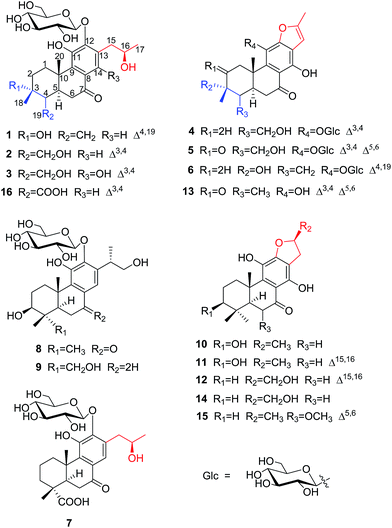
Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Isolation, identification and bioactivities of abietane diterpenoids from Premna szemaoensis†
De-Bing Pu‡
 acd,
Ting Wang‡b,
Xing-Jie Zhanga,
Jun-Bo Gaocd,
Rui-Han Zhanga,
Xiao-Nian Lic,
Yong-Mei Wangcd,
Xiao-Li Li*a,
He-Yao Wang*b and
Wei-Lie Xiao
acd,
Ting Wang‡b,
Xing-Jie Zhanga,
Jun-Bo Gaocd,
Rui-Han Zhanga,
Xiao-Nian Lic,
Yong-Mei Wangcd,
Xiao-Li Li*a,
He-Yao Wang*b and
Wei-Lie Xiao *ac
*ac
aKey Laboratory of Medicinal Chemistry for Natural Resource of Ministry of Education, School of Chemical Science and Technology, State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Yunnan University, Kunming 650091, People's Republic of China. E-mail: lixiaoli@ynu.edu.cn; xiaoweilie@ynu.edu.cn; Fax: +86 871-665033214; Fax: +86 871-67357014; Tel: +86 871-665033214 Tel: +86 871-67357014
bState Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, People's Republic of China. E-mail: hywang@simm.ac.cn; Fax: +86 021-50807088; Tel: +86 021-50805785
cState Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650201, People's Republic of China
dUniversity of Chinese Academy of Sciences, Beijing 100049, People's Republic of China
First published on 9th February 2018
Abstract
Investigation of the leaves and stems of Premna szemaoensis resulted in the isolation of twelve new abietane diterpenoids, szemaoenoids A–L (1–12), together with four known abietane diterpenoids (13–16). The structures involved two rearranged-abietane skeletons: 17(15 → 16)-abeo-abietane (7, 10–12, 14 and 15) and 17(15 → 16),18(4 → 3)-diabeo-abietane (1–6, 13 and 16). The structures of the new compounds were established mainly by analyzing NMR and HRESIMS data. The absolute configurations of 1, 3 and 10 were confirmed by single crystal X-ray diffraction analysis. In bioactivity assays, compounds 11, 12, 14 and 15 were active against two human colon cancer cell lines (HCT-116 and HT-29) with IC50 values ranging from 8.8 to 34.3 μM, and compounds 10, 13 and 14 exhibited effective free radical scavenging activity with IC50 values ranging from 35.6 to 41.5 μM by DPPH experiment.
Introduction
The genus Premna (family: Verbenaceae) comprises approximately 200 species, which are mainly distributed in the tropical zone of Asia and Africa.1 There are about 44 species and 5 varieties grown in the south of China, especially in Southwest China. The dried aerial parts of some Premna species have been used in traditional folk medicine for the treatment of pyogenic infections, trauma, fracture, dysentery, haemorrhoids, and rheumatic arthritis.2 Previous phytochemical investigations of Premna have indicated the presence of diterpenoids,3 flavonoids,3–5 iridoid glycosides,4–7 xanthones,8 phenylethanoid glycosides,9 triterpenoids,10,11 and lignins.12 Their pharmacological effects, including neuroprotective,13 analgesic,14,15 antioxidative, cytotoxic,16,17 anti-inflammatory,18 and α-glucosidase inhibition,19 have been reported for crude extracts and pure compounds from Premna plants.Premna szemaoensis Pei, locally called “simao dofu chai”, is mainly distributed in the south of Yunnan province of China.20 This plant has drawn the attention of local farmers and has been cultivated to be an important commercial crop due to its various applications. Its fresh leaves can be rubbed and squeezed in water to yield a mucilaginous juice, which was used to prepare a food named “green tofu” by local people through addition of materials containing Ca2+. In addition, the local villagers also used the leaves of this plant to cure injuries and fracture.20 However, the phytochemical investigation of this species was extremely rare, except a small number of flavonoids. In this investigation, we firstly afforded 12 new abietane diterpenoids (1–12) and four known abietane diterpenoids (13–16) from the aerial parts of P. szemaoensis. Most of the diterpenoids were with rearranged-abietane skeleton: 17(15 → 16)-abeo-abietane framework or 17(15 → 16),18(4 → 3)-diabeo-abietane framework, which were mainly isolated from the plants of genus Clerodendron and reported bioactivities including cytotoxic, angiotensin converting enzyme (ACE) inhibitory, antiviral actives.21–24 Herein, we describe the isolation and structural elucidation of these diterpenoids and the biological activities of selected compounds.
Results and discussion
The aerial parts of P. szemaoensis were extracted three times with 70% acetone aqueous. After recycling acetone, the rest of portion was partitioned by liquid–liquid extraction between n-butanol and H2O. The n-butanol-soluble portion was repeatedly subjected to silica gel, Sephadex LH-20, and RP-C18 gel column chromatography (CC) and semi-preparative HPLC to afford 16 abietane diterpenoids, including 12 new compounds. The structures and stereochemistry of these isolates were elucidated mainly using spectroscopic analysis, X-ray diffraction analysis, and compared to data in the literature. Ultimately, the new compounds were named as szemaoenoids A–L (1–12), and known compounds were identified as teuvincenone F (13),25, (16R)-12,16-epoxy-11,14,17-trihydroxy-17(15 → 16)-abeo-8,11,13-abieta-triene-7-one (villosin B) (14),26,27 12,16-epoxy-11,14,17-trihydroxy-6-methoxy-17(15 → 16)-abeo-5,8,11,13-abietatetraene-7-one (15),28 11,16-dihydroxy-12-O-β-D-glucopyranosyl-17(15 → 16),18(4 → 3)-diabeo-4-carboxy-3,8,11,13-abietatetraene-7-one (16),21 respectively.Compound 1 was isolated as an optically active, white monoclinic crystals (MeOH); [α]25D +36.3 (c 0.10, MeOH). Its HRESIMS data showed a sodium adduct ion [M + Na]+ at m/z 531.2205 (calcd. for C26H36NaO10, 531.2206), which together with 13C NMR (Table 2) and DEPT data were consistent with a molecular formula of C26H36O10, representing nine indices of hydrogen deficiency. The 1H NMR spectrum of 1 (Table 1) showed one doublet and two singlets for methyl groups at δH 1.12 (d, J = 6.2), 1.41 (s) and 1.20 (s), respectively; three olefinic proton signals at δH 7.46 (s), 5.20 (s), and 4.74 (s), and a double peak at δH 4.57 (d, J = 7.9). The 13C NMR and DEPT spectra of 1 (Table 2) showed signals of 20 carbons of an aglycon, attributable to a ketone group at δC 200.7, three methyls (two tertiary), five methylenes (one olefinic), three methines (one olefinic, one oxygenated), and eight quaternary carbons (six olefinic, one oxygenated), along with signals for a hexose unit. These data suggested that 1 is a diterpene glycoside and in accordance with the characteristics of a 17(15 → 16),18(4 → 3)-diabeo-8,11,13-abietatriene.
| Position | 1a | 2a | 3a | 4a | 5a | 6a | 7a | 8a | 9a | 10b | 11b | 12b |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a In CD3OD solution.b In acetone-d6 solution. | ||||||||||||
| 1α | 1.77 (overlap) | 1.51 (td, 12.7, 6.5) | 1.53 (td, 12.6, 6.0) | 1.68 (td, 12.6, 6.1) | 2.54 (d, 16.9) | 1.90 (td, 13.7, 4.1) | 1.18 (overlap) | 1.43 (overlap) | 1.75 (m) | 1.47 (br t, 14.2) | 1.55 (br t, 14.0) | 1.43 (overlap) |
| 1β | 3.16 (br d, 13.1) | 3.52 (overlap) | 3.52 (overlap) | 3.56 (overlap) | 4.29 (d, 16.9) | 3.22 (overlap) | 3.38 (d, 12.9) | 3.45 (overlap) | 1.87 (overlap) | 3.46 (br d, 13.7) | 3.55 (br d, 13.6) | 2.57 (br d, 17.0) |
| 2α | 1.74 (overlap) | 2.10 (dd, 18.5, 6.1) | 2.09 (dd, 18.5, 6.0) | 2.08 (dd, 18.2, 5.5) | 1.74 (overlap) | 1.46 (overlap) | 1.75 (overlap) | 1.55 (qd, 12.4, 5.1) | 1.74 (overlap) | 1.73 (m) | 1.55 (m) | |
| 2β | 1.74 (overlap) | 2.27 (m) | 2.28 (m) | 2.32 (m) | 1.74 (overlap) | 2.12 (m) | 1.81 (dd, 26.0, 12.8) | 1.90 (overlap) | 1.74 (overlap) | 1.79 (overlap) | 1.79 (overlap) | |
| 3α | 1.00 (td, 13.1, 3.4) | 3.28 (overlap) | 3.37 (overlap) | 3.27 (overlap) | 3.30 (dd, 11.5, 5.3) | 1.33 (td, 13.5, 3.6) | ||||||
| 3β | 2.25 (br d, 13.1) | 1.50 (overap) | ||||||||||
| 5 | 3.32 (overlap) | 2.94 (br d, 15.4) | 2.90 (overlap) | 2.88 (br d, 14.5) | 3.28 (overlap) | 1.81 (br d, 14.2) | 1.73 (overlap) | 1.29 (overlap) | 1.71 (overlap) | 1.80 (dd, 14.5, 1.7) | 1.84 (dd, 14.7, 2.1) | |
| 6α | 2.37 (dd, 16.0, 2.4) | 2.99 (dd, 16.8, 2.9) | 2.69 (br t, 12.0) | 3.04 (dd, 17.3, 3.2) | 6.80 (s) | 2.41 (dd, 16.6, 2.4) | 2.89 (br d, 16.9) | 2.66 (br t, 15.5) | 1.23 (overlap) | 2.49 (d, 17.0) | 2.57 (br d, 17.0) | 2.57 (d, 17.0) |
| 6β | 2.69 (overlap) | 2.57 (t, 15.9) | 3.01 (dd, 17.0, 2.7) | 2.70 (dd, 17.3, 15.2) | 2.85 (t, 15.7) | 3.27 (overlap) | 2.58 (d, 16.7) | 3.40 (overlap) | 2.70 (overlap) | 2.78 (overlap) | 2.76 (t, 15.9) | |
| 7α | 2.71 (overlap) | |||||||||||
| 7β | 2.76 (overlap) | |||||||||||
| 14 | 7.46 (s) | 7.46 (s) | 7.39 (s) | 7.44 (s) | 6.35 (s) | |||||||
| 15α | 2.71 (overlap) | 2.70 (dd, 13.3, 6.6) | 2.87 (overlap) | 6.55 (s) | 6.62 (d, 0.9) | 6.55 (s) | 2.66 (dd, 13.3, 6.9) | 3.77 (m) | 3.68 (overlap) | 2.73 (overlap) | 6.55 (s) | 6.77 (s) |
| 15β | 3.20 (dd, 13.4, 6.6) | 3.18 (dd, 13.3, 6.6) | 3.17 (dd, 13.0, 6.8) | 3.20 (m) | 3.26 (overlap) | |||||||
| 16α | 4.10 (ddd, 6.2, 6.4, 6.6) | 4.11 (ddd, 6.2, 6.6, 6.7) | 4.15 (dd, 12.8, 6.4) | 4.11 (ddd, 6.6, 6.4, 6.2) | 3.50 (overlap) | 3.42 (overlap) | 5.09 (m) | |||||
| 16β | 3.61 (dd, 10.5, 6.6) | 3.58 (dd, 10.5, 6.3) | ||||||||||
| 17 | 1.12 (d, 6.2) | 1.12 (d, 6.2) | 1.12 (d, 6.3) | 2.46 (s) | 2.49 (s) | 2.46 (s) | 1.11 (d, 6.2) | 1.15 (d, 6.9) | 1.11 (d, 6.9) | 1.43 (d, 6.2) | 2.40 (s) | 4.64 (d, 5.9) |
| 18α | 1.41 (s) | 1.26 (s) | 1.24 (s) | 1.68 (s) | 2.06 (s) | 1.40 (s) | 1.03 (s) | 3.45 (overlap) | 1.05 (s) | 1.07 (s) | 0.99 (s) | |
| 18β | 4.18 (d, 11.2) | |||||||||||
| 19α | 4.74 (s) | 4.07 (d, 11.7) | 4.07 (d, 11.9) | 4.09 (d, 12.0) | 4.61 (overlap) | 4.76 (s) | 1.18 (s) | 0.93 (s) | 1.26 (s) | 0.92 (s) | 0.94 (s) | 1.01 (s) |
| 19β | 5.20 (s) | 4.27 (d, 11.7) | 4.27 (d, 11.9) | 4.26 (d, 12.0) | 4.71 (overlap) | 5.20 (s) | ||||||
| 20 | 1.20 (s) | 1.78 (s) | 1.77 (s) | 1.36 (s) | 1.73 (s) | 1.32 (s) | 1.43 (s) | 1.40 (s) | 1.28 (s) | 1.39 (s) | 1.44 (s) | 1.47 (s) |
| 1′ | 4.57 (d, 7.9) | 4.57 (d, 7.9) | 4.65 (d, 8.0) | 5.41 (d, 7.2) | 5.90 (d, 7.3) | 5.59 (d, 7.4) | 4.55 (d, 7.9) | 4.46 (d, 7.9) | 4.37 (d, 7.8) | |||
| 2′ | 3.51 (t, 8.5) | 3.51 (t, 8.5) | 3.52 (m) | 3.49 (overlap) | 3.53 (overlap) | 3.47 (overlap) | 3.50 (t, 8.4) | 3.50 (overlap) | 3.45 (overlap) | |||
| 3′ | 3.29 (overlap) | 3.29 (overlap) | 3.27 (overlap) | 3.20 (m) | 3.34 (overlap) | 3.22 (m) | 3.29 (overlap) | 3.28 (overlap) | 3.25 (overlap) | |||
| 4′ | 3.44 (overlap) | 3.45 (overlap) | 3.44 (t, 9.1) | 3.33 (overlap) | 3.34 (overlap) | 3.29 (overlap) | 3.44 (overlap) | 3.27 (overlap) | 3.27 (overlap) | |||
| 5′ | 3.43 (overlap) | 3.42(overlap) | 3.42 (t, 9.1) | 3.49 (overlap) | 3.53 (overlap) | 3.47 (overlap) | 3.43 (overlap) | 3.41 (m) | 3.39 (overlap) | |||
| 6′α | ||||||||||||
| 6′β | 3.75 (dd, 12.0, 4.8) | 3.75 (dd, 12.0, 4.7) | 3.75 (dd, 12.0, 4.7) | 3.57 (overlap) | 3.70 (br, 11.9) | 3.54 (dd, 12.0, 5.7) | 3.75 (dd, 10.3, 4.7) | 3.66 (br d, 11.7) | 3.65 (overlap) | |||
| 3.84 (dd, 12.0, 1.8) | 3.85 (dd, 12.0, 1.9) | 3.84 (dd, 12.0, 1.6) | 3.73 (dd, 11.8, 2.0) | 3.54 (overlap) | 3.70 (dd, 12.0, 2.0) | 3.83 (br d, 10.3) | 3.89 (br d, 11.7) | 3.89 (d, 12.0) | ||||
| OH-3 | 3.60 (br s) | 3.63 (s) | ||||||||||
| OH-11 | 7.27 (s) | 8.16 (s) | 8.28 (s) | |||||||||
| OH-14 | 13.39 (s) | 13.8 (s) | 13.8 (s) | |||||||||
| OH-17 | 4.50 (s) | |||||||||||
| Position | 1a | 2a | 3a | 4a | 5a | 6a | 7a | 8a | 9a | 10b | 11b | 12b |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a In CD3OD solution.b In acetone-d6 solution. | ||||||||||||
| 1 | 31.9, CH2 | 32.6, CH2 | 32.7, CH2 | 33.7, CH2 | 47.9, CH2 | 33.4, CH2 | 37.4, CH2 | 35.8, CH2 | 29.2, CH2 | 35.6, CH2 | 36.1, CH2 | 37.6, CH2 |
| 2 | 37.8, CH2 | 31.1, CH2 | 31.2, CH2 | 31.2, CH2 | 201.1, C | 38.1, CH2 | 21.2, CH2 | 28.4, CH2 | 20.5, CH2 | 28.7, CH2 | 18.8, CH2 | 19.7, CH2 |
| 3 | 71.5, C | 133.8, C | 134.0, C | 133.9, C | 138.4, C | 71.3, C | 39.9, CH2 | 78.7, CH | 80.9, CH | 77.5, CH | 77.5, CH | 41.8, CH2 |
| 4 | 153.7, C | 129.6, C | 129.3, C | 129.4, C | 149.6, C | 153.6, C | 45.7, C | 40.2, C | 44.3, C | 39.8, C | 39.9, C | 34.0, C |
| 5 | 44.0, CH | 44.5, CH | 44.0, CH | 43.9, CH | 161.9, C | 43.7, CH | 53.6, CH | 51.4, CH | 55.0, CH | 50.4, CH | 50.4, CH | 50.9, CH |
| 6 | 38.6, CH2 | 38.0, CH2 | 38.3, CH2 | 38.5, CH2 | 124.9, CH | 38.7, CH2 | 39.2, CH2 | 36.2, CH2 | 34.4, CH2 | 35.6, CH2 | 36.0, CH2 | 36.2, CH2 |
| 7 | 200.7, C | 201.1, C | 207.1, C | 207.5, C | 191.8, C | 207.0, C | 203.2, C | 201.3, C | 35.6, CH2 | 205.2, C | 207.0, C | 207.0, C |
| 8 | 130.0, C | 130.2, C | 114.2, C | 112.1, C | 110.1, C | 111.9, C | 130.1, C | 130.2, C | 135.6, C | 110.7, C | 111.2, C | 111.5, C |
| 9 | 139.5, C | 139.7, C | 137.3, C | 139.9, C | 133.7, C | 139.1, C | 141.1, C | 140.3, C | 134.1, C | 140.6, C | 135.1, C | 135.6, C |
| 10 | 42.0, C | 39.1, C | 39.2, C | 39.9, C | 44.2, C | 42.7, C | 42.3, C | 41.4, C | 40.1, C | 41.2, C | 41.2, C | 41.6, C |
| 11 | 149.9, C | 149.7, C | 141.7, C | 133.7, C | 133.1, C | 134.0, C | 150.4, C | 149.3, C | 149.1, C | 132.7, C | 133.5, C | 133.4, C |
| 12 | 150.5, C | 150.5, C | 153.3, C | 153.4, C | 151.3, C | 153.0, C | 149.7, C | 150.0, C | 143.2, C | 156.3, C | 148.3, C | 152.1, C |
| 13 | 132.8, C | 132.7, C | 121.0, C | 119.8, C | 120.6, C | 119.9, C | 132.2, C | 138.2, C | 136.6, C | 111.7, C | 117.9, C | 117.3, C |
| 14 | 121.7, CH | 122.1, CH | 157.4, C | 156.8, C | 154.1, C | 156.7, C | 121.3, CH | 117.2, CH | 118.2, CH | 155.9, C | 153.6, C | 154.1, C |
| 15 | 40.9, CH2 | 40.9, CH2 | 33.6, CH2 | 101.5, CH | 101.2, CH | 101.4, CH | 41.0, CH2 | 35.1, CH | 34.9, CH | 34.5, CH2 | 101.6, CH | 102.5, CH |
| 16 | 68.3, CH | 68.3, CH | 68.3, CH | 156.5, C | 157.4, C | 155.7, C | 68.3, CH | 68.8, CH2 | 69.1, CH2 | 83.3, CH | 155.9, C | 158.8, C |
| 17 | 22.8, CH3 | 22.9, CH3 | 22.9, CH3 | 13.7, CH3 | 13.8, CH3 | 13.7, CH3 | 22.8, CH3 | 18.2, CH3 | 18.3, CH3 | 22.0, CH3 | 13.7, CH3 | 57.4, CH2 |
| 18 | 27.8, CH3 | 19.0, CH3 | 18.9, CH3 | 18.9, CH3 | 11.4, CH3 | 27.8, CH3 | 185.1, C | 28.5, CH3 | 65.3, CH2 | 28.7, CH3 | 28.4, CH3 | 21.9, CH2 |
| 19 | 108.2, CH2 | 59.2, CH2 | 59.2, CH2 | 59.4, CH2 | 59.7, CH2 | 108.4, CH2 | 30.1, CH3 | 16.0, CH3 | 23.6, CH3 | 16.7, CH3 | 16.0, CH3 | 33.4, CH2 |
| 20 | 14.2, CH3 | 15.6, CH3 | 15.4, CH3 | 18.3, CH3 | 25.9, CH3 | 15.8, CH3 | 15.6, CH3 | 17.4, CH3 | 20.4, CH3 | 17.9, CH3 | 18.4, CH3 | 18.4, CH2 |
| 1′ | 107.6, CH | 107.6, CH | 107.1, CH | 101.5, CH | 101.9, CH | 102.4, CH | 107.6, CH | 107.4, CH | 107.8, CH | |||
| 2′ | 75.4, CH | 75.4, CH | 75.4, CH | 75.9, CH | 75.7, CH | 75.9, CH | 75.4, CH | 75.5, CH | 75.6, CH | |||
| 3′ | 78.6, CH | 78.6, CH | 78.7, CH | 78.3, CH | 78.6, CH | 78.4, CH | 78.6, CH | 79.1, CH | 79.0, CH | |||
| 4′ | 70.8, CH | 70.8, CH | 70.7, CH | 71.8, CH | 71.4, CH | 71.7, CH | 70.8, CH | 71.4, CH | 71.5, CH | |||
| 5′ | 77.9, CH | 77.9, CH | 78.0, CH | 78.1, CH | 78.4, CH | 78.2, CH | 77.9, CH | 77.9, CH | 77.9, CH | |||
| 6′ | 62.1, CH2 | 62.1, CH2 | 62.1, CH2 | 62.6, CH2 | 62.3, CH2 | 62.6, CH2 | 62.1, CH2 | 63.0, CH2 | 63.0, CH2 | |||
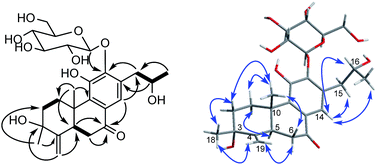
The proton and protonated carbon NMR signals of 1 were assigned unambiguously by the HSQC experiment. Partial structures and the whole connection were deduced from correlations observed in the 1H–1H COSY and HMBC spectra (Fig. 1). The HMBC correlations of CH3-18 (δH 1.41) to C-2 (δC 37.8), C-3 (δC 71.5, an oxygenated quaternary carbon), and C-4 (δC 153.7, an olefinic quaternary carbon) and of H2-19 (δH 4.74, 5.20) to C-3/C-4/C-5 (δC 44.0) suggested the presence of a 18(4 → 3)-abeo-abietane structural unit, established the location of an OH group at C-3 and an exocyclic double bond at C-4 and C-19. The ketone group was placed at C-7 from correlations of H-5 (δH 3.32), H2-6 (δH 2.37, 2.69), H-14 (δH 7.46, one aromatic proton) to a ketone-group carbon (δC 200.7). 1H–1H COSY correlations from H2-15 (δH 2.71, 3.20) through H-16 (δH 4.10, an oxygenated proton) to CH3-17 (δH 1.12), in combination with HMBC correlations from H2-15 to C-12 (δC 150.5) and C-14 (δC 121.7), from CH3-17 to C-16 (δC 68.3) and C-15 (δC 40.9), were suggestive of 17(15 → 16)-abeo-abietane moiety in this structure, and an hydroxyl group at C-16. The location of the sugar moieties was determined by the HMBC correlation of the anomeric proton H-1′ (δH 4.57) to C-12. In addition, 1H–1H COSY correlations: H2-1/H2-2, H-5/H2-6, and HMBC correlations of CH3-20 to C-1, C-9 and C-10, of H-5 to C-4 and C-10 were also key interactions to support this gross structure.
The β-D-configurational glucose unit was confirmed through coupling constant of H-1′ (d, J = 7.9), acid hydrolysis and comparison with reference standard. In the case of abietane diterpenoid derivatives, relative configuration of OH at C-3 could be assigned for the α- and β-epimers, by NOE effect between H-3/H-5 or H-3/CH3-20 respectively. However, the interaction of CH3-18/H-5 or CH3-18/CH3-20 was not observed in 1. Its ROESY spectrum provided interactions of CH3-18/H-2 and CH3-20/H-2 (Fig. 1), but α- and β-H connected to C-2 displayed an overlapped signal at 1.74 in the 1H NMR, which was not enough evidence to assign the C-3 configuration. Fortunately, appropriate crystals have been obtained, and the absolute configuration was assigned as 3R, 5R, 10S, 16R on the basis of the Flack parameter [0.17(14)] and Hooft parameter [0.10(6)] for 1225 Bijvoet pairs obtained by low-temperature [100(2) K] Cu Kα radiation X-ray crystallography (Fig. 2).29,30 Therefore, the structure of 1 was elucidated as (3R,16R)-12-O-β-D-glucopyranosyl-3,11,16-trihydroxy-17(15 → 16),18(4 → 3)-diabeo-4(19),8(9),11(12),13(14)-abietatetraene-7-one, named szemaoenoid A.
 | ||
| Fig. 2 ORTEP plot for the molecular structure of 1 drawn with 30% probability displacement ellipsoids. | ||
Compound 2 was isolated as a white amorphous powder with [α]25D −8.8 (c 0.14, MeOH). The HRESIMS gave a quasi-molecular ion at m/z 531.2203 [M + Na]+ (calcd. for C26H36NaO10, 531.2206). Thus, in conjunction with 13C NMR and DEPT data, the molecular formula was established as C26H36O10, representing nine indices of hydrogen deficiency. The 1H and 13C NMR data of 2 (Tables 1 and 2) were similar to those of compound 1, with the differences being the presence of an oxygenated methylene [δH 4.07 (d, J = 11.7), 4.27 (d, J = 11.7), δC 59.2] and two olefinic quaternary carbons (δC 133.8, δC 129.6) in 2 vs. an oxygenated quaternary carbon and an exocyclic double bond group in 1. Through HMBC experiment, observed correlations from H2-19 (δH 4.07, 4.27) to C-3/C-4/C-5, from CH3-18 (δH 1.26) to C-2/C-3/C-4 indicated that an OH group at C-19 and a double bond at C-3 and C-4. There was another hydroxyl group at C-16 (assigned the configuration as R) via comparing chemical shift and coupling constant of 2 with 1 [δH-16 4.11 (ddd, J = 6.2, 6.6, 6.7), δC-16 68.3 for 2; δH-16 4.10 (ddd, J = 6.2, 6.4, 6.6), δC-16 68.3 for 1], and the connecting correlations of 1H–1H COSY and HMBC data.
So far, the vast majority of natural abietane-type diterpenes from plants share the same carbon skeleton, with a trans-fused system of two six-membered rings A and B, a β-oriented methyl at C-10 and an α-oriented proton at C-5.31 From biogenetic considerations, 2 was inferred as possessing an identical absolute configuration to 1. Thus, the structure of 2 was established as (16R)-12-O-β-D-glucopyranosyl-11,16,19-trihydroxy-17(15 → 16),18(4 → 3)-diabeo-3(4),8(9),11(12),13(14)-abietatetraene-7-one, named szemaoenoid B.
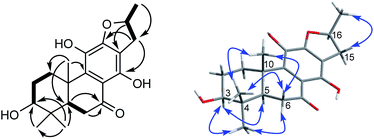
Compound 3 was obtained as white monoclinic crystals (MeOH). Its molecular formula assigned was determined to be C26H36O11 based on the negative HRESIMS (m/z 523.2177 [M − H]−). The NMR data for this compound were highly close to those of 2 (Tables 1 and 2), except for presence of one olefinic quaternary carbon (δC 157.4) in 3, correspondingly absence of an olefinic methine (δH 7.46, δC 122.1) vs. 2. In the HMBC spectrum, the correlations of H2-15 (δH 2.87, 3.17) to C-12 (δC 153.3), C-13 (δC 121.0), C-14 (δC 157.4) indicated that an OH group was at C-14, which made the chemical shifts of aromatic fields and ketone group (δC 207.1) obviously change in 13C NMR spectrum vs. 2. Ultimately, the absolute configuration was confirmed by single crystal X-ray diffraction analysis, which assigned C-16 as R configuration (Fig. 3). Therefore, the structure of 3 was determined as (16R)-12-O-β-D-glucopyranosyl-11,14,16,19-tetrahydroxy-17(15 → 16),18(4 → 3)-diabeo-3(4),8(9),11(12),13(14)-abietatetraene-7-one, named szemaoenoid C.
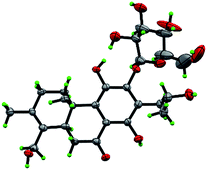 | ||
| Fig. 3 ORTEP plot for the molecular structure of 3 drawn with 30% probability displacement ellipsoids. | ||
Compound 4, a yellowish amorphous powder, had a molecular formula of C26H32O10 according to HRESIMS (m/z 527.1888 [M + Na]+). The 1H NMR and 13C NMR data were closely related to those of 3 (Tables 1 and 2). The differences were emergence of an olefinic double bond: one methine at C-15 (δH 6.55, s; δC 101.5), one quaternary carbon at C-16 (δC 156.5). The HMBC correlations from CH3-17 (δH 2.46, s) to C-15/C-16 and from H-15 to C-12 (δC 153.4)/C-13 (δC 119.8)/C-14 (δC 156.8) powerfully confirmed the locations of the double bond between C-15 and C-16, and an OH group at C-16. In consideration of its molecular formula as C26H32O10, and chemical stability, this structure must be absence of one H2O, and linked from C-12 to C-16 through a vinyl ether bond, likewise the known compound 13 with a furan ring. The sugar moieties were determined to be located at C-11, by the HMBC correlation of the anomeric proton H-1′ (δH 5.41, d, 7.2) to C-11 (δC 133.7). Thus, compound 4 was established as 11-O-β-D-glucopyranosyl-12,16-epoxy-14,19-dihydroxy-17(15 → 16),18(4 → 3)-diabeo-3(4),8(9),11(12),13(14),15(16)-abietapentaene-7-one, named szemaoenoid D.
Compound 5 was obtained as a brown amorphous powder. Its molecular formula was determined to be C26H28O11 by HRESIMS data, indicating 12 degrees of unsaturation (two more than that of compound 4). The 1H and 13C NMR resonances of 5 closely resembled those of 4 (Tables 1 and 2), except for appearance of a ketone group at C-2 and a double bond at C-5/C-6. The HMBC correlations from H2-1 (δH 2.54, d, J = 16.9 and δH 4.29, d, J = 16.9) and CH3-18 (δH 2.06, s) to C-2 (δC 201.1) and from H-6 (δH 6.80, s) to C-4 (δC 149.6)/C-8 (δC 110.1)/C-10 (δC 44.2) demonstrated above inference. Therefore, the structure of 5 was elucidated as 11-O-β-D-glucopyranosyl-12,16-epoxy-14,19-dihydroxy-17(15 → 16),18(4 → 3)-diabeo-3(4),5(6),8(9),11(12),13(14),15(16)-abietahexaene-2,7-dione, named szemaoenoid E.
Compound 6 was isolated as a yellowish amorphous powder. The HRESIMS gave its molecular formula as C26H32O10 (being identical with 4). The NMR data of 6 (Tables 1 and 2) were similar to those of 4, except for an oxygenated quaternary carbon (δC 71.3) and an exocyclic double bond [δC 153.6, δC 108.4; δH 4.76, 5.20] in 6 (in accord with compound 1) vs. an oxygenated methylene and two olefinic quaternary carbons in 4. In the HMBC spectrum, the cross-connection signals of CH3-18 (δH 1.40) to C-2 (δC 38.1), C-3 (δC 71.3) and C-4 (δC 153.6), and of H2-19 [δH 4.76 (s), 5.20 (s)] to C-3, C-4 and C-5 (δC 43.7), supported the presence of an OH group at C-3 and a double bond at C-4/C-19. In consideration of identical NMR data and biogenesis, absolute configuration of C-3 in 6 was identical with 1 (assigned as R configuration). Consequently, compound 6 was established as (3R)-11-O-β-D-glucopyranosyl-12,16-epoxy-3,14-dihydroxy-17(15 → 16),18(4 → 3)-diabeo-4(19),8(9),11(12), 13(14),15(16)-abietapentaene-7-one, named szemaoenoid F.
Compound 7, a white amorphous powder, had a molecular formula of C26H36O11 on the basis of the HRESIMS. From the NMR data, it was similar to those of 2 (Tables 1 and 2), except for absence of two olefinic quaternary carbons (δC 133.8, 129.6) and an oxygenated methylene (δC 59.2) vs. 2, correspondingly presence of an obvious carboxyl (δC 185.1), a quaternary carbon (δC 45.7) and a methylene (δC 39.9) in 7. The HMBC correlations of CH3-19 (δH 1.18, s) to C-3 (δC 39.9)/C-4 (δC 45.7)/C-5 (δC 53.6) and the carboxyl C-18 (δC 185.1), of H-5(δH 1.81, br d, J = 14.2) to C-4/C-18/CH3-19 (δC 30.1), and the 1H–1H COSY correlations of H2-1/H2-2/H2-3, all indicated that CH3-19 and the carboxyl C-18 were both linked to C-4. CH3-19 was assigned as β-orientation from the NOE effect of CH3-19/CH3-20. In view of identical NMR data and biogenesis, absolute configuration of C-16 in 7 was also identical with 1 (assigned as R configuration). Therefore, the structure of 7 was determined as (16R)-12-O-β-D-glucopyranosyl-11,16-dihydroxy-17(15 → 16)-abeo-8(9),11(12),13(14)-abietatriene-7-one-18-acid, named szemaoenoid G.
Compound 8, a white amorphous powder, exhibited a molecular formula of C26H38O10 according to HRESIMS. The 1H and 13C NMR spectra of 8 (Tables 1 and 2) were comparable with those of a known compound 12-O-D-glucopyranosyl-3,11,16-trihydroxy-8,11,13-abietatriene.21 The evident difference was appearance of carbonyl group (δC 201.3) in 8, but the known compound was absent of this group. The HMBC correlations of H-5 (δH 1.73)/H2-6 (δH 2.58, 2.66)/H-14 (δH 7.44) with C-7 (δC 201.3), of CH3-18 (δH 1.03)/CH3-19 (δH 0.93) with C-3 (δC 78.7), and 1H–1H COSY connections of H2-1/H2-2/H-3, suggested the carbonyl group at C-7 and an OH group at C-3, respectively. 1H–1H COSY correlations from CH3-17 (δH 1.15, d, J = 6.3) through H-15 (δH 3.77, m) to CH2-16 (δH 3.50, 3.61, an oxygenated methylene), and HMBC correlations from CH3-17 to C-13 (δC 138.2) and C-15 (δC 35.1), from H-15 to C-16 (δC 68.8), C-17 (δC 18.2), C-12 and C-14, were suggestive of an 1-hydroxy-isopropyl moiety linked to C-13 in this structure. Configurations of C-15 was established as S, by comparing the chemical shifts at C-13, C-15, C-16 with that of two known compounds (Table 3): (15S)-12-O-D-glucopy-ranosyl-3,11,16-trihydroxy-8,11,13-abie-tatriene21 and (15R)-cyrtophyllone B,32 whose structures were undoubtedly established by Mosher method and X-ray crystallography respectively. The β orientation of OH-3 was confirmed by the NOE effect of H-3/H-5. Therefore, this structure was established as (15S)-12-O-β-D-glucopyranosyl-3β,11,16-trihydroxy-8(9),11(12),13(14)-abietatriene-7-one, named szemaoenoid H.
| Position | δH | Position | δC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| a | b | c | d | a | b | c | d | ||
| a The a was (15S)-12-O-D-glucopyranosyl-3,11,16-trihydroxy-8,11,13-abietatriene;21 b was (15R)-cyrtophyllone B;32 c was szemaoenoid H (8); d was szemaoenoid I (9). | |||||||||
| H-15 | 3.69 | 3.15 | 3.77 | 3.68 | C-13 | 135.7 | 130 | 138.2 | 136.6 |
| H-16a | 3.42 | 3.78 | 3.50 | 3.42 | C-15 | 35.1 | 39.1 | 35.1 | 34.9 |
| H-16b | 3.58 | 3.94 | 3.61 | 3.58 | |||||
The HREIMS and NMR data of compound 9 were consistent with the molecular formula of C26H40O10. Its 1H and 13C NMR spectra (Tables 1 and 2) were almost identical with those of the known compound: 12-O-D-glucopyranosyl-3,11,16-trihydroxy-8,11,13-abietatriene.21 In fact, the only difference was an oxygenated methylene (δC 65.3) in 9 instead of a methyl group (δC 17.0) in the known structure. Moreover, the oxygenated methylene signals at δH 3.45 and 4.18 showed the HMBC correlations with the signals at C-3 (δC 80.9), C-4 (δC 44.3) and C-5 (δC 55.0), and the NOE effect with H-5/H-3, which evidently inferred that the OH was located at C-18, in combination with NOE effect of CH3-19/CH3-20. The β orientation of OH-3 and S configuration of C-15 were confirmed like that of compound 8. Consequently, compound 9 was assigned as (15S)-12-O-β-D-glucopyranosyl-3β,11,16,18-tetrahydroxy-8(9),11(12),13(14)-abietatriene, named szemaoenoid I.
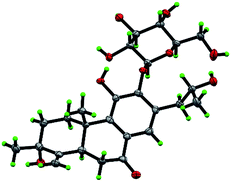
Compound 10, yellowish monoclinic crystals (MeOH), its chemical formula as C20H26O5 was determined by HRESIMS, indicating 8 degrees of unsaturation in the structure. From the 1H and 13C NMR spectrum (Tables 1 and 2), its data was extremely similar to known compound 14, with a 17(15 → 16)-abeo-abietane framework. Detailed HMBC and 1H–1H COSY NMR spectroscopic analyses (Fig. 4) suggested the appearance of an OH at C-3 [δH 3.27; δC 77.5] and the absence of a hydroxy group at C-17 in 10 by comparing with 14. A correlation observed in the ROESY spectrum (Fig. 4) between H-3 and H-5 (δH 1.71) indicated the β-orientation of OH-3. The absolute configuration of this compound was established as 3S, 5R, 10S, 16S by single crystal X-ray diffraction analysis (Fig. 5). Accordingly, the structure of 10 was assigned as (3S,16S)-12,16-epoxy-3,11,14-trihydroxy-17(15 → 16)-abeo-8(9),11(12),13(14)-abietatriene-7-one, named szemaoenoid J.
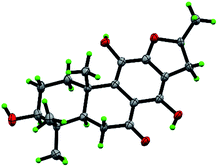 | ||
| Fig. 5 ORTEP plot for the molecular structure of 10 drawn with 30% probability displacement ellipsoids. | ||
Compound 11 and 12 were assigned to an identical molecular formula of C20H24O5 by HRESIMS data, with one more degree of unsaturation then 10. As seen from 1H NMR and 13C NMR spectrum (Tables 1 and 2), the structures of 11 and 12 were highly analogous to compound 10, except for the appearance of a double bond between C-15 and C-16 in 11 and 12, besides the presence of oxygenated methylene at C-17 [δH 4.64, d, J = 5.9; δC 57.4] and the absence of OH-3 in 12. The positions of all functional groups in their structures were assigned by correlations of HMBC and 1H–1H COSY spectrum. The β-orientation of OH-3 in 11 was confirmed by ROESY correlation of H-3/H-5 and comparing with NMR data with those of 10. Therefore, their structures were established as 12,16-epoxy-3β,11,14-trihydroxyl-17(15 → 16)-abeo-8(9),11(12),13(14),15(16)-abietatetraene-7-one (11), named szemaoenoid K; and 12,16-epoxy-11,14,17-trihydroxy-17(15 → 16)-abeo-8(9),11(12), 13(14),15(16)-abietatetraene-7-one (12), named szemaoenoid L.
In our cognition, many diterpenoides had been reported from plants of Premna genus,33–36 but this was also the first to report diterpenoids isolated from the Premna plants distributed in China, and the described rearranged-abietane skeletons were firstly isolated from Premna genus. Abietane diterpenoids represented a large group of secondary metabolites that have shown interesting biological activities.31 But the rearranged abietane diterpenoids with 17(15 → 16)-abeo-abietane or 17(15 → 16),18(4 → 3)-diabeo-abietane were not common in nature. Structurally, the rearranged diterpenoids contain abundant hydroxyl groups and aromatic carbons, as well as trans-fused rings A and B according to biosynthetic pathway. However, the stereocenter of C-16 replaced with OH had never been established previously. Within this work, we firstly employed X-ray crystallography to assign absolute configuration of C-16 for a small series of 16-hydroxy-17(15 → 16)-abeo-abietane diterpenoids, which might assist future unambiguous identification of structurally related compounds.
All the above compounds, except 3, 4 and 10, were evaluated for cytotoxicity in vitro against two human colon carcinoma cell lines (HCT-116 and HT-29). As a result (Table 4), compounds 11–15 showed antiproliferative activity against HCT-116 cell line, and compounds 11, 12 and 15 also exhibited potent cytotoxicity on HT-29 colon carcinoma cell line. Impressively, the antiproliferation activity of compound 14 was comparable (IC50 8.7 ± 1.4 μM) with positive control (sorafenib, IC50 8.4 ± 1.3 μM) (Table 4). Almost all the diterpene aglycones showed effective cytotoxicity, but none of the diterpene glucosides exhibited remarkable activity. The reason might be that the glucosides with strong chemical polarity failed to penetrate the liposoluble cell membrane.
| Compound | IC50 (mean ± SD, μM) | |
|---|---|---|
| HCT-116 | HT-29 | |
| a NA means that compounds exhibited indistinctive activity against tumor cells, and IC50 values were not evaluated. | ||
| 11 | 30.5 ± 4.7 | 21.3 ± 2.9 |
| 12 | 24.1 ± 4.5 | 34.6 ± 3.4 |
| 13 | 34.3 ± 2.8 | NA |
| 14 | 17.7 ± 4.6 | 8.7 ± 1.4 |
| 15 | 20.1 ± 3.0 | 14.2 ± 2.6 |
| Sorafenib | 8.5 ± 1.1 | 8.4 ± 1.3 |
As most of natural products possessing phenolic hydroxy exhibited antioxidant activity,37–39 some selective compounds of the diterpenosides were executed free radical scavenging activity assay in the DPPH experiment (Table 5). Among the tested compounds, diterpene aglycones 13, 14 showed strong free radical scavenging activity with IC50 values of 41.5 ± 17.0 and 39.9 ± 12.9 μM respectively, and compound 10 was especially the strongest activity with IC50 35.6 ± 9.8 μM (more potent than the positive control trolox and vitamin C). Compound 12 exhibited slightly weaken antioxidant activity with IC50 values of 74.9 ± 6.9 μM. None of the tested diterpene glucosides (1, 2, 3, 5, 9 and 16) showed potent free radical scavenging activity. Concerning the structure–activity relationship, a reasonable conclusion was reasoned out that the more the structure possessed phenolic hydroxyl groups, the more its scavenging activity was strong.
| Compound | IC50 (mean ± SD, μM) |
|---|---|
| a IC50, 50% inhibitory concentration. Mean activity of IC50 was exhibited by mean ± standard deviation, n ≥ 3. | |
| 1 | >200 |
| 2 | >200 |
| 3 | >200 |
| 5 | 149.5 ± 51.1 |
| 9 | >200 |
| 10 | 35.6 ± 9.8 |
| 12 | 74.9 ± 6.9 |
| 13 | 41.5 ± 17.0 |
| 14 | 39.9 ± 12.9 |
| 16 | >200 |
| Trolox | 36.3 ± 5.6 |
| Ascorbic acid (vitamin C) | 39.1 ± 6.7 |
Experimental section
General experimental procedures
X-ray data were collected using a Bruker APEX DUO instrument. Optical rotations were measured with Horiba SEPA-300 and JASCO P-1020 polarimeters. UV spectra were recorded on a Shimadzu UV-2401A spectrophotometer. IR spectra were obtained on a Tenor 27 spectrophotometer with KBr pellets. One-dimensional (1D) and two-dimensional (2D) NMR spectra were recorded on Bruker DRX-600 spectrometers with TMS as the internal standard. Chemical shifts (δ) were expressed in parts per million with reference to the solvent signals. HRESIMS was performed on an Agilent G6230 TOF MS. Semi-preparative HPLC was performed on an Agilent 1260 liquid chromatograph with a Zorbax SB-C18 (9.4 mm × 25 cm) column. Column chromatography (CC) was performed on silica gel (100–200 mesh and 200–300 mesh; Qingdao Marine Chemical Inc., Qingdao, People's Republic of China), Lichroprep RP-18 gel (40–63 μm, Merck, Darmstadt, Germany), MCI gel (75–150 μm, Mitsubishi Chemical Corporation, Tokyo, Japan), and Sephadex LH-20 (Pharmacia). Fractions were monitored by TLC, and spots were visualized by UV light (254 nm) and sprayed with 8% H2SO4 in ethanol, followed by heating.Plant materials
Aerial parts of Premna szemaoensis were collected in February 2012 from Puer city, Yunnan Province, People's Republic of China, and identified by Researcher Xi-Wen Li, Kunming Institute of Botany. A voucher specimen (XWL20140403) has been deposited in the Herbarium of the Kunming Institute of Botany, Chinese Academy of Sciences.Extraction and isolation
The air-dried and powdered aerial parts of P. szemaoensis (10 kg) were extracted with 70% aqueous acetone (40 L) four times (two days each time) at room temperature and then filtered. The filtrate was evaporated under reduced pressure at 40 °C and then partitioned between n-butanol and H2O. The n-butyl alcohol soluble portion (600 g) was subjected to silica gel CC (2.5 kg, 100–200 mesh), eluted with a CHCl3–Me2CO gradient system (1:0–0:1) that afforded fractions A–E. The fractions were then decolorized using MCI gel and eluted with 95% MeOH–H2O.Fraction B (33 g) was subjected to silica gel CC (200–300 mesh), eluted with a CHCl3–MeOH gradient (150:1–1:1), to yield fractions B1–B5. Fraction B1 was purified by repeated silica gel CC (petroleum ether–Me2CO gradient, 12:1–0:1) to yield compound 14 (10.2 mg). Fraction B3 was purified by Sephadex LH-20 (MeOH) to yield fractions B31–B34. Then compound 13 (75.0 mg) was crystallized from fraction B34, and compound 15 (8.4 mg) was isolated by HPLC (78% MeOH–H2O, Rt = 15.2 min). B4 was purified by RP-18 CC (MeOH–H2O gradient, 30–100%) to yield fractions B41–B45, then B43 was isolated by semi-preparative HPLC (72% MeOH–H2O, Rt = 13.5 min) to obtain compound 10 (10.1 mg). B5 was subjected to Sephadex LH-20 CC (MeOH) to give B51–B55, then compounds 11 (8.7 mg) and 12 (15.4 mg) were isolated from fraction B52 by semi-preparative HPLC (75% MeOH–H2O, Rt = 13.1 and 14.8 min, respectively).
Fraction C (120 g) was separated by Sephadex LH-20 (MeOH) to give fractions C1–C5. Fraction C2 was subjected to repeated silica gel CC (200–300 mesh), eluted with CHCl3–MeOH (gradient system: 120:1–1:1) to yield fractions C21–C27. C23 was isolated by semi-preparative HPLC (42% MeOH–H2O, Rt = 15.1 and 13.2 min, respectively) to afford compounds 1 (8.5 mg) and 2 (11.7 mg). C25 was purified by HPLC (44% MeOH–H2O, Rt = 10.3, 15.3 and 14.5 min, respectively) to yield compounds 4 (5.2 mg), 5 (4.6 mg), and 6 (2.3 mg).
Fraction D (100 g) was subjected to RP-18 CC (MeOH–H2O, 10–100%) to give fractions D1–D6. Compound 16 (20.1 mg) was crystallized from fractions D2. Fraction D4 was separated to by Sephadex LH-20 eluted with MeOH to give D41–D47, then D43 was isolated by semi-preparative HPLC (41% MeOH–H2O, Rt = 14.5 and 7.8 min, respectively) to yield compounds 3 (6.1 mg) and 7 (4.3 mg). Fraction D5 was separated to silica gel CC (200–300 mesh) eluted with CHCl3–MeOH (50:1–1:2) to yield D51–D56, then D54 was followed by HPLC (44% MeOH–H2O, Rt = 15.5 and 14.2 min, respectively) to afford compounds 8 (4.6 mg) and 9 (2.3 mg).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 213 (4.23), 269 (3.91), 318 (3.49) nm; IR (KBr) νmax 3441, 1632, 1049 cm−1; 1H and 13C NMR data, see Tables 1 and 2; positive-ion ESIMS m/z 531 [M + Na]+; positive-ion HRESIMS [M + Na]+ m/z 531.2205 (calcd for 531.2206).
ε) 213 (4.23), 269 (3.91), 318 (3.49) nm; IR (KBr) νmax 3441, 1632, 1049 cm−1; 1H and 13C NMR data, see Tables 1 and 2; positive-ion ESIMS m/z 531 [M + Na]+; positive-ion HRESIMS [M + Na]+ m/z 531.2205 (calcd for 531.2206).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 204 (4.17), 270 (3.96), 318 (3.32) nm; IR (KBr) νmax 3441, 1632, 1071 cm−1; 1H and 13C NMR data, see Tables 1 and 2; positive-ion ESIMS m/z 531 [M + Na]+; positive-ion HRESIMS [M + Na]+ m/z 531.2203 (calcd for 531.2206).
ε) 204 (4.17), 270 (3.96), 318 (3.32) nm; IR (KBr) νmax 3441, 1632, 1071 cm−1; 1H and 13C NMR data, see Tables 1 and 2; positive-ion ESIMS m/z 531 [M + Na]+; positive-ion HRESIMS [M + Na]+ m/z 531.2203 (calcd for 531.2206).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 203 (4.55), 239 (1.55), 283 (1.51) nm; IR (KBr) νmax 3414, 1616, 1424, 1072 cm−1; 1H and 13C NMR data, see Tables 1 and 2; negative-ion ESIMS m/z 523 [M − H]−; negative-ion HRESIMS [M − H]− m/z 523.2177 (calcd for 523.2179).
ε) 203 (4.55), 239 (1.55), 283 (1.51) nm; IR (KBr) νmax 3414, 1616, 1424, 1072 cm−1; 1H and 13C NMR data, see Tables 1 and 2; negative-ion ESIMS m/z 523 [M − H]−; negative-ion HRESIMS [M − H]− m/z 523.2177 (calcd for 523.2179).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 204 (4.47), 239(4.41), 254 (4.33), 355 (3.61) nm; IR (KBr) νmax 3425, 2924, 1631, 1384, 1069, 586 cm−1; 1H and 13C NMR data, see Tables 1 and 2; positive-ion ESIMS m/z 527 [M + Na]+; positive-ion HRESIMS [M + Na]+ m/z 527.1888 (calcd for 527.1893).
ε) 204 (4.47), 239(4.41), 254 (4.33), 355 (3.61) nm; IR (KBr) νmax 3425, 2924, 1631, 1384, 1069, 586 cm−1; 1H and 13C NMR data, see Tables 1 and 2; positive-ion ESIMS m/z 527 [M + Na]+; positive-ion HRESIMS [M + Na]+ m/z 527.1888 (calcd for 527.1893).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 202 (4.13), 295 (4.06) nm; IR (KBr) νmax 3426, 1629, 1466, 1216, 580 cm−1; 1H and 13C NMR data, see Tables 1 and 2; positive-ion ESIMS m/z 539 [M + Na]+; positive-ion HRESIMS [M + Na]+ m/z 539.1519 (calcd for 539.1529).
ε) 202 (4.13), 295 (4.06) nm; IR (KBr) νmax 3426, 1629, 1466, 1216, 580 cm−1; 1H and 13C NMR data, see Tables 1 and 2; positive-ion ESIMS m/z 539 [M + Na]+; positive-ion HRESIMS [M + Na]+ m/z 539.1519 (calcd for 539.1529).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 202 (4.27), 240 (4.34), 254 (4.27), 356 (3.52) nm; IR (KBr) νmax 3422, 2924, 1635, 1357, 1201, 1077 cm−1; 1H and 13C NMR data, see Tables 1 and 2; negative-ion ESIMS m/z 503 [M − H]−; negative-ion HREIMS [M − H]− m/z 503.1912 (calcd for 503.1917).
ε) 202 (4.27), 240 (4.34), 254 (4.27), 356 (3.52) nm; IR (KBr) νmax 3422, 2924, 1635, 1357, 1201, 1077 cm−1; 1H and 13C NMR data, see Tables 1 and 2; negative-ion ESIMS m/z 503 [M − H]−; negative-ion HREIMS [M − H]− m/z 503.1912 (calcd for 503.1917).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 215 (4.25), 269 (3.84), 316 (3.44) nm; IR (KBr) νmax 3441, 1632, 1068 cm−1; 1H and 13C NMR data, see Tables 1 and 2; negative-ion ESIMS m/z 523 [M − H]−; negative-ion HRESIMS [M − H]− m/z 523.2176 (calcd for 523.2179).
ε) 215 (4.25), 269 (3.84), 316 (3.44) nm; IR (KBr) νmax 3441, 1632, 1068 cm−1; 1H and 13C NMR data, see Tables 1 and 2; negative-ion ESIMS m/z 523 [M − H]−; negative-ion HRESIMS [M − H]− m/z 523.2176 (calcd for 523.2179).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 215 (4.11), 269 (3.76), 316 (3.33) nm; IR (KBr) νmax 3428, 1632, 1069 cm−1; 1H and 13C NMR data, see Tables 1 and 2; negative-ion ESIMS m/z 509 [M − H]−; negative-ion HRESIMS [M − H]− m/z 509.2388 (calcd for 509.2387).
ε) 215 (4.11), 269 (3.76), 316 (3.33) nm; IR (KBr) νmax 3428, 1632, 1069 cm−1; 1H and 13C NMR data, see Tables 1 and 2; negative-ion ESIMS m/z 509 [M − H]−; negative-ion HRESIMS [M − H]− m/z 509.2388 (calcd for 509.2387).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 204 (4.65), 275 (3.27) nm; IR (KBr) νmax 3424, 1632, 1422, 1063, 596 cm−1; 1H and 13C NMR data, see Tables 1 and 2; negative-ion ESIMS m/z 511 [M − H]−; negative-ion HRESIMS [M − H]− m/z 511.2540 (calcd for 511.2543).
ε) 204 (4.65), 275 (3.27) nm; IR (KBr) νmax 3424, 1632, 1422, 1063, 596 cm−1; 1H and 13C NMR data, see Tables 1 and 2; negative-ion ESIMS m/z 511 [M − H]−; negative-ion HRESIMS [M − H]− m/z 511.2540 (calcd for 511.2543).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 203 (4.84), 212 (4.70), 298 (3.10), 353 (1.67) nm; IR (KBr) νmax 3427, 1632, 1462, 1351, 1015 cm−1; 1H and 13C NMR data, see Tables 1 and 2; negative-ion ESIMS m/z 345 [M − H]−; negative-ion HRESIMS [M − H]− m/z 345.1708 (calcd for 345.1702).
ε) 203 (4.84), 212 (4.70), 298 (3.10), 353 (1.67) nm; IR (KBr) νmax 3427, 1632, 1462, 1351, 1015 cm−1; 1H and 13C NMR data, see Tables 1 and 2; negative-ion ESIMS m/z 345 [M − H]−; negative-ion HRESIMS [M − H]− m/z 345.1708 (calcd for 345.1702).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 202 (4.23), 237 (4.25), 258 (4.18), 369 (3.52) nm; IR (KBr) νmax 3441, 1631, 1458, 1372, 1277, 1179, 1069, 602 cm−1; 1H and 13C NMR data, see Tables 1 and 2; negative-ion ESIMS m/z 343 [M − H]−; negative-ion HRESIMS [M − H]− m/z 343.1551 (calcd for 343.1546).
ε) 202 (4.23), 237 (4.25), 258 (4.18), 369 (3.52) nm; IR (KBr) νmax 3441, 1631, 1458, 1372, 1277, 1179, 1069, 602 cm−1; 1H and 13C NMR data, see Tables 1 and 2; negative-ion ESIMS m/z 343 [M − H]−; negative-ion HRESIMS [M − H]− m/z 343.1551 (calcd for 343.1546).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 202 (3.99), 237 (4.06), 260 (4.02), 366 (3.36) nm; IR (KBr) νmax 3429, 2926, 1631, 1456, 1370 cm−1; 1H and 13C NMR data, see Tables 1 and 2; negative-ion ESIMS m/z 343 [M − H]−; negative-ion HRESIMS [M − H]− m/z 343.1548 (calcd for 343.1546).
ε) 202 (3.99), 237 (4.06), 260 (4.02), 366 (3.36) nm; IR (KBr) νmax 3429, 2926, 1631, 1456, 1370 cm−1; 1H and 13C NMR data, see Tables 1 and 2; negative-ion ESIMS m/z 343 [M − H]−; negative-ion HRESIMS [M − H]− m/z 343.1548 (calcd for 343.1546).X-ray crystal structure analysis
Crystals of 1, 3 and 10 were obtained in MeOH, respectively. Intensity data were collected at 100 K on a Bruker APEX DUO diffractometer equipped with an APEX II CCD using Cu Kα radiation. Cell refinement and data reduction were performed with Bruker SAINT. The structures were solved by direct methods using SHELXS-97.40 Refinements were performed with SHELXL-97 and SHELXL-2014 using full-matrix least-squares, with anisotropic displacement parameters for all the nonhydrogen atoms. The H-atoms were placed in calculated positions and refined using a riding model. Molecular graphics were computed with PLATON.41 Crystallographic data (excluding structure factor tables) for the structures reported have been deposited with the Cambridge Crystallographic Data Center as supplementary publications no. CCDC 1554050 for 1, CCDC 1554052 for 3, and CCDC 1554051 for 10.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 232 reflections measured, 3499 independent reflections (Rint = 0.0328). The final R1 values were 0.0300 (I > 2σ(I)). The final wR(F2) values were 0.0884 (I > 2σ(I)). The final R1 values were 0.0300 (all data). The final wR(F2) values were 0.0885 (all data). The goodness of fit on F2 was 1.113. Flack parameter = 0.17(14).29 The Hooft parameter is 0.10(6) for 1225 Bijvoet pairs.30
232 reflections measured, 3499 independent reflections (Rint = 0.0328). The final R1 values were 0.0300 (I > 2σ(I)). The final wR(F2) values were 0.0884 (I > 2σ(I)). The final R1 values were 0.0300 (all data). The final wR(F2) values were 0.0885 (all data). The goodness of fit on F2 was 1.113. Flack parameter = 0.17(14).29 The Hooft parameter is 0.10(6) for 1225 Bijvoet pairs.30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 714.3(8) Å3, T = 100(2) K, space group P212121, Z = 4, μ(CuKα) = 0.728 mm−1, 112641 reflections measured, 23
714.3(8) Å3, T = 100(2) K, space group P212121, Z = 4, μ(CuKα) = 0.728 mm−1, 112641 reflections measured, 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 429 independent reflections (Rint = 0.0484). The final R1 values were 0.0744 (I > 2σ(I)). The final wR(F2) values were 0.2065 (I > 2σ(I)). The final R1 values were 0.0759 (all data). The final wR(F2) values were 0.2083 (all data). The goodness of fit on F2 was 1.044. Flack parameter = 0.11(3).42
429 independent reflections (Rint = 0.0484). The final R1 values were 0.0744 (I > 2σ(I)). The final wR(F2) values were 0.2065 (I > 2σ(I)). The final R1 values were 0.0759 (all data). The final wR(F2) values were 0.2083 (all data). The goodness of fit on F2 was 1.044. Flack parameter = 0.11(3).42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 384 reflections measured, 4801 independent reflections (Rint = 0.0536). The final R1 values were 0.0627 (I > 2σ(I)). The final wR(F2) values were 0.1682 (I > 2σ(I)). The final R1 values were 0.0701 (all data). The final wR(F2) values were 0.1743 (all data). The goodness of fit on F2 was 1.058. Flack parameter = 0.0(2).29 The Hooft parameter is 0.01(14) for 1718 Bijvoet pairs.30
384 reflections measured, 4801 independent reflections (Rint = 0.0536). The final R1 values were 0.0627 (I > 2σ(I)). The final wR(F2) values were 0.1682 (I > 2σ(I)). The final R1 values were 0.0701 (all data). The final wR(F2) values were 0.1743 (all data). The goodness of fit on F2 was 1.058. Flack parameter = 0.0(2).29 The Hooft parameter is 0.01(14) for 1718 Bijvoet pairs.30Acid hydrolysis of szemaoenoid A
Compound 1 (4 mg) was hydrolyzed with 2 M HCl/dioxane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4 mL) under reflux for 8 h, respectively. The reaction mixture was partitioned between H2O and CHCl3 (2 mL × 3). The aqueous layer was neutralized with 2 M NaOH and then dried to give a monosaccharide. A solution of the sugar in pyridine (2 mL) was added to L-cysteine methyl ester hydrochloride (about 1.0 mg) and kept at 60 °C for 1 h. Then trimethylsilylimidazole (about 1.0 mL) was added to the reaction mixture and kept at 60 °C for 30 min. The mixture was subjected to GC analysis, run on a Shimadzu GC-14C gas chromatograph equipped with an H2 flame ionization detector. The column was a 30 m × 0.32 mm i.d. 30QC2/AC-5 quartz capillary column with the following conditions: column temperature, 180–280 °C; programmed increase, 3 °C min−1; carrier gas, N2 (1 mL min−1); injector and detector temperature, 250 °C; injection volume, 4 μL; and split ratio, 1/50. The configuration of the sugar moiety was determined by comparing the retention time with the derivatives of the authentic samples. The retention times of D-/L-glucose were 21.115/21.565 min.43 The configuration of the sugar moiety from compound 1 was D-glucose (Rt = 21.117 min).
1, 4 mL) under reflux for 8 h, respectively. The reaction mixture was partitioned between H2O and CHCl3 (2 mL × 3). The aqueous layer was neutralized with 2 M NaOH and then dried to give a monosaccharide. A solution of the sugar in pyridine (2 mL) was added to L-cysteine methyl ester hydrochloride (about 1.0 mg) and kept at 60 °C for 1 h. Then trimethylsilylimidazole (about 1.0 mL) was added to the reaction mixture and kept at 60 °C for 30 min. The mixture was subjected to GC analysis, run on a Shimadzu GC-14C gas chromatograph equipped with an H2 flame ionization detector. The column was a 30 m × 0.32 mm i.d. 30QC2/AC-5 quartz capillary column with the following conditions: column temperature, 180–280 °C; programmed increase, 3 °C min−1; carrier gas, N2 (1 mL min−1); injector and detector temperature, 250 °C; injection volume, 4 μL; and split ratio, 1/50. The configuration of the sugar moiety was determined by comparing the retention time with the derivatives of the authentic samples. The retention times of D-/L-glucose were 21.115/21.565 min.43 The configuration of the sugar moiety from compound 1 was D-glucose (Rt = 21.117 min).
Cytotoxicity assay
Human colon adenocarcinoma cell lines, HCT-116 and HT-29 were obtained from the American Type Culture Collection (ATCC). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS in a 5% CO2 atmosphere. HCT-116 (3 × 103 per well) and HT-29 (6 × 103 per well) were seeded onto 96-well plates and allowed to grow for 24 h prior to treatment. Different concentrations of compounds were then added and further incubated for 3 days. Sorafenib (purity > 99%; Medchem Express) was used as positive control. The culture medium was replaced by fresh DMEM containing 0.5 mg mL−1 of MTT. After incubation for another 4 h, the medium was removed and the reduced formazan blue was solubilized by adding 100 μL DMSO to each well. The absorbance at 492 nm was measured using a microplate reader (Multiskan MK3, Thermo). The IC50 values were calculated from concentration–response curves using Graphpad Prism software.Antioxidant activity assay
Trolox (purity > 98%; Sigma) and vitamin C (Ascorbic acid, purity > 98%; Sigma) were used as positive control. A 0.1 mM solution of DPPH radical in ethanol was prepared, and 100 μL of this solution was mixed with 100 μL of sample solution. The mixture was incubated for 5 min in a dark room at room temperature. Scavenging capacity was read spectropho-tometrically by monitoring the decrease in absorbance at 517 nm. DPPH scavenging activity (%) = [1 − (S − B)/(C − B)] × 100%, where S, B and C are the absorbencies of the sample, the blank and the control, respectively.44Conclusions
In summary, we have firstly reported twelve new abietane diterpenoids (1–12) isolated from P. szemaoensis, together with four known compounds (13–16). Structurally, these compounds involved two rearranged-abietane skeletons: 17(15 → 16)-abeo-abietane and 17(15 → 16),18(4 → 3)-diabeo-abietane. Their structures with absolute configurations were characterized by a series of spectroscopic methods and X-ray diffraction. In bioactivity assays, compounds 11, 12, 14 and 15 were active against two human colon cancer cell lines (HCT-116 and HT-29) with IC50 values ranging from 8.8 to 34.3 μM, and compounds 10, 13 and 14 exhibited effective free radical scavenging activity with IC50 values ranging from 35.6 to 41.5 μM by DPPH experiment. In short, the current study adds to understanding of the chemical composition and biological effects of this plant prepared for green food and ethnodrugs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project was supported financially by the NSFC (81422046, 21762048 and U1702286), the State Key Laboratory of Drug Research (SIMM1705KF-05), and Program for Changjiang Scholars and Innovative Research Team in University (IRT_17R94).Notes and references
- J. W. Kadereit, The Families and Genera of Vascular Plants, Springer, Berlin, 2004 Search PubMed.
- J. Pei and S. L. Chen, Zhongguo Zhiwu Zhi, Science Press, Beijing, 1982 Search PubMed.
- S. Habtemariam, BMC Pharmacol., 2003, 3, 6 CrossRef PubMed.
- H. Sudo, T. Ide, H. Otsuka, E. Hirata, A. Takushi and Y. Takeda, Phytochemistry, 1997, 46, 1231–1236 CrossRef CAS.
- H. Sudo, A. Takushi, E. Hirata, T. Ide, H. Otsuka and Y. Takeda, Phytochemistry, 1999, 52, 1495–1499 CrossRef CAS.
- N. T. B. Hang, P. T. Ky, C. Van Minh, N. X. Cuong, N. P. Thao and P. Van Kiem, Nat. Prod. Commun., 2008, 3, 1449–1452 CAS.
- V. Shukla, S. C. Phulara, D. Yadav, S. Tiwari, S. Kaur, M. M. Gupta, A. Nazir and R. Pandey, CNS Neurol. Disord.: Drug Targets, 2012, 11, 984–992 CAS.
- D. Y. Wang and S. Y. Xu, Nat. Prod. Res., 2003, 17, 75–77 CrossRef CAS PubMed.
- H. Sudo, A. Takushi, T. Ide, H. Otsuka, E. Hirata and Y. Takeda, Phytochemistry, 1997, 46, 1147–1150 CrossRef CAS.
- S. B. Lirio, A. P. G. Macabeo, E. M. Paragas, M. Knorn, P. Kohls, S. G. Franzblau, Y. H. Wang and M. A. M. Aguinaldo, J. Ethnopharmacol., 2014, 154, 471–474 CrossRef CAS PubMed.
- K. Y. Niu, L. Y. Wang, S. Z. Liu and W. M. Zhao, J. Asian Nat. Prod. Res., 2013, 15, 1–8 CrossRef CAS PubMed.
- D. Yadav, N. Masood, S. Luqman, P. Brindha and M. M. Gupta, Ind. Crops Prod., 2013, 41, 397–402 CrossRef CAS.
- J. Lin, B. Yang and J. H. Chen, J. Guangxi Med. Univ., 2001, 2, 207–208 Search PubMed.
- N. Narayanan, P. Thirugnanasambantham, S. Viswanathan, M. Kannappa Reddy, V. Vijayasekaran and E. Sukumar, Fitoterapia, 2000, 71, 147–153 CrossRef CAS PubMed.
- H. P. Pan, Y. Chen, S. H. Jing, M. Lu and G. H. Zhang, Chin. J. Hosp. Pharm., 2007, 07, 861–864 Search PubMed.
- G. Suresh, K. S. Babu, V. R. S. Rao, M. S. A. Rao, V. L. Nayak and S. Ramakrishna, Tetrahedron Lett., 2011, 52, 1273–1276 CrossRef CAS.
- M. Biradi and K. Hullatti, J. Pharmacol., 2015, 10, 205–208 Search PubMed.
- A. W. Salae, A. Rodjun, C. Karalai, C. Ponglimanont, S. Chantrapromma, A. Kanjana-Opas, S. Tewtrakul and H.-K. Fun, Tetrahedron, 2012, 68, 819–829 CrossRef CAS.
- S. R. Ayinampudi, R. Domala, R. Merugu, S. Bathula and M. R. Janaswamy, Fitoterapia, 2012, 83, 88–92 CrossRef CAS PubMed.
- Z. Y. Wu, Yunnan Zhiwu Zhi, Science Press, Yunnan province, 1977 Search PubMed.
- S. Liu, H. Zhu, S. Zhang, X. Zhang, Q. Yu and L. Xuan, J. Nat. Prod., 2008, 71, 755–759 CrossRef CAS PubMed.
- M. F. Xu, L. Q. Shen, K. W. Wang, