provided the corresponding product 3g in 73% yield with the PhCl as solvent. Symmetric diynones bearing electron-donating substituents such as Me, MeO, and Et or electron-withdrawing groups such as F and Cl on the benzene ring were found to be good substrates for this reaction and provided the desired products (3l–3q) in moderate to high yields, which showed that the position of the substituents on the benzene ring did not affect the transformation significantly. In addition, the diynones reacted with 2-methylquinoline which has an electron-deficient substituent at C-6 or C-7, furnishing the corresponding 4-(quinolin-2-yl)-phenols products in good yields (3h, 3i). It was found that the reaction of the 3-methylbenzo[f]quinoline and 2,6-dimethylpyridine also proceeded smoothly and afforded the desired product 3j and 3k in 82% and 68% yields, respectively. Unfortunately, the reaction of 2-methylpyridine and 1,5-diphenylpenta-1,4-diyn-3-one (2a) under the standard conditions only give a trace amount of the desired product. Then, we investigated the reaction with heterocycle substituted diynones, and found the thiophene substrates furnishing the desired product in higher yield (3v). Subsequently, other asymmetrical diynones which have two different substituents were also tested for the present reaction and the corresponding products were isolated in good to excellent yields (3r–3u, 3x–3z). We found that strained cyclopropyl was tolerated for this transformation and provided the corresponding products 3w and 3y in moderate yields.
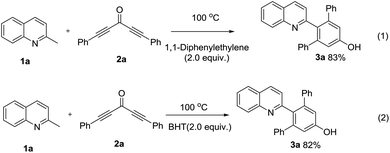
To support the proposed reaction pathway, additional control experiments were carried out and the results were presented in Scheme 2. It was observed that the presence of 2 equiv. of 1,1-diphenylethylene or BHT (2,6-di-tert-butyl-4-methylphenol) didn't suppress the synthesis of 3a. These results suggested that a radical mechanism wasn't likely involved.
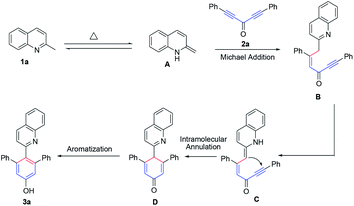
A possible mechanism is proposed in Scheme 3. At first, the enamine intermediate A was formed from 1a via tautomerization,22 followed by, Michael addition to diynones, giving the intermediate B. And then, the enamine intermediate C was generated from B via the requisite disruption of aromaticity. Subsequently, intermediate C was transformed into intermediate D by intramolecular annulation reaction and the intermediate D was rapidly aromatized to form the stable product 3a.
In summary, we have developed a rapid, simple, efficient, catalyst-free, and solvent-free reaction to access 4-(quinolin-2-yl)-phenols through a [5 + 1] annulation directly from 2-methylquinolines and diynones. The synthetic process was atom-economic, applicable to wide range of substrates, and has functional group tolerance, and these features would render this method attractive for academic and industrial use.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank the National Natural Science Foundation of China (41465009 and 21362002), Guangxi Natural Science Foundation of China (2017GXNSFDA198043, 2016GXNSFEA380001, 2016GXNSFGA380005 and AA17204058), Ministry of Education of China (IRT_16R15) and BAGUI Scholar Program of Guangxi Province of China (2016A13).Notes and references
- (a) Y. Hitora, K. Takada, Y. Ise, S. Okada and S. Matsunaga, J. Nat. Prod., 2016, 79, 2973 CrossRef CAS PubMed; (b) H. Fan, J.-G. Peng, M. T. Hamann and J.-F. Hu, Chem. Rev., 2008, 108, 264 CrossRef CAS PubMed; (c) B. Boucherle, R. Haudecoeur, E. F. Queiroz, M. de Waard, J.-L. Wolfender, R. J. Robins and A. Boumendjel, Nat. Prod. Rep., 2016, 33, 1034 RSC; (d) H.-T. Tang, K. Xiong, R.-H. Li, Z.-C. Ding and Z.-P. Zhan, Org. Lett., 2015, 17, 326 CrossRef CAS PubMed; (e) J.-J. Wen, H.-T. Tang, K. Xiong, Z.-C. Ding and Z.-P. Zhan, Org. Lett., 2014, 16, 5940 CrossRef CAS PubMed; (f) X. Tang, Z. Zhu, C. Qi, W. Wu and H. Jiang, Org. Lett., 2016, 18, 180 CrossRef CAS PubMed; (g) P. Li, X. Zhang and X. Fan, J. Org. Chem., 2015, 80, 7508 CrossRef CAS PubMed; (h) S.-S. Zhang, J.-Q. Wu, X. Liu and H. Wang, ACS Catal., 2015, 5, 210 CrossRef CAS.
