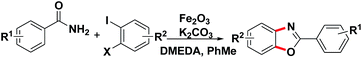Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of benzoxazoles via an iron-catalyzed domino C–N/C–O cross-coupling reaction†
Bo Yang,
Weiye Hu and
Songlin Zhang *
*
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, P. R. China. E-mail: zhangsl@suda.edu.cn
First published on 9th January 2018
Abstract
An eco-friendly and efficient method has been developed for the synthesis of 2-arylbenzoxazoles via a domino iron-catalyzed C–N/C–O cross-coupling reaction. Some of the issues typically encountered during the synthesis of 2-arylbenzoxazoles in the presence of palladium and copper catalysts, including poor substrate scope and long reaction times have been addressed using this newly developed iron-catalyzed method.
2-Arylbenzoxazoles are an important class of structures in natural products, and pharmaceuticals and has shown a wide range of biological activities, such as antitumor, antiviral, and antimicrobial activities.1 In particular, they show a marvellous efficacy in the treatment of duchenne muscular dystrophy (DMD) which is one of the most common of the muscular dystrophies that is caused by a mutation in the gene DMD, located in humans on the X chromosome (Xp21).2 So the synthesis of 2-arylbenzoxazoles has been intensively studied for use in organic and medicinal chemistry over the past few years.
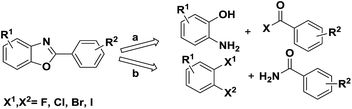
Numerous methods have been reported to synthesise this motif, one of the common methods is transition-metal-catalyzed (like Pd,3 Ni,4 Cu,5 Mn6 etc.) cross-coupling from pre-existing benzoxazoles with aryl halide or arylboronic acid. And another method is the classic one employing a cyclocondensation approach between an aminophenol and either a carboxylic acid7 or benzaldehyde8 (Scheme 1, path a). In 2004, Frank Glorius' group reported a domino copper-catalyzed C–N and C–O cross-coupling for the conversion of primary amides into benzoxazoles9 (Scheme 1, path b) which is a new reaction type for the synthesis of benzoxazoles. Bunch et al. apply this domino reaction in the synthesis of planar heterocycles in 2014.10 In addition the cyclization of o-halobenzenamides to benzoxazoles has been reported several times.11,12 Nevertheless, some limitations in the reported methods need to be overcome, such as the use of palladium complexes and narrow substrate range.
In the last few years, there has been a significant increase in the number of reports pertaining to the development of iron-catalyzed reactions in organic synthesis, where iron has shown several significant advantages over other metals, such as being more abundant, commercially inexpensive, environmentally friendly and drug safety.13 Compared with palladium and copper, the use of iron is particularly suitable for reactions involving the preparation of therapeutic agents for human consumption. With this in mind, it was envisaged that an new method should be developed for the synthesis of benzoxazoles via an iron-catalyzed domino C–N/C–O cross-coupling reaction.
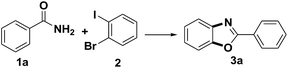
The reaction of benzamide (1a) with 1-bromo-2-iodobenzene was used as model transformation to identify the optimum reaction conditions by screening a variety of different iron salts, bases, ligands and solvents (Table 1). Several iron salts were screened in this reaction, including FeCl3, FeCl2·4H2O, FeSO4·7H2O, Fe(acac)3, Fe2O3, Fe3O4, Fe3O4(nano), Fe2O3(nano), Fe2(SO4)3 and Fe(NO3)3·9H2O, Fe2O3 was found to give the best results with the desired product 3a being formed in a yield of 15% while most of the iron salt have no effect on the reaction (Table 1, entries 1–10). Then, several other bases, including LiOtBu, Na2CO3, NaOAc, KOH and K2CO3 were also evaluated under the same conditions using Fe2O3, but all of them failed to provided the desired product 3a except K2CO3 with a yield of 37% (Table 1, entries 11–15). When the reaction was stirred for 24 h at 110 °C in the presence of 20% mol of Fe2O3, 20% mol N,N′-dimethylethanediamine (DMEDA) and 1 equiv. of K2CO3 in PhMe under nitrogen, (N-(2-bromophenyl)benzamide) was obtained as an intermediate which could be converted to the final product with a yield of 87% if extend the reaction time from 24 h to 48 h (Table 1, entries 15, 16). Several ligands were also screened in the model reaction, and the results revealed that the nature of the ligand has a dramatic impact on the yield of the reaction. For example, the use of DMEDA gave 2-phenylbenzo[d]oxazole in 85% yield, whereas 1,10-phenanthroline, dipyridyl and L-proline provided no product (Table 1, entries 16–19). The reaction was conducted in DMSO, DMF and PhMe2 respectively and none of them provided a much higher of the desired product than toluene (Table 1, entries 20–22). Control experiments was taken in the absence of Fe2O3, no product was obtained (Table 1, entry 23). In view of the fact that the trace metals in catalytic, as is well-known, sometime could play an important role in the reaction,14 high-purity Fe2O3 (99.999%) and K2CO3 (99.999%) were applied in the reaction (Table 1, entry 24). The product was formed in a yield of 86% which was similar with the one of the entry 16.
| Entry | Iron salt | Ligand | Base | Solvent | Yb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: benzamides (0.5 mmol), 1-bromo-2-iodobenzene (1.5 eq.), iron salt (20% mol), base (1 eq.), ligand (20%) were added to a solvent (2 mL) and react at 110 °C for 48 h under N2.b Isolated yield based on 1a after silica gel chromatography.c Fe2O3 and K2CO3 were applied in purity of 99.999% from alfa.d with Fe2O3 in a dosage of 10 mmol%. | |||||
| 1 | FeCl3 | DMEDA | KOtBu | PhMe | Trace |
| 2 | FeCl2·4H2O | DMEDA | KOtBu | PhMe | Trace |
| 3 | FeSO4·7H2O | DMEDA | KOtBu | PhMe | 0 |
| 4 | Fe(acac)3 | DMEDA | KOtBu | PhMe | 0 |
| 5 | Fe2O3 | DMEDA | KOtBu | PhMe | 15 |
| 6 | Fe3O4 | DMEDA | KOtBu | PhMe | 0 |
| 7 | Fe3O4(nano) | DMEDA | KOtBu | PhMe | 10 |
| 8 | Fe2O3(nano) | DMEDA | KOtBu | PhMe | 0 |
| 9 | Fe2(SO4)3 | DMEDA | KOtBu | PhMe | 0 |
| 10 | Fe(NO3)3·9H2O | DMEDA | KOtBu | PhMe | 0 |
| 11 | Fe2O3 | DMEDA | LiOtBu | PhMe | 0 |
| 12 | Fe2O3 | DMEDA | Na2CO3 | PhMe | 0 |
| 13 | Fe2O3 | DMEDA | NaOAc | PhMe | 0 |
| 14 | Fe2O3 | DMEDA | KOH | PhMe | 0 |
| 15 | Fe2O3 | DMEDA | K2CO3 (24 h) | PhMe | 37 |
| 16 | Fe2O3 | DMEDA | K2CO3 (48 h) | PhMe | 87 |
| 17 | Fe2O3 | Phen | K2CO3 | PhMe | Trace |
| 18 | Fe2O3 | L-Proline | K2CO3 | PhMe | 0 |
| 19 | Fe2O3 | Dpy | K2CO3 | PhMe | 0 |
| 20 | Fe2O3 | DMEDA | K2CO3 | DMSO | 0 |
| 21 | Fe2O3 | DMEDA | K2CO3 | DMF | 0 |
| 22 | Fe2O3 | DMEDA | K2CO3 | PhMe2 | 0 |
| 23 | — | DMEDA | K2CO3 | PhMe | 0 |
| 24 | Fe2O3 | DMEDA | K2CO3 | PhMe | 86c |
| 25 | Fe2O3 | DMEDA | K2CO3 | PhMe | 58d |
At last, the dosage of Fe2O3 was reduce to 10 mmol%, but only 58% yield was obtained (Table 1, entry 26). Taken together, the results of these screening experiments revealed that the optimal conditions for the reaction were Fe2O3 (20 mol%), DMEDA (20 mol%) and K2CO3 (1 eq.) in toluene at 110 °C for 48 h.
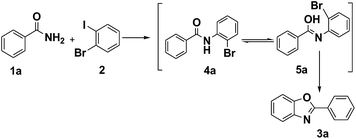
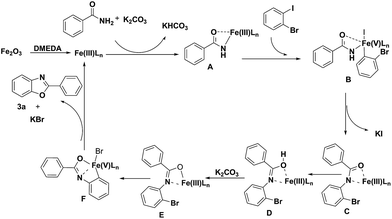
It is noteworthy that the intermediate product 4a was formed under the optimized conditions via the C–N cross coupling reaction of benzamide (1a) with 1-bromo-2-iodobenzene (2). So a possible pathway of the reaction was proposed as shown in Scheme 2.
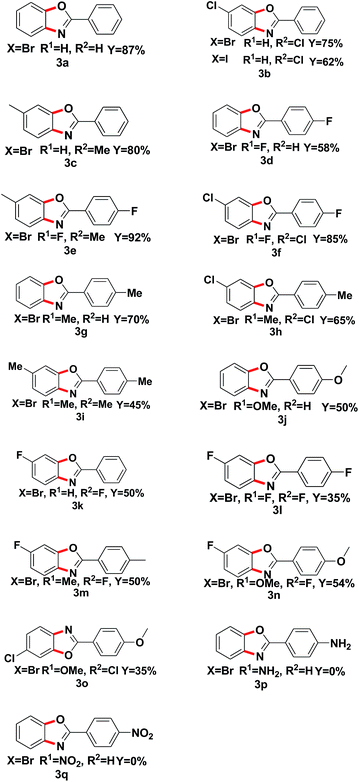
With the optimized reaction conditions in hand, we proceeded to investigate the substrate scope of the reaction using a variety of different 1,2-dihalobenzene substrates and aryl formamide (Table 2). Benzamide containing electron poor (3d–f, 3l), electron-neutral (3a–c, 3k), and electron-rich (3g–j, 3m–n) substituents were all obtained in moderate to excellent yields. But some functional groups are intolerated in the reaction, like amino (3p) and nitro (3q).
| a Reaction conditions: 1a (0.5 mmol), o-dihalo substrate (1.5 eq.), Fe2O3 (20% mol), K2CO3 (1 eq.), DMEDA (20%) were added to PhMe (2 mL) and react at 110 °C for 48 h under N2. |
|---|
 |
Based on the results observed in the current study and Goldberg reaction,15 we have proposed a reaction mechanism for this transformation, which is shown in Scheme 3. The initial transmetalation of benzamide with Fe2O3Ln in the presence of K2CO3 would give rise to the iron(III) species A. Complex A would then undergo an oxidative addition reaction with 1-bromo-2-iodobenzene to give the iron(V) species B, which would undergo a reductive elimination reaction to give iron(III) species C with the concomitant formation of a C–N bond. Followed the tautomerism of intermediate C to D, the intermediate iron(III) species E was formed in the presence of K2CO3, which would undergo another oxidative addition reaction to afford iron(V) species F. Compound 3a would then be obtained via a reductive elimination reaction from iron(V) species F.
In summary, we have demonstrated that the cheap and environmental friendly catalyst system composed of Fe2O3 and ligand DMEDA is highly effective for the synthesis of 2-arylbenzoxazoles. The new catalyzed system can be effective for both C–N coupling and C–O coupling.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, The Project of Scientific and Technologic Infrastructure of Suzhou (No. SZS201207) and the National Natural Science Foundation of China (No. 21072143) for financial support.Notes and references
- (a) S. Tzanopoulou, M. Sagnou, M. Paravatou-Petsotas, E. Gourni, G. Loudos, S. Xanthopoulos, D. Lafkas, H. Kiaris, A. Varvarigou, I. C. Pirmettis, M. Papadopoulos and M. Pelecanou, J. Med. Chem., 2010, 53, 4633–4641 CrossRef CAS PubMed; (b) M. C. V. Zandt, M. L. Jones, D. E. Gunn, L. S. Geraci, J. H. Jones, D. R. Sawicki, J. Sredy, J. L. Jacot, A. T. DiCioccio, T. Petrove, A. Mitschler and A. D. Podjarny, J. Med. Chem., 2005, 48, 3141–3152 CrossRef PubMed; (c) L. Katz, J. Am. Chem. Soc., 1953, 75, 712–714 CrossRef CAS; (d) D. K. Dalvie, A. S. Kalgutkar, S. C. Khojasteh-Bakht, R. S. Obach and J. P. O_Donnell, Chem. Res. Toxicol., 2002, 15, 269 CrossRef CAS PubMed; (e) H. Z. Boeini and K. H. Najafabadi, Eur. J. Org. Chem., 2009, 2009, 4926–4929 CrossRef.
- D. R. Chancellor, K. E. Davies, O. D. Moor, C. R. Dorgan, P. D. Johnson, A. G. Lambert, D. Lawrence, C. Lecci, C. Maillol, P. J. Middleton, G. Nugent, S. D. Poignant, A. C. Potter, P. D. Price, R. J. Pye, R. Storer, J. M. Tinsley, R. V. Well, R. Vickers, J. Vile, F. J. Wilkes, F. X. Wilson, S. P. Wren and G. M. Wynne, J. Med. Chem., 2011, 54, 3241–3250 CrossRef CAS PubMed.
- (a) M. R. Kumar, K. Park and S. Lee, Adv. Synth. Catal., 2010, 352, 3255–3266 CrossRef CAS; (b) A. M. Hamdy, N. Eleya, H. H. Mohammed, T. Patonay, A. Spannenberg and P. Langer, Tetrahedron, 2013, 69, 2081–2086 CrossRef CAS; (c) F. Shibahara, E. Yamaguchi and T. Murai, Chem. Commun., 2010, 46, 2471–2473 RSC; (d) J. Huang, J. Chan, Y. Chen, C. J. Borths, K. D. Baucom, R. D. Larsen and M. M. Faul, J. Am. Chem. Soc., 2010, 132, 3674–3675 CrossRef CAS PubMed; (e) R. S. Sánchez and F. A. Zhuravlev, J. Am. Chem. Soc., 2007, 129, 5824–5825 CrossRef PubMed; (f) S. Ranjit and X.-G. Liu, Chem.–Eur. J., 2011, 17, 1105–1108 CrossRef CAS PubMed.
- (a) T. Yamamoto, K. Muto, M. Komiyama, J. Canivet, J. Yamaguchi and K. Itami, Chem.–Eur. J., 2011, 17, 10113–10122 CrossRef CAS PubMed; (b) J. Canivet, J. Yamaguchi, I. Ban and K. Itami, Org. Lett., 2009, 11, 1733–1736 CrossRef CAS PubMed; (c) H. Hachiya, K. Hirano, T. Satoh and M. Miura, ChemCatChem, 2010, 2, 140–1406 CrossRef.
- (a) H.-Q. Do and O. Daugulis, J. Am. Chem. Soc., 2007, 129, 12404–12405 CrossRef CAS PubMed; (b) W. Zhang, Q.-L. Zeng, X.-M. Zhang, Y.-J. Tian, Y. Yue, Y.-J. Guo and Z.-H. Wang, J. Org. Chem., 2011, 76, 4741–4745 CrossRef CAS PubMed; (c) W.-Y. Hu, P.-P. Wang and S.-L. Zhang, Synthesis, 2015, 47, 42–48 CAS; (d) F.-Z. Yang, Z.-Q. Xu, Z. Wang, Z.-K. Yu and R. Wang, Chem.–Eur. J., 2011, 17, 6321–6325 CrossRef CAS PubMed.
- S. K. Guchhait, M. Kashyap and S. Saraf, Synthesis, 2010, 2010, 1166–1170 CrossRef.
- (a) Y. Wang, K. Sarris, D. R. Sauer and S. W. Djuric, Tetrahedron Lett., 2006, 47, 4823–4826 CrossRef CAS; (b) C. O. Kangani, D. E. Kelley and B. W. Day, Tetrahedron Lett., 2006, 47, 6497–6499 CrossRef CAS.
- (a) Y. Riadi, R. Mamouni, R. Azzalou, M. E. Haddad, S. Routier and G. Guillaumet, Tetrahedron Lett., 2011, 52, 3492–3495 CrossRef CAS; (b) S. Banerjee, S. Payra, A. Saha and G. Sereda, Tetrahedron Lett., 2014, 55, 5515–5520 CrossRef CAS; (c) Y.-X. Chen, L.-F. Qian, W. Zhang and B. Han, Angew. Chem., Int. Ed., 2008, 47, 9330–9333 CrossRef CAS PubMed; (d) L. Tang, X.-F. Guo, Y. Yang, Z.-G. Zha and Z.-Y. Wang, Chem. Commun., 2014, 50, 6145–6148 RSC; (e) A. K. Nezhad and F. Panahi, ACS Catal., 2014, 4, 1686–1692 CrossRef; (f) K. Osowska and O. S. Miljanic, J. Am. Chem. Soc., 2011, 133, 724–727 CrossRef CAS PubMed.
- G. Altenhoff and F. Glorius, Adv. Synth. Catal., 2004, 346, 1661–1664 CrossRef CAS.
- C. S. Demmer, J. C. Hansen, J. Kehler and L. Bunch, Adv. Synth. Catal., 2014, 356, 1047–1055 CrossRef CAS.
- (a) D. Xue and Y.-Q. Long, J. Org. Chem., 2014, 79, 4727–4734 CrossRef CAS PubMed; (b) Q.-C. Xu, Z.-N. Li and H.-Y. Chen, Chin. J. Chem., 2011, 29, 925–932 CrossRef CAS.
- (a) J.-Y. Lu, X.-Y. Gong, H.-J. Yang and H. Fu, Chem. Commun., 2010, 46, 4172–4174 RSC; (b) P. Saha, M. Ashif Ali, P. Ghosh and T. Punniyamurthy, Org. Biomol. Chem., 2010, 8, 5692–5699 RSC; (c) N. Khatun, S. Guin, S. K. Rout and B. K. Patel, RSC Adv., 2014, 4, 10770–10778 RSC; (d) R. J. Perry and B. D. Wilson, J. Org. Chem., 1992, 57, 6351–6354 CrossRef CAS; (e) G. Evindar and R. A. Batey, J. Org. Chem., 2006, 71, 1802–1808 CrossRef CAS PubMed; (f) P. Saha, T. Ramana, N. Purkait, M. A. Ali, R. Paul and T. Punniyamurthy, J. Org. Chem., 2009, 74, 8719–8725 CrossRef CAS PubMed; (g) Y.-T. Park, C. H. Jung and K.-W. Kim, J. Org. Chem., 1999, 64, 8546–8556 CrossRef CAS; (h) J. Bonnamour and C. Bolm, Org. Lett., 2008, 10, 2665–2667 CrossRef CAS PubMed.
- (a) C. Bolm, J. Legros, J. L. Paih and L. Zani, Chem. Rev., 2004, 104, 6217–6254 CrossRef CAS PubMed; (b) D. Damodara, R. Arundhathi and P. R. Likhar, Catal. Sci. Technol., 2013, 3, 797–802 RSC; (c) O. Bistri, A. Correa and C. Bolm, Angew. Chem., 2008, 120, 596–598 CrossRef; (d) C. L. Sun, B. J. Li and Z. J. Shi, Chem. Rev., 2011, 111, 1293–1314 CrossRef CAS PubMed; (e) H. B. Wang, L. Wang, J. S. Shang, X. Li, H. Y. Wang, J. Guiand and A. W. Lei, Chem. Commun., 2012, 48, 76–78 RSC; (f) K. Gopalaiah, Chem. Rev., 2013, 113, 3248–3296 CrossRef CAS PubMed; (g) I. Bauer and H.-J. Knolker, Chem. Rev., 2015, 115, 3170–3387 CrossRef CAS PubMed.
- (a) S. L. Buchwald and C. Bolm, Angew. Chem., Int. Ed., 2009, 48, 5586 CrossRef CAS PubMed; (b) Z. Gonda, G. L. Tolnai and Z. Novak, Chem.–Eur. J., 2010, 16, 11822 CrossRef CAS PubMed.
- (a) A. Klapars, X.-H. Huang and S. L. Buchwald, J. Am. Chem. Soc., 2002, 124, 7421–7428 CrossRef CAS PubMed; (b) E. R. Strieter, D. G. Blackmond and S. L. Buchwald, J. Am. Chem. Soc., 2005, 127, 4120–4121 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra13080e |
| This journal is © The Royal Society of Chemistry 2018 |