Open Access Article
Open Access ArticleThe utilization of a three-dimensional reduced graphene oxide and montmorillonite composite aerogel as a multifunctional agent for wastewater treatment†
Yunyun Zhanga,
Xueru Yana,
Yayuan Yana,
Dengjie Chena,
Langhuan Huanga,
Jingxian Zhanga,
Yu Keb and
Shaozao Tan *a
*a
aGuangdong Engineering and Technology R & D Center of Graphene or Its Like Materials and Products, Department of Chemistry, Jinan University, Guangzhou 510632, P. R. China. E-mail: tsztan@jnu.edu.cn
bDepartment of Biomedical Engineering, Jinan University, Guangzhou 510632, P. R. China
First published on 24th January 2018
Abstract
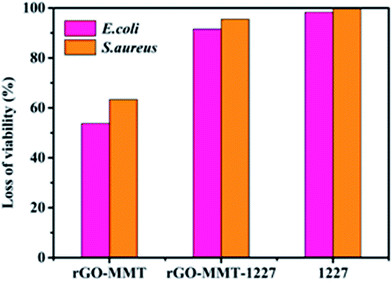
Mankind has witnessed a dramatically growing challenge regarding water pollution during the past decades. Meanwhile, intensive attention has been paid to developing functional materials which can comprehensively treat wastewater which always includes bacteria, organic and inorganic contaminants. In this work, a reduced graphene oxide and montmorillonite composite (rGO–MMT) aerogel was synthesized by a one-step green method to treat multiple pollutants in wastewater. The Langmuir isotherm model and the pseudo-second-order kinetic model were well fitted to the adsorption of both dyes and heavy metal ions. The rGO–MMT aerogel exhibited 97.31% and 94.87% removal efficiencies for methylene blue (MB) and Cr(VI), respectively. In addition, the antibacterial ratios of the rGO–MMT loaded with quaternary ammonium salt reached 91.57% and 95.53% for E. coli and S. aureus, respectively. Our results demonstrated that the aerogel had a high multi-component adsorption capacity and antibacterial activity. Attractively, the rGO–MMT aerogel exhibited excellent recyclable properties thanks to its special three-dimensional structure with outstanding mechanical strength.
Introduction
There has been a major global challenge to purify sewage water since it contains organic pollutants, heavy metals and pathogens.1 Even an extremely low concentration of 1.0 mg L−1 dye can contaminate drinking water and make it unavailable for human beings.2 Moreover, most dyes are stable to photo-degradation, bio-degradation and oxidizing agents.3 Similarly, heavy metal ions like Cr(VI), Cu(II) and Pb(II) at high dosage are poisonous and carcinogenic.4,5 At present, several physicochemical and biological methods have been applied to mediate water pollution, including flotation,6–8 membrane separation,9,10 adsorption,11–13 chemical precipitation,14,15 electrodialysis,16 photocatalytic degradation,17–20 and so on. Among these techniques, the adsorption process is one of the most popular methods for the removal of trace amounts of organic and inorganic pollutants from sewage due to its advantages of convenience, high removal efficiency and low cost.21High-efficient adsorbent is one of the research focuses to resolve wastewater problem. Several types of materials have been investigated as adsorbents to adsorb dyes and metal ions from wastewater, including activated carbon, montmorillonite (MMT), chitosan, clay mineral, chitosan, graphene oxide (GO), reduce graphene oxide (rGO), and their composites.22–24 Although these materials have shown excellent adsorption properties, their activity is limited in killing or inhibiting pathogens. Therefore, it has drawn intensive attention to develop adsorption material that shows antimicrobial activity for water purification. rGO, a new carbon-based material, exhibits excellent performances like high surface area, strong mechanical strength and rich surface chemistry, so it has received increasing attention.25 Among various rGO materials, rGO aerogel is three-dimensional (3D) porous network (held together by π–π interaction and hydrogen bonding) with large specific surface area, low density and plentiful interpenetrating pore structure. Hence, it is used in the removal of oil and other organic solvents in water, which is economical and efficient.26,27 For example, Guo et al. prepared chitosan–graphene oxide aerogel whose adsorption capacity of oil is 900 g g−1, so it can be used to treat the industrial oil leakage or effluent in the natural water.28 MMT is one type of layered silicate, and it has attracted extensive attention due to its abundance in various industries and low cost.29 In view of it, it would be significant to synthesize an rGO and MMT complex to resolve wastewater problem.
In this work, we synthesized a three-dimensional reduced graphene oxide and montmorillonite composite (rGO–MMT) aerogel with multi-component adsorption capacity and antibacterial activity. More importantly, the three-dimensional aerogel could sustain its initial structure after vigorous shaking when it was reused. Therefore, the three-dimensional rGO–MMT aerogel was a potential candidate as efficient adsorbent in water purification.
Experimental
Synthesis of rGO–MMT aerogel
GO originated from graphite powder was synthesized using a modified Hummers method.30,31 The rGO–MMT aerogel was obtained by sol–gel method. Briefly, GO powder (30 mg) and ascorbic acid (30–45 mg) were added into 10 mL of ultrapure water (>18 MΩ). Meanwhile, 4 mL of MMT aqueous solution with the content ranging from 2 wt% to 6 wt% was added in order to optimize the concentration. After ultrasonic treatment for 0.5 h, the mixed solution was transferred into a 25 mL glass bottle with cover and heated at 95 °C for 6 h (for antibacterial experiment, 1 mol of dodecyl dimethyl benzyl ammonium chloride (1227) was added to the mixed solution). Then, rGO–MMT hydrogel and 1227-loaded rGO–MMT hydrogel were immersed into 6 mg mL−1 polyvinyl alcohol (PVA) aqueous solution for 2 days. Finally, the hydrogel was washed with ultrapure water for several times, and then fully freeze-dried at −55 °C for 12 h to acquire rGO–MMT aerogel and rGO–MMT–1227 aerogel.Characterizations
X-ray diffraction (XRD) patterns were obtained using X-ray analyzer (MSAL-XD2) with a Cu Kα radiation source (λ = 1.5405 Å) at a voltage of 48 kV and a current of 30 mA. Characterization was performed under the 2θ range of 5.0–80.0° at a scanning rate of 2° min−1. Fourier transform infrared (FT-IR) spectra were recorded using a Nicolet 6700 spectrometer. The UV-vis spectra were measured by a Hitachi 330 UV-vis spectrophotometer. Transmission electron microscopy (TEM) images and the corresponding mappings were obtained by using Philips TECNAL-10 transmission electron microscope. Scanning electron microscopy (SEM) images and energy dispersive X-ray spectroscopy (EDX) patterns were performed on a field emission-scanning electron microscope (PHILIPS XL-30 ESEM, Netherlands). The surface area and porosity of aerogel were measured by ASAP-2010 and calculated with Brunner–Emmett–Teller (BET) method. The heavy metal content of the aqueous solution was determined by inductively coupled plasma (ICP) (Optima 2000DV).Adsorption investigation
| R% = (A − B) ÷ A × 100% |
Results and discussion
Characterization of aerogel
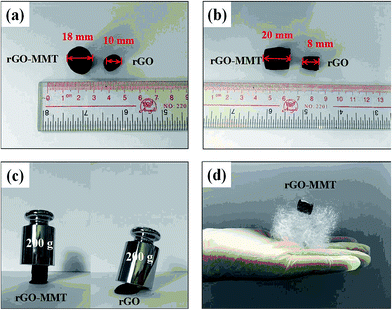
According to the digital photos of rGO–MMT aerogel (Fig. 1a and b), it was obviously that the volume of rGO–MMT aerogel was larger than that of rGO aerogel. The compressive strength of rGO–MMT aerogel was examined as shown in Fig. 1c. Fig. 1d depicted a digital image of kapok with rGO–MMT aerogel was placed on the top. The insignificant deformation of kapok indicated the low density of the rGO–MMT aerogel.XRD patterns of GO, rGO, MMT and rGO–MMT aerogel were shown in Fig. 2a. For GO, a diffraction peak at 2θ = 10.30° was observed, and the calculated interlayer spacing was 0.87 nm, corresponding to the (001) plane. Meanwhile, a new broad diffraction peak at 2θ = 24.9° was observed for the reduced GO, revealing the successful formation of rGO. For MMT, a well-defined diffraction peak at 2θ = 6.12° appeared, which indicated the formation of a calcium montmorillonite.32 The diffraction pattern of rGO–MMT aerogel contained the diffraction peaks of both rGO and MMT, indicating the successful incorporation of MMT into rGO. FT-IR spectra of GO, rGO, MMT and rGO–MMT aerogel were shown in Fig. 2b. The strong peak at 3452 cm−1 was attributed to the stretching vibration of the O–H. The peaks at 1731, 1621, 1384 and 1049 cm−1 could be assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–H–O, C
O, C–H–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–O–C, respectively. However, the O
C and C–O–C, respectively. However, the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O was negligible for rGO. The abundance of oxygen-containing functional group was much more on GO than that on rGO. For the FT-IR spectrum of MMT, the peaks at 3620 and 796 cm−1 corresponded to the stretching vibrations of the Al–OH and Si–O groups, and the strong speaks at 1089 and 1035 cm−1 originated from the Si–O vibration of amorphous silica and the tetrahedral sheet. Furthermore, the bands at 914, 844, 624 and 518 cm−1 were ascribed to deformation vibrations of Al–Al–OH, Al–Mg–OH and Si–O–Al. For rGO–MMT aerogel, all peaks belonging to rGO and MMT appeared simultaneously, and the intensity of the peak belonging to rGO became weak. These results demonstrated that rGO and MMT were combined together successfully.
C–O was negligible for rGO. The abundance of oxygen-containing functional group was much more on GO than that on rGO. For the FT-IR spectrum of MMT, the peaks at 3620 and 796 cm−1 corresponded to the stretching vibrations of the Al–OH and Si–O groups, and the strong speaks at 1089 and 1035 cm−1 originated from the Si–O vibration of amorphous silica and the tetrahedral sheet. Furthermore, the bands at 914, 844, 624 and 518 cm−1 were ascribed to deformation vibrations of Al–Al–OH, Al–Mg–OH and Si–O–Al. For rGO–MMT aerogel, all peaks belonging to rGO and MMT appeared simultaneously, and the intensity of the peak belonging to rGO became weak. These results demonstrated that rGO and MMT were combined together successfully.
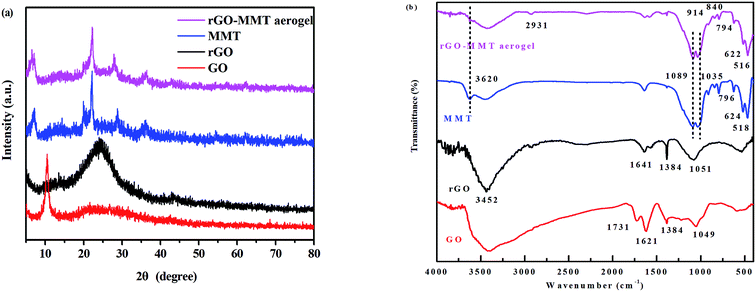 | ||
| Fig. 2 XRD patterns of rGO, GO, MMT and rGO–MMT aerogel (a), FT-IR spectra of GO, rGO, MMT and rGO–MMT aerogel (b). | ||
The N2 adsorption–desorption isotherms of rGO, rGO–MMT-0.02, rGO–MMT-0.04 and rGO–MMT-0.06 aerogels (Fig. S1†) exhibited type IV isotherms, according to the IUPAC classification.33 According to the Table S1 and Fig. S2† the application of 0.04 wt% MMT was beneficial for the dispersion of rGO and the enhanced adsorption capability. Thus, rGO–MMT-0.04 was selected in subsequent experiments.
According to the SEM and TEM images (Fig. 3 and 4), there were many apparent wrinkles on the surface of rGO due to the scrolled rGO sheets. Fig. 3b, c, 4d and 3e showed that the surface of rGO–MMT aerogel was quite loose with large pore compared to rGO aerogel. The EDX (Fig. 3f) and mapping (Fig. 4d) results of rGO–MMT aerogel suggested the existence of C, O, Mg, Al, Si and Ca elements. According to the nominal existence of Mg, Al, Si and Ca elements, it could be inferred that the small particles on rGO–MMT aerogel were MMT.32
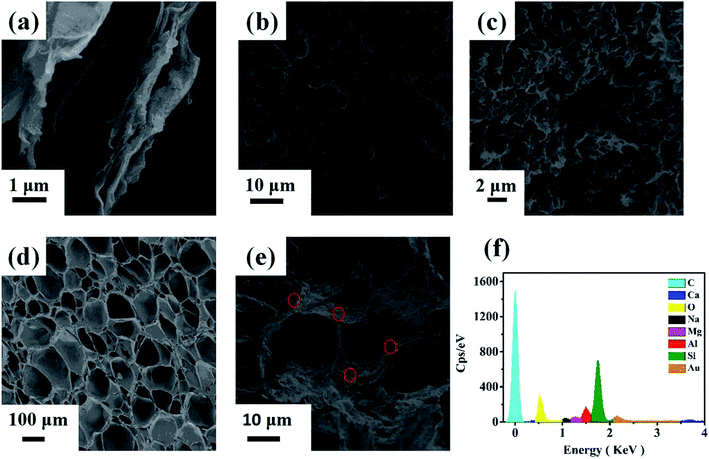 | ||
| Fig. 3 SEM images of rGO (a), rGO aerogel (b and c) and rGO–MMT aerogel (d and e). EDS of rGO–MMT aerogel (f). | ||
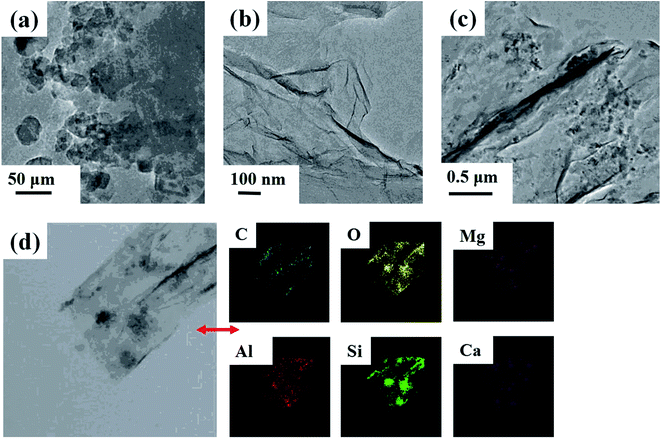 | ||
| Fig. 4 TEM images of MMT (a), rGO aerogel (b) and rGO–MMT aerogel (c). Mapping of rGO–MMT aerogel (d). | ||
Adsorption experiments
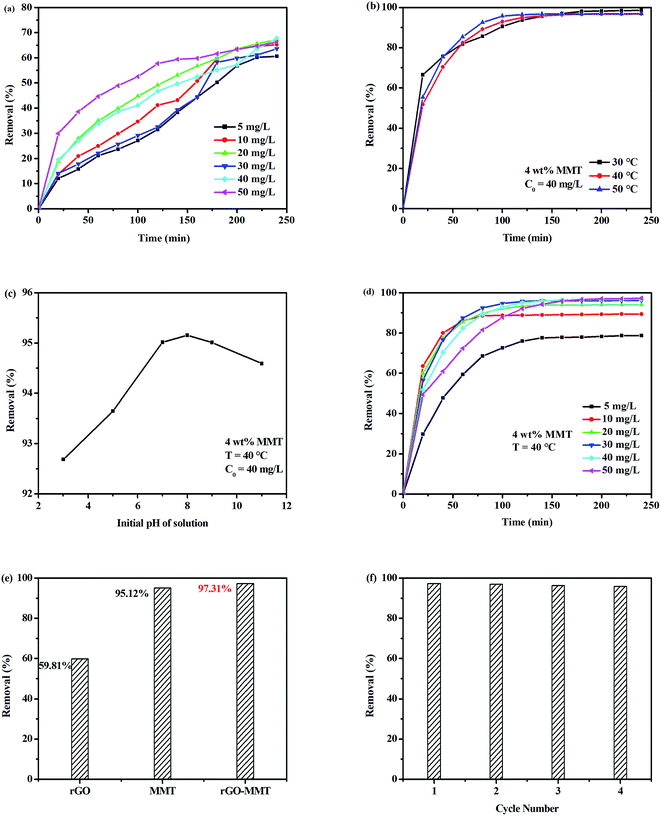
Fig. 5a showed the removal of MO with different contact time. The results indicated that there was a rapid uptake within the first 20 min and then the trend became smooth. This phenomenon indicated that the surface of adsorbent was gradually blocked by dye molecules during the adsorption process, and then the surface was completely covered after certain time.34 The adsorption curves showed that rGO–MMT rapidly adsorbed MO at the concentration of 50 mg L−1, so this concentration of MO was applied in the mixed aqueous solution in subsequent experiments.
The selective adsorption of MB from mixed aqueous solution was studied, where the MO aqueous solution (50 mg L−1, 200 mL) was mixed with different concentrations of MB aqueous solution (from 5 to 50 mg L−1). As shown in Fig. 5b, the influence of temperature (30 °C, 40 °C and 50 °C) on the selective adsorption capacity of MB (C0 = 40 mg L−1) was investigated. A high temperature promoted the diffusion rate of dye molecule, which influenced the selective adsorption of MB. However, high temperature operation would increase the cost. Thus, temperature of 40 °C was adopted for the following experiments. The pH in the solution might influence dye adsorption by changing the surface charge of the adsorbent and also the distribution of the dye in solution phase.35 Fig. 5c showed the maximum selective adsorption capacity of MB from the mixed aqueous solution by rGO–MMT aerogel at pH = 8. The selective adsorption capacity of MB from mixed aqueous solution by rGO–MMT aerogel increased with increasing pH when pH < 8, while the selective adsorption capacity of MB decreased with increasing pH from 8 to 11. Hydroxyl ions increased, and negative charges of hydroxyl ions competed with negative charges of rGO–MMT aerogel about MB. While we analyzed residual dye concentration in the aqueous solution. There were little MB combined by hydroxyl ions in aqueous solution, so the selective adsorption capacity of MB decreased. The adsorption curves showed that rGO–MMT largely adsorbed MO at the concentration of 50 mg L−1 (Fig. 5d). Thus, temperature of 40 °C, 4 h, pH = 8 and the concentration of 50 mg L−1 were selected in subsequent experiments.
Fig. 5e presented the comparison of MB selective removal rate by rGO–MMT aerogel, pristine MMT powder and rGO aerogel. rGO–MMT showed the highest removal rate of MB compared with rGO and MMT. rGO showed negligible removal rate of MB, while the removal rate of MB on MMT was 95.12%. It was difficult to recollection for suspended MMT powder in solution. From Fig. 5f, after four adsorption/desorption cycles, it was observed that in the first cycle the selective removal rate of MB onto the rGO–MMT aerogel was 97.31% at 40 °C and pH = 8. Moreover, after four absorption/desorption cycles the removal rate still remains at 95.87%, which just reduced 1.44% compared with that of the first cycle. Remarkably, after four times cycles, the aerogels could still maintain their 3D structure. This showed that aerogel could be an environmental, high-efficient adsorbent for selective removing MB.
In this study, Langmuir (1) and Freundlich isothermal models (2) were used to understand the thermodynamics of selective adsorption of MB.36,37
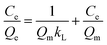 | (1) |
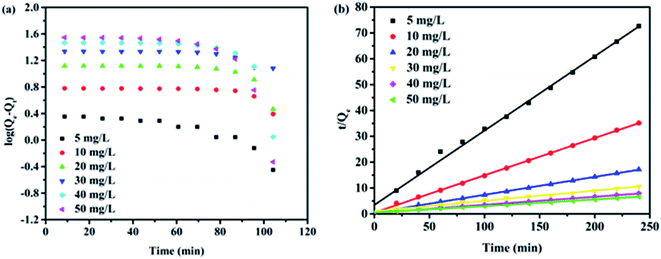
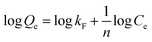 | (2) |
Kinetics analysis was deployed for a better understanding of the selective adsorption process. The pseudo-first-order and pseudo-second-order equations were further used to model the kinetics data using a linear fitting.36,37 The models were expressed in eqn (3) and (4):
ln(Qe − Qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe − k1t Qe − k1t
| (3) |
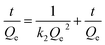 | (4) |
The fitted parameters in Langmuir and Freundlich models for selective adsorption were shown in Table 1. By comparing the R2 values of both models, Langmuir model was better to describe the selective adsorption onto the rGO–MMT aerogel than Freundlich model, suggesting the monolayer or homogeneous adsorption for MB.37
| T (K) | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qmax (mg g−1) | KL | R2 | KF | n | R2 | |
| 303 | 167.79 | 0.94 | 0.973 | 33.28 | 5.51 | 0.836 |
| 313 | 182.15 | 0.12 | 0.979 | 33.52 | 6.04 | 0.855 |
| 323 | 227.27 | 0.19 | 0.987 | 32.31 | 9.92 | 0.825 |
The kinetics data of the adsorbent were fitted to the pseudo-first and second-order model, as shown in Fig. 6. The calculated kinetics parameters from two models were listed in Table 2. In Fig. 6a and b, the pseudo-second-order model displayed a good fit with the selective adsorption of MB on rGO–MMT aerogel, while the pseudo-first-order model did not fit well, suggesting that the selective adsorption process might be based on chemisorption, involving valence force via sharing or exchanging electron between adsorbent and adsorbate.38 As known, the abundant functional groups were located in rGO–MMT aerogel, such as hydroxy groups, epoxide groups and carboxylic groups. Accordingly, MB could be adsorbed via interaction between MB molecule and these functional groups of rGO in the rGO–MMT aerogel.
 | ||
| Fig. 6 Pseudo-first-order (a) and pseudo-second-order (b) equations in the modeling of adsorption kinetics. | ||
| Initial MB (mg L−1) | Pseudo-first-order | Pseudo-second-order | ||||
|---|---|---|---|---|---|---|
| K1 | Qe (mg g−1) | R2 | K2 | Qe (mg g−1) | R2 | |
| 5 | 0.016 | 3.44 | 0.770 | 0.00024 | 3.48 | 0.995 |
| 10 | 0.0053 | 7.23 | 0.415 | 0.00035 | 6.94 | 0.999 |
| 20 | 0.0092 | 18.12 | 0.442 | 4.46 | 14.45 | 0.999 |
| 30 | 0.0053 | 25.95 | 0.606 | 1.47 | 25.19 | 0.984 |
| 40 | 0.019 | 57.45 | 0.403 | 2.74 | 32.05 | 0.996 |
| 50 | 0.029 | 94.01 | 0.506 | 1.36 | 39.22 | 0.992 |
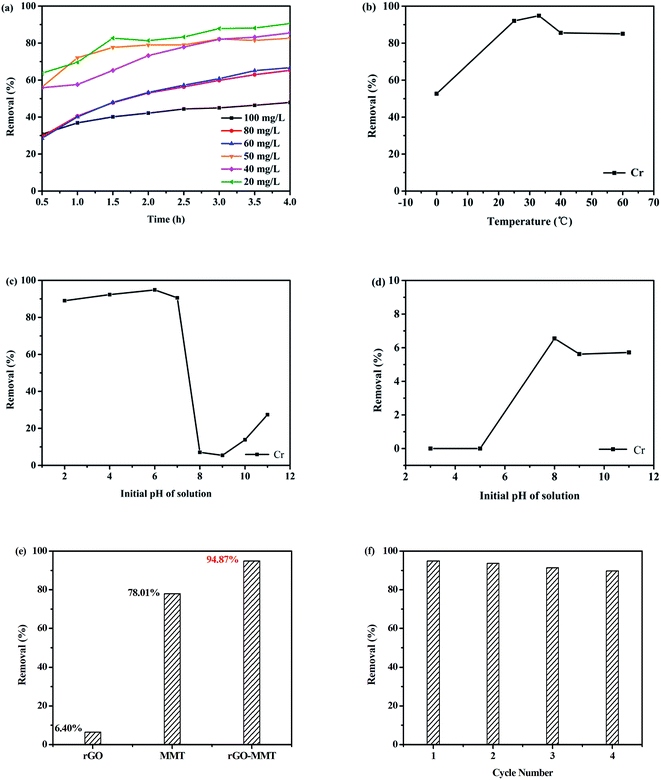
Fig. 7a showed the effects of initial concentration and contact time on the removal rate. It was evident that the adsorption of heavy metal ion on the aerogel increased gradually with increasing contact time. Therefore, we selected the contact time of 4 h in this work. Totally, a low concentration of Cr(VI) was beneficial for improving removal rate. Fig. 7b showed that the adsorption capacities of Cr(VI) were 52.64%, 92.05%, 94.87%, 85.60% and 85.06% for rGO–MMT aerogel at 0 °C, 20 °C, room temperature (35 °C), 40 °C and 60 °C, respectively. The pH of the initial solution determined the charge on the surface of adsorbent and the degree of ionization and speciation of the metal ion.39 The effect of pH on the adsorption of Cr(VI) was evaluated by changing the initial pH from 2 to 11. According to Fig. 7c and d, maximum removal rate of metal ion could be observed at pH = 6. Under the alkaline condition, the surface of the adsorbent was surrounded with OH− that competed with CrO42−. Meanwhile, Cr6+ would be reduced to Cr3+ to react with OH−, and Cr(OH)3 was formed. Therefore, the removal rate increased slightly when pH ranged from 8 to 11. In contrast, heavy metal ions were adsorbed due to the electrostatic adsorption at low pH.40
Fig. 7e presented the comparison of Cr(VI) removal rate by rGO–MMT, pristine MMT and rGO. rGO–MMT showed the highest removal rate of Cr(VI) compared with rGO and MMT. Compared with sole rGO and MMT, the effectiveness of rGO–MMT might be ascribed to a relatively large surface area, high pore volume and loose structure. We thus proposed that the synergy between rGO and MMT contributed to the enhancement of the removal rate of Cr(VI).
The adsorption of Cr(VI) was performed at pH = 6, 4 h and room temperature, and desorption with NaOH solution at pH = 13, 12 h, room temperature to evaluate the regenerate of rGO–MMT. As shown in Fig. 7f. It had no obvious decrement after four recycles, suggesting the good removal stability of the aerogel. In conclusion, our results had demonstrated that environment-friendly rGO–MMT aerogel was a robust and efficient adsorbent.
To evaluate the multiple adsorption of rGO–MMT for heavy metal ions, 10 mg L−1 Cu(NO3)2, 10 mg L−1 Pb(NO3)2 and 20 mg L−1 K2Cr2O7 were mixed together in the solution. As shown in Fig. S3† it was evident that rGO–MMT could not only adsorb Cr(VI), but also adsorb Cu(II) and Pb(II).
Adsorption isotherms were important for analyzing the adsorption mechanism. The Langmuir and Freundlich models were employed to derive possible mechanism.36,37 As shown in Table 3, it was easy to find that the fitting degree (R2) of Langmuir model was larger than that of Freundlich model, suggesting the monolayer adsorption.37 Besides, the Qmax values calculated from the Langmuir isotherm and Freundlich isotherm were 77.519 mg g−1 and 27.613 mg g−1, respectively.
| T (K) | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qmax (mg g−1) | KL | R2 | KF | n | R2 | |
| 303 | 77.52 | 0.344 | 0.989 | 27.61 | 3.497 | 0.815 |
To analyze the kinetics of Cr(VI) adsorption onto rGO–MMT aerogel, the pseudo-first-order and pseudo-second-order models were applied at different initial concentrations of Cr(VI). According to Fig. 8 and Table 4, the correlation factors of the pseudo-second order model of all initial Cr(VI) concentrations were higher than those of the pseudo-first order model. In addition, the calculated Qe values were well in accord with the experiments. Thus, the adsorption process of Cr(VI) onto rGO–MMT aerogel followed the pseudo-second order model, suggesting the rate limiting step was the chemisorption involving the valence force.37
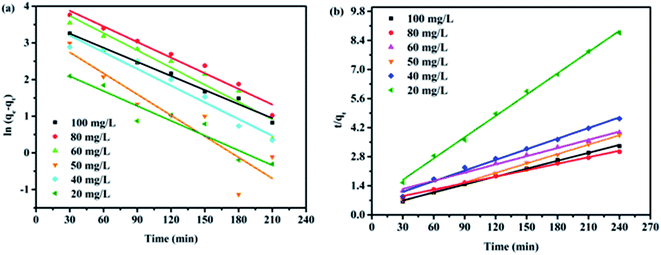 | ||
| Fig. 8 Pseudo-first-order and pseudo-second-order equations in the modeling of Cr(VI) removal kinetics. | ||
| Initial Cr (mg L−1) | Pseudo-first-order | Pseudo-second-order | ||||
|---|---|---|---|---|---|---|
| K1 | Qe (mg g−1) | R2 | K2 | Qe (mg g−1) | R2 | |
| 20 | 0.0135 | 9.48 | 0.9924 | 0.00176 | 29.24 | 0.998 |
| 40 | 0.0152 | 38.83 | 0.9547 | 0.000490 | 58.48 | 0.992 |
| 50 | 0.0191 | 27.59 | 0.8349 | 0.00109 | 66.23 | 0.999 |
| 60 | 0.0157 | 67.20 | 0.9198 | 0.00205 | 75.76 | 0.987 |
| 80 | 0.0142 | 73.99 | 0.9674 | 0.000182 | 98.15 | 0.997 |
| 100 | 0.0126 | 36.84 | 0.9881 | 0.000122 | 78.74 | 0.998 |
Conclusions
MMT had been successfully embedded in layer of rGO, which was demonstrated by various characterizations. The prepared rGO–MMT had shown a porous structure and large specific surface area compared to sole rGO. According to adsorption studies, the Langmuir isotherm model and the pseudo-second-order kinetics model could be applied to explain the adsorption mechanisms of dyes and heavy metal ions. We had proposed that the monolayer or homogeneous adsorption was dominant, and the chemisorption involving valence force was the rate limiting step. Moreover, the maximum selective adsorption capacity of MB from the mixed aqueous solution was 227.27 mg g−1 and the adsorption capacity of Cr(VI) was 77.52 mg g−1. In addition, the antibacterial ratios of rGO–MMT-1227 reached 91.57% and 95.53% for E. coli and S. aureus. The prepared rGO–MMT aerogel could be served as an efficient and robust material with multiple functions to handle dyes, heavy metal ions and pathogens. Therefore, the rGO–MMT aerogel was promising for water purification.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledged financial support from the National Natural Science Foundation of China (21676116, 21271087 and 21476052), the Foundation of Enterprise-University-Research Institute Cooperation from Guangdong Province and the Ministry of Education of China (2015B090903072, 2015A010105019 and 2013B090600148), and the Science and Technology Innovation Platform Project of Foshan City (2015AG10020 and 2014AG100171).References
- X. L. Qu, P. J. J. Alvarez and Q. L. Li, Water Res., 2013, 47, 3931–3946 CrossRef CAS PubMed
.
- M. T. Yagub, T. K. Sen, S. Afroze and H. M. Ang, Adv. Colloid Interface Sci., 2014, 209, 172–184 CrossRef CAS PubMed
.
- L. Peng, P. F. Qin, M. Lei, Q. R. Zeng, H. J. Song, J. Yang, J. H. Shao, B. H. Liao and J. D. Gu, J. Hazard. Mater., 2012, 209, 193–198 CrossRef PubMed
.
- R. K. Misra, S. K. Jain and P. K. Khatri, J. Hazard. Mater., 2011, 185, 1508–1512 CrossRef CAS PubMed
.
- V. C. Renge, S. V. Khedkar and S. Pandey, Sci. Rev. Chem. Commun., 2012, 2(4), 580–584 CAS
.
- M. X. Wu, J. J. Liang, J. Tang, G. Li, S. P. Shan, Z. H. Guo and L. Deng, J. Hazard. Mater., 2017, 337, 189–197 CrossRef CAS PubMed
.
- S. Aoudj, A. Khelifa and N. Drouiche, Chemosphere, 2017, 180, 379–387 CrossRef CAS PubMed
.
- M. Kaya, Waste Manag., 2016, 57, 64–90 CrossRef CAS PubMed
.
- M. C. Tomei, D. M. Angelucci, V. Stazi and A. J. Daugulis, Sci. Total Environ., 2017, 599, 1056–1063 CrossRef PubMed
.
- Y. J. Juang, E. Nurhayati, C. P. Huang, J. R. Pan and S. M. Huang, Sep. Purif. Technol., 2013, 120, 289–295 CrossRef CAS
.
- M. R. Awual, Chem. Eng. J., 2016, 303, 539–546 CrossRef CAS
.
- D. Ocinski, I. Jacukowicz-Sobala, P. Mazur, J. Raczyk and E. Kociolek-Balawejder, Chem. Eng. J., 2016, 294, 210–221 CrossRef CAS
.
- Y. D. Zou, X. X. Wang, A. Khan, P. Y. Wang, Y. H. Liu, A. Alsaedi, T. Hayat and X. K. Wang, Environ. Sci. Technol., 2016, 50, 7290–7304 CrossRef CAS PubMed
.
- C. E. Barrera-Diaz, V. Lugo-Lugo and B. Bilyeu, J. Hazard. Mater., 2012, 223, 1–12 CrossRef PubMed
.
- J. Wu, L. M. Ma, Y. L. Chen, Y. Q. Cheng, Y. Liu and X. S. Zha, Water Res., 2016, 92, 140–148 CrossRef CAS PubMed
.
- A. Abou-Shady, Chem. Eng. J., 2017, 323, 1–18 CrossRef CAS
.
- S. G. Kumar and K. S. R. K. Rao, Appl. Surf. Sci., 2017, 391, 124–148 CrossRef CAS
.
- O. Gimeno, J. F. Garcia-Araya, F. J. Beltran, F. J. Rivas and A. Espejo, Chem. Eng. J., 2016, 290, 12–20 CrossRef CAS
.
- X. F. Yang, J. L. Qin, Y. Jiang, K. M. Chen, X. H. Yan, D. Zhang, R. Li and H. Tang, Appl. Catal., B, 2015, 166, 231–240 CrossRef
.
- M. Mehrjouei, S. Muller and D. Moller, Chem. Eng. J., 2015, 263, 209–219 CrossRef CAS
.
- M. Hua, S. J. Zhang, B. C. Pan, W. M. Zhang, L. Lv and Q. X. Zhang, J. Hazard. Mater., 2012, 211, 317–331 CrossRef PubMed
.
- X. Guo, B. Du, Q. Wei, J. Yang, L. Hu, L. Yan and W. Xu, J. Hazard. Mater., 2014, 278, 211–220 CrossRef CAS PubMed
.
- M. Karnib, A. Kabbani, H. Holail and Z. Olama, Energy Procedia, 2014, 50, 113–120 CrossRef CAS
.
- S. Pandey, J. Mol. Liq., 2017, 241, 1091–1113 CrossRef CAS
.
- Y. Q. Guo, X. Y. Sun, Y. Liu, W. Wang, H. X. Qiu and J. P. Gao, Carbon, 2012, 50, 2513–2523 CrossRef CAS
.
- C. Li and G. Q. Shi, Nanoscale, 2012, 4, 549–5563 Search PubMed
.
- J. Y. Cai, W. J. Liu and Z. H. Li, Appl. Surf. Sci., 2015, 358, 146–151 CrossRef CAS
.
- X. Guo, L. Qu, S. Zhu, M. Tian, X. Zhang, K. Sun and X. Tang, Water Environ. Res., 2016, 88, 768–778 CrossRef CAS PubMed
.
- K. Peng, L. J. Fu, X. Y. Li, J. Ouyang and H. M. Yang, Appl. Clay Sci., 2017, 138, 100–106 CrossRef CAS
.
- D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, ACS Nano, 2010, 4, 4806–4814 CrossRef CAS PubMed
.
- L. Z. Li, Z. Wang, P. M. Ma, H. Y. Bai, W. F. Dong and M. Q. Chen, J. Polym. Res., 2015, 22, 150 CrossRef
.
- M. H. Yeh and W. S. Hwang, Mater. Trans., 2006, 47, 2753–2758 CrossRef CAS
.
- W. Mulewa, M. Tahir and N. A. S. Amin, Chem. Eng. J., 2017, 326, 956–969 CrossRef CAS
.
- R. Boopathy, S. Karthikeyan, A. B. Mandal and G. Sekaran, Environ. Sci. Pollut. Res., 2013, 20, 533–542 CrossRef CAS PubMed
.
- T. Santhi, S. Manonmani, V. S. Vasantha and Y. T. Chang, Arabian J. Chem., 2016, 9, 466–474 CrossRef
.
- M. A. P. Cechinel, S. M. A. G. U. de Souza and A. A. U. de Souza, J. Cleaner Prod., 2014, 65, 342–349 CrossRef CAS
.
- W. Y. Huang, D. Li, Z. Q. Liu, Q. Tao, Y. Zhu, J. Yang and Y. M. Zhang, Chem. Eng. J., 2014, 236, 191–201 CrossRef CAS
.
- N. Tekin, A. Safakli and D. Bingol, Desalin. Water Treat., 2015, 54, 2023–2035 CrossRef CAS
.
- R. Garcia, J. Campos, J. Alfonso Cruz, M. Elena Calderon, M. Elena Raynal and G. Buitron, TIP, Rev. Espec. Cienc. Quim.-Biol., 2016, 19, 5–14 Search PubMed
.
- T. Li, J. F. Shen, S. T. Huang, N. Li and M. X. Ye, Appl. Clay Sci., 2014, 93, 48–55 CrossRef
.
- A. Azam, A. S. Ahmed, M. Oves, M. S. Khan, S. S. Habib and A. Memic, Int. J. Nanomed., 2017, 7, 6003–6009 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra13103h |
| This journal is © The Royal Society of Chemistry 2018 |