- (a) W. R. Gutekunst, R. Gianatassio and P. S. Baran, Angew. Chem., Int. Ed., 2012, 124, 7625 CrossRef; (b) P. B. Arockiam, C. Bruneau and P. H. Dixneuf, Chem. Rev., 2012, 112, 5879 CrossRef CAS PubMed; (c) S. H. Cho, J. Y. Kim, J. Kwak and S. Chang, Chem. Soc. Rev., 2011, 40, 5068 RSC; (d) X. Chen, K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 121, 5196 CrossRef.
- R. W. Friesen, C. Brideau, C. C. Chan, S. Charleson, D. Deschênes, D. Dubé, D. Ethier, R. Fortin, J. Y. Gauthier, Y. Girard, R. Gordon, G. M. Greig, D. Riendeau, C. Savoie, Z. Wang, E. Wong, D. Visco, L. J. Xu and R. N. Young, Bioorganic Med. Chem. Lett., 1998, 8, 2777 CrossRef CAS PubMed.
- J. Quirk, M. Thornton and P. Kirkpatrick, Nature, 2003, 2, 769 CAS.
- R. Capdeville, E. Buchdunger, J. Zimmermann and A. Matter, Nature, 2002, 1, 493 CAS.
- (a) G. Chelucci, G. Orru and G. A. Pinna, Tetrahedron, 2003, 59, 9471 CrossRef CAS; (b) N. W. Alcock, J. M. Brown and D. I. Hulmes, Tetrahedron: Asymmetry, 1993, 4, 743 CrossRef CAS; (c) J. S. Carey, D. Laffan, C. Thomson and M. T. Williams, Org. Biomol. Chem., 2006, 4, 2337 RSC; (d) V. Bonnet, F. Mongin, F. Trécourt, G. Breton, F. Marsais, P. Knochel and G. Quéquiner, Synlett, 2002, 1008 CrossRef CAS.
- (a) N. Kaila, K. Janz, S. DeBernardo, P. W. Bedard, R. T. Camphausen, S. Tam, D. H. H. Tsao, J. C. Keith Jr, C. Nickerson-Nutter, A. Shilling, R. Young-Sciame and Q. Wang, J. Med. Chem., 2007, 50, 21 CrossRef CAS PubMed; (b) A. Zarghi, R. Ghodsi, E. Azizi, B. Daraie, M. Hedayati and O. G. Dadrass, Bioorg. Med. Chem., 2009, 17, 5312 CrossRef CAS PubMed; (c) R. Ghodsi, A. Zarghi, B. Daraei and M. Hedayati, Bioorg. Med. Chem., 2010, 18, 1029 CrossRef CAS PubMed.
- M. J. Iglesias, A. Prieto and M. C. Nicasio, Org. Lett., 2012, 17, 4318 CrossRef PubMed.
- (a) V. Arumugam, W. Kaminsky and D. Nallasamy, RSC Adv., 2015, 5, 77948 RSC; (b) D.-H. Lee, J. Young and M.-J. Jin, Green Chem., 2010, 12, 2024 RSC; (c) J.-F. Yang, S.-J. Liu, J.-F. Zheng and J.-R. Zhou, Eur. J. Org. Chem., 2012, 6248 CrossRef CAS; (d) Y.-J. Zou, G.-Z. Yue, J.-W. Xu and J.-R. Zhou, Eur. J. Org. Chem., 2014, 5901 CrossRef CAS.
- (a) D. Haas, J. M. Hammann, F. H. Lutter and P. Knochel, Angew. Chem., Int. Ed., 2016, 55, 3809 CrossRef CAS PubMed; (b) Z.-L. Liu, N.-N. Dong, M.-Z. Xu, Z.-M. Sun and T. Tu, J. Org. Chem., 2013, 78, 7436 CrossRef CAS PubMed.
- (a) A. Lützen and M. Hapke, Eur. J. Org. Chem., 2002, 2292 CrossRef; (b) D. C. Blakemore and L. A. Marples, Tetrahedron Lett., 2011, 52, 4192 CrossRef CAS.
- (a) L.-H. Kong, S.-J. Yu, X.-K. Zhou and X.-W. Li, Org. Lett., 2016, 18, 588 CrossRef CAS PubMed; (b) D.-B. Zhao, W.-H. Wang, F. Yang, J.-B. Lan, L. Yang, G. Gao and J.-S. You, Angew. Chem., Int. Ed., 2009, 48, 3296 CrossRef CAS PubMed; (c) L.-C. Campeau, D. R. Stuart, J.-P. Leclerc, M. Bertrand-Laperle, E. Villemure, H.-Y. Sun, S. Lasserre, N. Guimond, M. Lecavallier and K. Fagnou, J. Am. Chem. Soc., 2009, 131, 3291 CrossRef CAS PubMed; (d) Q.-Q. Lu, S. Vásquez-Céspedes, T. Gensch and F. Glorius, ACS Catal., 2016, 6, 2352 CrossRef CAS; (e) Q.-Q. Yan, Z.-K. Chen, Z.-X. Liu and Y.-H. Zhang, Org. Chem. Front., 2016, 3, 678 RSC.
- (a) S.-M. Li, J. Huang, G.-J. Chen and F.-S. Han, Chem. Commun., 2011, 47, 12840 RSC; (b) C. K. Haley, C. D. Gilmore and B. M. Stoltz, Tetrahedron, 2013, 69, 5732 CrossRef CAS PubMed; (c) K. Muto, T. Hatakeyama, K. Itami and J. Yamaguchi, Org. Lett., 2016, 18, 5106 CrossRef CAS PubMed.
- (a) A. M. Bergman, R. G. Bergman and J. A. Ellman, J. Org. Chem., 2010, 75, 7863 CrossRef PubMed; (b) A. M. Bergman, J. C. Lewis, R. G. Bergman and J. A. Ellman, J. Am. Chem. Soc., 2008, 130, 14926 CrossRef PubMed.
- A. Deb, S. Manna, A. Maji, U. Dutta and D. Maiti, Eur. J. Org. Chem., 2013, 5251 CrossRef CAS.
- (a) I. Hyodo, M. Tobisu and N. Chatani, Chem.–Asian J., 2012, 7, 1357 CrossRef CAS PubMed; (b) M. Tobisu, I. Hyodo and N. Chatani, J. Am. Chem. Soc., 2009, 131, 12070 CrossRef CAS PubMed.
- (a) H. V. Mierde, P. V. D. Voort, D. D. Vos and F. Verpoort, Eur. J. Org. Chem., 2008, 1625 CrossRef; (b) R. Martínez, D. J. Ramón and M. Yus, Eur. J. Org. Chem., 2007, 1599 CrossRef; (c) D. Srimani, Y. Ben-David and D. Milstein, Chem. Commun., 2013, 49, 6632 RSC; (d) C. S. Cho, B. T. Kim, T.-J. Kim and S. C. Shim, Chem. Commun., 2001, 2576 RSC; (e) B. Pan, B. Liu, E. Yue, Q.-B. Liu, X.-Z. Yang, Z. Wang and W.-H. Sun, ACS Catal., 2016, 6, 1247 CrossRef CAS.
- M. Mastalir, M. Glatz, E. Pittenauer, G. Allmaier and K. Kirchner, J. Am. Chem. Soc., 2016, 138, 15543 CrossRef CAS PubMed.
- (a) V. Balaram, TrAC, Trends Anal. Chem., 2016, 80, 83 CrossRef CAS; (b) C. E. Garrett and K. Prasad, Adv. Synth. Catal., 2004, 346, 889 CrossRef CAS.
- (a) F.-S. Wu, H.-Y. Zhao, Y.-L. Xu, K. Hu, Y.-M. Pan and X.-L. Ma, J. Org. Chem., 2017, 82, 4289 CrossRef CAS PubMed; (b) X. Wang, S.-Y. Li, Y.-M. Pan, H.-S. Wang, H. Liang and Z.-F. Chen, Org. Lett., 2014, 16, 580 CrossRef CAS PubMed.
- CCDC 1534893 (3a) contains the supplementary crystallographic data for this paper.†.
- L.-B. Xu, Z.-Z. Shao, L. Wang, H.-L. Zhao and J. Xiao, Tetrahedron Lett., 2014, 55, 6856 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data and spectra data. CCDC 1534893. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra12716b |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |