Open Access Article
Open Access ArticleMetabolism of five diterpenoid lactones from Dioscorea bulbifera tubers in zebrafish†
Wei Shi a,
Jie Lingb,
Li-Long Jianga,
Dong-Sheng Zhaoa,
Ling-Li Wanga,
Zi-Tian Wua,
Ping Li
a,
Jie Lingb,
Li-Long Jianga,
Dong-Sheng Zhaoa,
Ling-Li Wanga,
Zi-Tian Wua,
Ping Li a,
Ying-Jie Wei*b and
Hui-Jun Li
a,
Ying-Jie Wei*b and
Hui-Jun Li *a
*a
aState Key Laboratory of Natural Medicines, China Pharmaceutical University, No. 24 Tongjia Lane, Nanjing 210009, China. E-mail: cpuli@163.com
bThe Third Clinical School of Medicine, Nanjing University of Chinese Medicine, 100 Shizi Street, Nanjing 210028, China. E-mail: wyj970@163.com
First published on 19th February 2018
Abstract
Diterpenoid lactones (DLs) have been reported to be the main hepatotoxic constituents in Dioscorea bulbifera tubers (DBT), a traditional Chinese medicinal herb. The acquisition of early information regarding its metabolism is critical for evaluating the potential hepatotoxicity of DLs. We investigated, for the first time, the main metabolites of diosbulbin A (DIOA), diosbulbin C (DIOC), diosbulbin (DIOG), diosbulbin (DIOM) and diosbulbin (DIOF) in adult zebrafish. By using ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UHPLC-QTOF MS), 6, 2, 7, 5 and 4 metabolites of DIOA, DIOC, DIOF, DIOM and DIOG were identified in the zebrafish body and the aqueous solution, respectively. Both phase-I and phase-II metabolites were observed in the metabolic profiles and the metabolic pathways involved in hydroxyl reduction, glucuronidation, glutathione conjugation and sulfation. The above results indicated that hepatocytic metabolism might be the major route of clearance for DLs. This study provided important information for the understanding of the metabolism of DLs in DBT.
Introduction
The tuber of Dioscorea bulbifera (DBT), called “Huang-Yao-Zi” in Chinese, has been widely used in traditional Chinese medicine as a remedy for leprosy, tumors and cancers, especially for thyroid diseases.1,2 Due to analogous morphologies, DBT may be mistakenly eaten as wild yams.3 Early studies have demonstrated that chronic and excessive use of DBT-containing prescriptions are associated with toxicity in clinical practice, and liver is the main toxic target organ.4,5 Experimental studies have shown that DBT induces severe liver injury in mice after consecutive administrations for fourteen days, along with oxidative stress changes.6It has been reported that diterpenoid lactones (DLs), saponins, flavonoids and polysaccharides are the major components of DBT.7,8 Among these components, DLs namely diosbulbins A–P (DIOA–P) and 8-epidiosbulbin E acetate (EEA)9–13 have attracted much attention due to potential hepatotoxicity. For instance, DIOB and EEA, two abundant DLs in the herb, have been known to cause serious hepatotoxicity in experimental animals.14–17 Studies indicated that the DLs-induced liver injuries required cytochrome P450 (CYP)-mediated metabolism. Furthermore, recent findings suggested that glutathione (GSH) conjugate was observed in the bile of rats treated with DIOB.18 The metabolic generation of six cyclic GSH/N-acetyl lysine conjugates from EEA were detected both in vitro and in vivo.19 To fully understand the hepatotoxicity effects and mechanism of the DLs, it is essential to obtain early information regarding its metabolism. However, compared with extensive researches on DIOB and EEA, the knowledge of the metabolites and metabolic pathways focusing on other DLs are limited.
Due to the practical limitations of applying metabolism on human beings, animal models are of great importance in studying the metabolism of toxicants. In terms of the metabolism studies, rats are often chosen as the major object.20–22 With mammal-like genes, complex organ system and typical drug-metabolizing enzymes, the zebrafish model has proven to be a versatile tool for studying the metabolism of herbal components.23–26 Moreover, zebrafish-based metabolism studies have significant advantages of less amount of compound needed, lower cost, easier operation and higher efficiency.24,27 Owning to these distinguished characteristics, the zebrafish has become an important animal model and has provided new insights into metabolism studies.
In the present study, an ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UHPLC-QTOF MS) method was employed to characterize the metabolic profiles of DIOA, DIOC, DIOF, DIOG and DIOM in zebrafish biological samples. The metabolic pathways of these compounds and the fragmentation patterns of the metabolites were proposed.
Experimental
Chemicals and materials
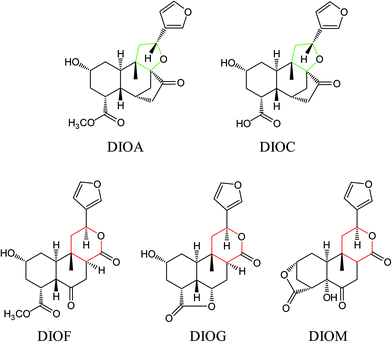
The DBT was purchased from Yunnan province, China. The sample was authenticated by Prof. Hui-Jun Li and deposited at State Key Laboratory of Natural Medicines (China Pharmaceutical University). Five DLs including DIOA, DIOC, DIOM, DIOG and DIOF (Fig. 1) were isolated from DBT in our laboratory. Their chemical structures were determined by extensive spectroscopic analyses.9,13,28,29 The purity of each compound was more than 98%, by normalization of the peak area detected by UPLC analysis. Dimethyl sulfoxide (DMSO) was purchased from Sigma-Aldrich (St. Louis, MO, USA). HPLC grade acetonitrile, methanol and formic acid were purchased from ROE (Newark, NJ, USA). Deionized water (18 MΩ cm−1) was prepared by distilled water through a Milli-Q system (Millipore, Milford, MA, USA). Other reagents and solvents were of analytical grade.Chromatographic and mass spectrometric conditions
Chromatographic analysis was performed on an Agilent series 1290 UPLC system equipped with a quaternary pump, a degasser, an autosampler and a thermostated column compartment (Agilent Technologies, Palo Alto, CA, USA). Chromatographic separation was carried out at 30 °C on an Agilent Zorbax SB-C18 analytical column (4.6 mm i.d. × 50 mm, 1.8 μm, Agilent Technologies, Palo Alto, CA, USA). The mobile phase was a mixture of 0.1% formic acid in water (A) and acetonitrile (B) with a gradient elution as follows: 5–95% B at 0–20 min. The flow rate was 0.4 mL min−1, and the column temperature was set at 30 °C.The mass spectrometric analysis was performed on a 6545 QTOF mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) equipped with electrospray ionization source in positive mode. The mass spectrometric conditions were as follows: nebulizer pressure, 35 psi; capillary voltage, 3500 V; fragmentor voltage, 135 V; drying gas flow, 10 L min−1; drying gas temperature, 350 °C; sheath gas flow, 11 L min−1; sheath gas temperature, 350 °C. The mass rang was recorded from m/z 50 to 1500 Da. Data acquisition was performed with MassHunter Workstation (Agilent Technologies, Palo Alto, CA, USA). The TOF mass spectrometer was calibrated every day before sample analysis using reference masses at m/z 121.0508 and 922.0098.
Animal experiments
Male adult zebrafish (AB strain; 5 month old; weight, 0.4–0.5 g) were supplied by Nanjing Ezerinka Biotechnology Co., Ltd (Nanjing, China), and acclimatized to tap water in a glass aquarium for at least 10 d preceding experimentation. Fish were cultured at 25 ± 1 °C in a 12 h:12 h day/night cycle. The fish were fed daily during the acclimatization period, and were fasted 12 h before the day of the experiment. Animal studies were conducted in accordance with the Provision and General Recommendation of Chinese Experimental Animals Administration Legislation and were approved by Department of Science and Technology of Jiangsu Province (License number: SYXK (SU) 2016-0011).Drug administration and sample collection
Zebrafish were randomly separated into six groups with six fish in each group. After fasting for 12 h, the fish was kept individually in brown glass bottles maintaining at a temperature of 25 ± 1 °C in a water bath with 40 mL solution: one blank control group was exposed to 0.5% DMSO purified water (blank zebrafish group), and five groups were exposed to 40 mL solution of DIOA (15.41 μg mL−1), DIOC (17.03 μg mL−1), DIOG (17.08 μg mL−1), DIOM (16.74 μg mL−1) or DIOF (16.55 μg mL−1) in 0.5% DMSO purified water (drug-treated groups). Zebrafish body and the solution were sampled at 24 h. The zebrafish bodies of each group were combined and washed quickly with purified water three times, then weighed after sacrifice and removal of fins and scales of fish and stored at −80 °C until analysis; the solution of each group were also combined. The combined solutions of each group were sampled and also stored at −80 °C until analysis.Sample preparation
The aqueous solution (40 mL) was freeze-dried to dryness, and the residue dissolved in 90% (v/v) methanol (1 mL). The solution was filtered through a 0.22 μm filter and 2 μL was injected into the UHPLC-QTOF MS system for analysis. The zebrafish samples were cut with scissors, and 1 g was sampled and homogenized with physiological saline (5 mL), followed by centrifugation at 3500 rpm for 15 min, then the supernatant was mixed by vortexing with methanol at the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (v/v) three times, followed by centrifugation at 3500 rpm for 15 min. The supernatant was evaporated to dryness with nitrogen at room temperature, and the residue was dissolved in 90% methanol (1 mL). After filtering through a 0.22 μm filter, 2 μL of the solution containing 1 g fish tissue per mL was injected into the UHPLC-QTOF MS system for analysis.
4 (v/v) three times, followed by centrifugation at 3500 rpm for 15 min. The supernatant was evaporated to dryness with nitrogen at room temperature, and the residue was dissolved in 90% methanol (1 mL). After filtering through a 0.22 μm filter, 2 μL of the solution containing 1 g fish tissue per mL was injected into the UHPLC-QTOF MS system for analysis.
Results and discussion
Mass spectrometric behaviors of DLs
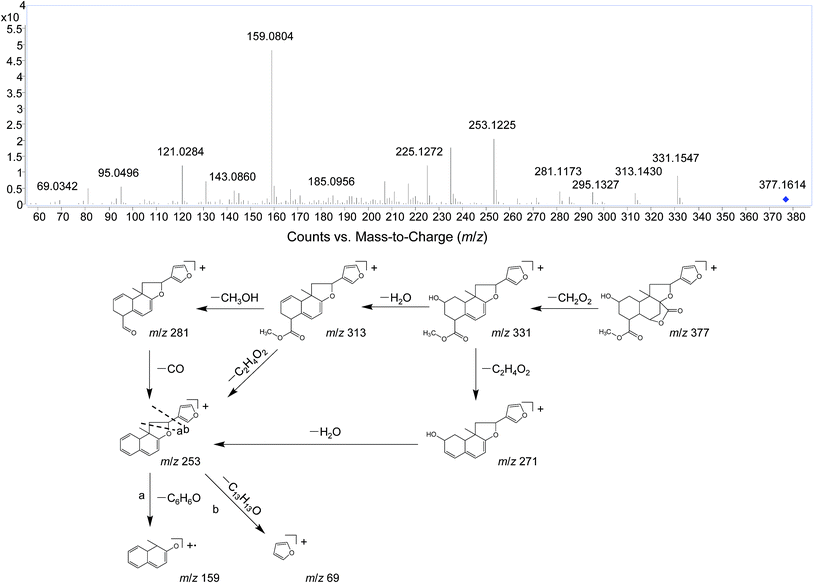
Several studies have confirmed that the mass fragmentation patterns of metabolites are similar to the parent compound, therefore the analysis of fragmentation pattern of parent compound is very important for the metabolite characterizations.30 In the present study, the mass spectrometric behaviors and fragmentation patterns of the DLs were investigated firstly. It was noted that the response of ESI (+) mode was much higher than that of ESI (−) mode, therefore the positive mode was employed for the rest of the study.DIOA provided a protonated molecule [M + H]+ and sodium adduct ion [M + Na]+ at m/z 377 and 399, respectively. The product ion at m/z 69 was generated from the furan moiety. Additionally, the mass spectrum showed major fragment ions at m/z 159 (C11H11O), 253 (C17H17O2), 271 (C17H19O3), 281 (C18H17O3), 295 (C19H19O3), 313 (C19H21O4) and 331 (C19H23O5). The product ion spectrum of DIOA under high collision energy scan and the fragmentation pathways of DIOA are proposed in Fig. 2. DIOC provided a protonated molecule [M + H]+ and the ammonium adduction ion [M + NH4]+ at m/z 363 and 380, respectively. The mass spectrum showed major fragment ions at m/z 159 (C11H11O), 253 (C17H17O2), 271 (C17H19O3), 281 (C18H17O3), 299 (C18H19O4) and 317 (C18H21O5) (Fig. S1†). As expected, these observations clearly demonstrated that DIOA and DIOC shared the same fragment ions and fragmentation pathways due to their similar chemical skeleton.
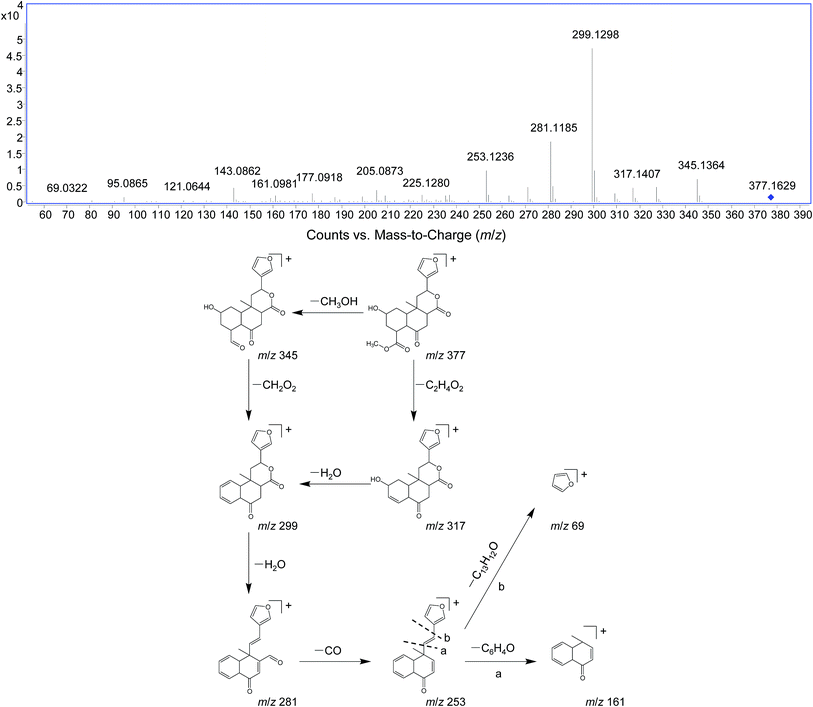
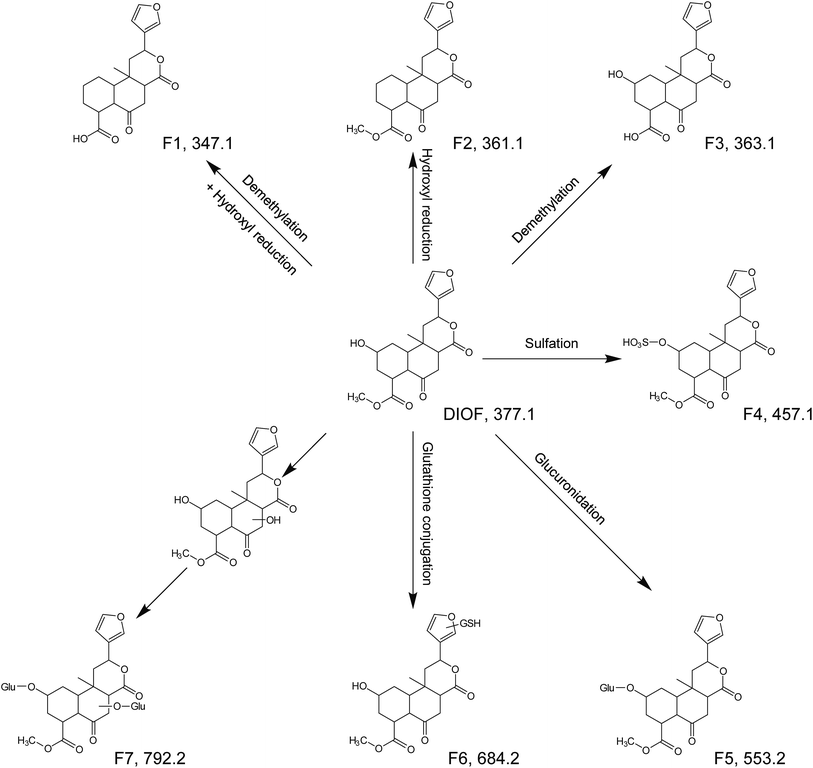
DIOF showed [M + H]+ ion at m/z 377.1629 in positive mode, corresponding to the molecular formula C20H24O7. The neutral loss of 60 Da generated from ion at m/z 377 to 317; suggested the presence of a terminal acetic acid unit (Fig. 3). Apart from the characteristic ions at m/z 281, 271, and 253 which were also found in DIOA and DIOC, the predominant ion at m/z 345 (−32 Da) was supposed to be formed by a neutral loss of CH3OH at the position of ester group.
DIOM provided a protonated molecule [M + H]+ and the ammonium adduction ion [M + NH4]+ at m/z 361 and 378, respectively. DIOM exhibited a series of fragmentation ions at m/z 159, 251, 297, 315, 333 and 343, and the specific ion at m/z 69 corresponded to furan group. For better understanding of fragmentation pathway of DIOM, the fragmentation behaviors are given in Fig. S2.†
DIOG gave precursor ion [M + H]+ at m/z 347.1468 (with 6.11 ppm error compared with theoretically calculated value) in positive mode, suggesting the molecular formula C19H22O6. In the MS/MS spectra, the parent compound gave a series of fragmentation ions at m/z 145, 217, 237, 265, 283, 311 and 329. The fragmentation pathways of DIOG were proposed in Fig. S3.†
The fragment ion of the furan ring at m/z 69 presented in the MS/MS spectra of all these DLs. The characteristic neutral losses and product ions from the parent compounds could be used to identify the metabolites formed in vivo from DIOA, DIOC, DIOF, DIOG and DIOM.
Structure elucidation of DLs metabolites in zebrafish
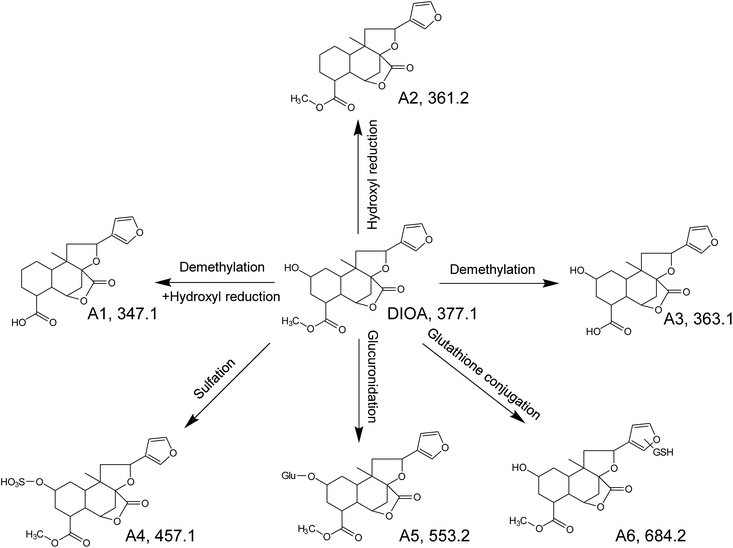
As the core of the present study, metabolites of the DLs in zebrafish were identified using UHPLC-QTOF MS by matching accurate masses. After comparing the results of drug samples with the corresponding blank samples, a total of 6, 2, 7, 5 and 4 metabolites of DIOA, DIOC, DIOF, DIOM and DIOG were detected and identified in the zebrafish body and the aqueous solution, respectively. The chromatographic retention times and mass spectrometric data of the parent compounds and metabolites were listed in Tables 1, 2. In addition, the proposed metabolic pathways of the five analytes in zebrafish were depicted in Fig. 4 (DIOA), Fig. 5 (DIOF), Fig. S4† (DIOC), Fig. S5† (DIOG) and Fig. S6† (DIOM), respectively.| Compound | No. | Formula | Adduct type | tR (min) | Calcd (m/z) | Exptl (m/z) | Diff (ppm) | Fragment ions (m/z) | Description | Part | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zebrafish body | The aqueous solution | ||||||||||
| DIOA | Parent | C20H24O7 | [M + H]+ | 6.526 | 377.1595 | 377.1614 | −5.13 | 331.1, 313.1, 295.1, 281.1, 271.1, 253.1, 159.1 | — | + | + |
| A1 | C19H22O6 | [M + H]+ | 6.494 | 347.1489 | 347.1485 | 1.20 | 159.1, 295.1, 235.1, 313.1, 253.1, 69.0, 331.1 | Demethylation + hydroxyl reduction | − | + | |
| A2 | C20H24O6 | [M + H]+ | 7.723 | 361.1646 | 361.1635 | 2.96 | 184.1, 313.2, 331.2 | Hydroxyl reduction | − | + | |
| A3 | C19H22O7 | [M + H]+ | 4.828 | 363.1438 | 363.1438 | 0.08 | 331.1, 253.1, 159.1 | Demethylation | − | + | |
| A4 | C20H24O10S | [M + H]+ | 3.355 | 457.1163 | 457.1163 | −0.01 | 457.1, 377.2 | Sulfation | + | − | |
| A5 | C26H32O13 | [M + H]+ | 3.323 | 553.1916 | 553.1946 | −5.46 | 553.2, 377.2, 159.1 | Glucuronidation | + | − | |
| A6 | C30H41N3O13S | [M + H]+ | 3.672 | 684.2433 | 684.2448 | −2.23 | 684.2, 377.2, 187.1 | Glutathione conjugation | + | − | |
| Compound | No. | Formula | Adduct type | tR (min) | Calcd (m/z) | Exptl (m/z) | Diff (ppm) | Fragment ions (m/z) | Description | Part | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zebrafish body | The aqueous solution | ||||||||||
| DIOC | Parent | C19H22O7 | [M + NH4]+ | 3.428 | 380.1704 | 380.1726 | −6.13 | 317.1, 299.1, 271.1, 253.1, 159.1 | — | + | + |
| C1 | C19H22O10S | [M + H]+ | 2.665 | 443.1006 | 443.1018 | −2.61 | 382.1, 345.2, 271.1, 166.1 | Sulfation | + | − | |
| C2 | C25H30O13 | [M + H]+ | 2.124 | 539.1759 | 539.1738 | 3.93 | 474.1, 403.1, 271.1, 137.0 | Glucuronidation | + | − | |
| DIOF | Parent | C20H24O7 | [M + H]+ | 5.377 | 377.1595 | 377.1629 | −9.09 | 345.1, 317.1, 299.1, 281.1, 271.1, 253.1, 225.1, 143.1 | — | + | + |
| F1 | C19H22O6 | [M + H]+ | 5.968 | 347.1489 | 347.1483 | 1.78 | 311.1, 265.1, 145.1 | Demethylation + hydroxyl reduction | − | + | |
| F2 | C20H24O6 | [M + H]+ | 6.351 | 361.1646 | 361.1627 | 5.18 | 345.1, 299.1, 281.1, 253.1, 143.1 | Hydroxyl reduction | − | + | |
| F3 | C19H22O7 | [M + H]+ | 5.131 | 363.1438 | 363.1430 | 2.29 | 345.1, 299.1, 281.1, 253.1, 161.1 | Demethylation | − | + | |
| F4 | C20H24O10S | [M + H]+ | 3.265 | 457.1163 | 457.1163 | −0.01 | 317.1, 184.1 | Sulfation | + | − | |
| F5 | C26H32O13 | [M + H]+ | 3.290 | 553.1916 | 553.1911 | 0.85 | 478.1, 184.1 | Glucuronidation | + | − | |
| F6 | C30H41N3O13S | [M + H]+ | 3.334 | 684.2433 | 684.2431 | 0.27 | 578.0, 184.1, 156.1 | Glutathione conjugation | + | − | |
| F7 | C32H40O19 | [M + H]+ | 2.669 | 729.2237 | 729.2220 | 2.27 | 475.2, 184.1, 156.1 | Bis-glucuronidation | + | − | |
| DIOM | Parent | C19H20O7 | [M + H]+ | 5.263 | 361.1282 | 361.1284 | −0.61 | 343.1, 333.1, 315.1, 297.1, 269.1, 251.1, 177.1, 159.1 | — | + | + |
| M1 | C19H20O6 | [M + H]+ | 5.841 | 345.1332 | 345.1323 | 2.80 | 315.1, 297.1, 184.1, 177.1, 159.1, 104.1 | Hydroxyl reduction | − | + | |
| M2 | C19H20O10S | [M + H]+ | 4.195 | 441.0850 | 441.0864 | −3.19 | 309.1, 184.1, 104.1 | Sulfation | + | − | |
| M3 | C25H28O13 | [M + H]+ | 2.821 | 537.1603 | 537.1606 | −0.62 | 433.2, 184.1, 156.1, 104.1 | Glucuronidation | + | − | |
| M4 | C29H37N3O13S | [M + H]+ | 3.170 | 668.2120 | 668.2118 | 0.28 | 455.2, 251.1, 184.1, 156.1, 104.1 | Glutathione conjugation | + | − | |
| M5 | C31H36O19 | [M + H]+ | 2.781 | 713.1924 | 713.1925 | −0.20 | 470.1, 258.1, 184.1, 156.1 | Bis-glucuronidation | + | − | |
| DIOG | Parent | C19H22O6 | [M + H]+ | 5.566 | 347.1416 | 347.1468 | 6.11 | 329.1, 311.1, 283.1, 265.1, 253.1, 217.1, 145.1 | — | + | + |
| G1 | C19H22O5 | [M + H]+ | 6.721 | 331.1540 | 331.1528 | 3.64 | 316.3, 228.2, 184.1 | Hydroxyl reduction | − | + | |
| G2 | C19H22O9S | [M + H]+ | 1.300 | 427.1057 | 427.1076 | −4.39 | 313.0, 291.1, 159.0 | Sulfation | + | − | |
| G3 | C25H30O12 | [M + H]+ | 2.342 | 523.1737 | 523.1788 | 4.22 | 427.2, 184.1 | Glucuronidation | + | − | |
| G4 | C29H39N3O12S | [M + H]+ | 5.536 | 654.2327 | 654.2303 | 3.71 | 483.2, 184.1 | Glutathione conjugation | + | + | |
Metabolites of DIOA
DIOA, exhibited the protonated molecular ion at 377.1614 in positive mode, was confirmed by a general analysis of the MS and MS/MS fragmentation behaviors and retention time. The parent component was identified and detected in both zebrafish body and the aqueous solution after the metabolism for 24 h.The metabolite named as A1 was observed as a protonated molecule [M + H]+ at m/z 347.1485, with a retention time of 6.49 min. The relative abundant fragment ion at m/z 331 was 16 Da less than that of the parent ion, suggesting the existence of an oxygen-containing group. The characteristics of other fragments observed at m/z 313 ([M + H–O–H2O]+), 295 ([M + H–O–2H2O]+), 253 ([M + H–O–H2O–CH3OH–CO]+), 235 ([M + H–O–2H2O–CH3OH–CO]+) and 159 ([M + H–O–H2O–CH3OH–CO–C6H6O]+), which shared the same fragmentation pattern as DIOA.
A2, eluted at 7.72 min, was characterized as the main metabolite, with the predominant quasi-molecular ion [M + H]+ at m/z 361.1635, which was 16 Da less than that of DIOA, suggesting a dehydration followed by reduction.
A3, eluted at 4.80 min, was observed as a protonated molecule [M + H]+ at m/z 363.1438, which was 14 Da less than DIOA, suggesting a methyl ester of carboxylic acid in the side chain. The characteristics of other fragments observed at m/z 331.1984 ([M + H–2O]+), and 159 ([M + H–2O–H2O–CH3OH–CO–C6H6O]+). The mass fragment of m/z 159.0807 was consistent with the fragmentation pattern of DIOA.
A4, eluted at 3.36 min, was characterized as the main metabolite, with the predominant quasi-molecular ion [M + H]+ at m/z 457.1163 (C20H24O10S), which generated a fragment ion at m/z 377 corresponding to the neutral loss of 80 Da (SO3), suggesting that A4 were deduced as DIOA sulfate conjugate. Similarly, A5 was observed as a protonated molecule [M + H]+ at m/z 553.1946, with a retention time of 3.32 min. The product ion at m/z 377 was corresponding to a neutral loss of 176 Da (C6H8O6) from the parent [M + H]+ ion. Further analysis of A5 by UHPLC-QTOF MS demonstrated its protonated molecular ion [M + H]+ at m/z 159.0893 in positive ion mode, which matched the elemental composition of DIOA. Therefore, A5 was tentatively identified the glucuronidated metabolite of the DIOA.
A6, eluted at 3.67 min, was characterized as the main metabolite, with the predominant quasi-molecular ion [M + H]+ at m/z 684.2448. The product ion at m/z 377 was derived from the loss of GSH moiety (−307 Da) from m/z 684. This indicated that the participation of GSH in the formation of A6.
Metabolites of DIOC/DIOF/DIOM/DIOG
In all, two metabolites (C1–C2) of DIOC, seven metabolites (F1–F7) of DIOF, five metabolites (M1–M5) of DIOM and four metabolites (G1–G4) of DIOG were tentatively characterized by MS and MS/MS in the zebrafish body and the aqueous solution, respectively. All metabolites were detected and characterized based on the accurate mass measurement, the fragmentation pattern of the parent compounds and relevant drug biotransformation knowledge.Like mammals, the zebrafish has a serious of drug-metabolizing enzymes, such as CYP450 isoforms or conjugation enzymes including glutathione-S-transferase, uridine diphosphoglucuronyl transferases, and sulfotransferases.31–34 The xenobiotic metabolism is often divided into two phases: modification (phase-I) and conjugation (phase-II).23 CYP450, belonging to monoxygenases, is the prevailing group of phase I enzymes.35–37 The present study showed that, after 24 h metabolism, the phase-I metabolites (such as A1/A2/A3) were detected in the solution sample of zebrafish. In subsequent phase-II reactions, these activated xenobiotic metabolites are prone to be conjugated with charged species such as glutathione (GSH), sulfate, glycine, or glucuronic acid.38,39 The obtained results indicated that sulfation, glucuronidation and glutathione conjugations (such as A4/A5/A6) were the main physiological process of metabolism of the DLs. It should be noted that the GSH conjugate was found in zebrafish body after metabolism, which facilitate the metabolic investigation of DB and EEA in rats.
Structurally, unlike DB and EEA, these DLs have a hydroxyl group, which might lead to distinction of solubility and metabolism between DLs. The sulfation, and glucuronidation, along with hydroxyl reduction products were detected and identified. Additionally, fewer phase-I metabolites were detected and identified in DIOC-treated group compared with the zebrafish given DIOA, which might partly be related to the solubility difference between the two DLs. Overall, the results indicated that the hydroxyl group possibly plays an essential role in the metabolism of the DLs.
Conclusions
In our study, an UHPLC-QTOF MS method was successfully applied for the analysis of the metabolites of five DLs in zebrafish. The main phase-I and phase-II metabolites, including hydroxyl reduction metabolites, sulfate conjugated metabolites, glutathione adducts and glucuronic acid conjugated metabolites, were detected and identified on the basis of the mass-to-charge ratios and fragments. The investigation confirmed that the reaction pathways for degradation of DLs in zebrafish involving hydroxyl reduction, glucuronidation, glutathione conjugation and sulfation. Toxic effects of compounds in an organism are substantially dependent on their metabolites. The metabolites observed in zebrafish, which will be greatly helpful in elucidating the potential toxic effects of DLs in DBT.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81773993 and 81573833), the Natural Science Foundation of Jiangsu Province (No. BK20151442) and the Project Funded by the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.References
- F. M. Niyas, Res. J. Pharm. Technol., 2015, 8, 1059–1062 CrossRef
.
- Y. X. Tang, China J. Chin. Mater. Med., 1995, 20, 435–438 Search PubMed
.
- T. Natascha, P. Iffat and A. K. Ikhlas, Genome, 2016, 60, 201–207 Search PubMed
.
- W. J. Lee, H. W. Kim, H. Y. Lee and C. G. Son, Food Chem. Toxicol., 2015, 84, 47–54 CrossRef CAS PubMed
.
- H. Yang, J. X. Li, X. Q. Cui, C. Yang, L. Y. Li, J. L. Liu, L. C. Mu, J. C. Yuan and B. Zhang, Clin. Misdiagn. Misther., 2006, 19, 85–87 Search PubMed
.
- J. M. Wang, L. L. Ji, H. Liu and Z. T. Wang, BioSci. Trends, 2010, 4, 79–85 Search PubMed
.
- H. Y. Gao, A. L. Shui, Y. H. Chen, X. Y. Zhang and L. J. Wu, J. Shenyang Pharm. Univ., 2003, 20, 178–180 CAS
.
- S. Ghosh, V. S. Parihar, P. More, D. D. Dhavale and B. A. Chopade, Med. Chem., 2014, 5, 160–172 Search PubMed
.
- H. Liu, G. X. Chou, Y. L. Guo, L. L. Ji, J. M. Wang and Z. T. Wang, Phytochemistry, 2010, 71, 1174–1180 CrossRef CAS PubMed
.
- G. Wang, J. S. Liu, B. B. Lin, G. K. Wang and J. K. Liu, Chem. Pharm. Bull., 2009, 57, 625–627 CrossRef CAS PubMed
.
- T. Kawasaki, T. Komori and S. Setoguchi, Chem. Pharm. Bull., 1968, 16, 2430–2435 CrossRef CAS
.
- Y. Tang, Y. B. Xue, L. Zhou, J. W. Zhang, G. M. Yao, Z. W. Luo, G. Du and Y. H. Zhang, Chem. Pharm. Bull., 2014, 62, 719–724 CrossRef CAS PubMed
.
- Y. Ida, S. Kubo, M. Fujita, T. Komori and T. Kawasaki, Justus Liebigs Ann. Chem., 1978, 818–833 CrossRef CAS
.
- B. H. Yang, W. Liu, K. X. Chen, Z. T. Wang and C. H. Wang, Drug Metab. Dispos., 2014, 42, 1737–1750 CrossRef PubMed
.
- D. J. Lin, C. Y. Li, Y. Peng, H. Y. Gao and J. Zheng, Drug Metab. Dispos., 2014, 42, 1727–1736 CrossRef PubMed
.
- W. W. Li, D. J. Lin, H. Y. Gao, Y. J. Xu, D. Y. Meng, C. V. Smith, Y. Peng and J. Zheng, Arch. Toxicol., 2016, 90, 863–872 CrossRef CAS PubMed
.
- D. J. Lin, W. W. Li, Y. Peng, C. F. Jiang, Y. J. Xu, H. Y. Gao and J. Zheng, Chem. Res. Toxicol., 2016, 29, 359–366 CrossRef CAS PubMed
.
- C. Y. Li, D. J. Lin, H. Y. Gao, H. M. Hua, Y. Peng and J. Zheng, Chem. Res. Toxicol., 2015, 28, 384–393 CrossRef CAS PubMed
.
- D. J. Lin, X. C. Guo, H. Y. Gao, L. Cheng, M. S. Cheng, S. J. Song, Y. Peng and J. Zheng, Chem. Res. Toxicol., 2015, 28, 1737–1746 CrossRef CAS PubMed
.
- L. Guo, L. Duan, X. Dong, L. L. Dou, P. Zhou, P. Li and E. H. Liu, J. Pharm. Biomed. Anal., 2015, 107, 473–479 CrossRef CAS PubMed
.
- R. Xing, L. J. Zhou, L. Xie, K. Hao, T. Rao, Q. Wang, W. Ye, H. Fu, X. W. Wang, G. J. Wang and Y. Liang, Anal. Chim. Acta, 2015, 867, 56–66 CrossRef CAS PubMed
.
- R. D. Arnold, J. E. Slack and R. M. Straubinger, J. Chromatogr. B, 2004, 808, 141–152 CrossRef CAS PubMed
.
- Y. J. Wei, P. Li, H. W. Fan, Y. R. Peng, W. Liu, C. M. Wang, L. Shu and X. B. Jia, Molecules, 2011, 16, 6621–6633 CrossRef CAS PubMed
.
- Y. J. Wei, P. Li, C. M. Wang, Y. R. Peng, L. Shu, X. B. Jia, W. Q. Ma and B. Wang, Molecules, 2012, 17, 8617–8632 CrossRef CAS PubMed
.
- B. Chen, J. Y. Wei, D. Wang and B. X. Jia, Arch. Pharmacal Res., 2014, 38, 1468–1476 CrossRef PubMed
.
- G. W. Wang, Z. K. Du, H. Y. Chen, Y. Su, S. X. Gao and L. Mao, Environ. Sci. Technol., 2016, 50, 13555–13564 CrossRef CAS PubMed
.
- Y. J. Wei, Q. Ning, X. B. Jia and Z. A. Gong, Arch. Pharmacal Res., 2009, 40, 1009–1011 Search PubMed
.
- A. Lentini, J. Webster, B. Ternai and T. Komori, Magn. Reson. Chem., 1986, 24, 646–648 CrossRef CAS
.
- R. B. Teponno, A. L. Tapondjou, E. Abou-Mansour, H. Stoeckli-Evans, P. Tane and L. Barboni, Phytochemistry, 2008, 69, 2374–2379 CrossRef CAS
.
- L. Wang, H. Ye, D. Sun, T. Meng, L. Cao, M. Wu, M. Zhao, Y. Wang, B. Chen, X. Xu, G. Wang and H. Hao, Anal. Chem., 2017, 89, 1229–1237 CrossRef CAS PubMed
.
- L. M. Félix, A. M. Vidal, C. Serafim, A. M. Valentim, L. M. Antunes, S. Campos, M. Matos, S. M. Monteiro and A. M. Coimbra, RSC Adv., 2016, 6, 61254–61266 RSC
.
- A. Tierbach, K. J. Groh, R. Schonenberger, K. Schirmer and M. J. Suter, Toxicol. Sci., 2018 DOI:10.1093/toxsci/kfx293
.
- M. Dong, L. S. Zhu, B. Shao, S. Y. Zhu, J. H. Wang, H. Xie, J. H. Wang and F. H. Wang, Ecotoxicol. Environ. Saf., 2013, 92, 1–9 CrossRef CAS PubMed
.
- M. L. Scornaienchi, C. Thornton, K. L. Willett and J. Y. Wilson, Arch. Biochem. Biophys., 2010, 502, 17–22 CrossRef CAS PubMed
.
- H. P. Song, H. Zhang, Y. Fu, H. Y. Mo, M. Zhang, J. Chen and P. Li, J. Chromatogr. B, 2014, 961, 56–61 CrossRef CAS PubMed
.
- G. W. Wang, H. Y. Chen, Z. K. Du, J. H. Li, Z. Y. Wang and S. X. Gao, Sci. Total Environ., 2017, 590–591, 50–59 CrossRef CAS PubMed
.
- H. P. Song, J. Chen, J. Y. Hong, H. Hao, L. W. Qi, J. Lu, Y. Fu, B. Wu, H. Yang and P. Li, Chem. Commun., 2015, 51, 1494–1497 RSC
.
- J. Petra and M. Šiller, InTech, 2012, 35–60 Search PubMed
.
- Y. X. Wang, H. P. Hao, G. J. Wang, P. F. Tu, Y. Jiang, Y. Liang, L. Dai, H. Yang, L. Lai and C. N. Zheng, Talanta, 2009, 80, 572–580 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra12910f |
| This journal is © The Royal Society of Chemistry 2018 |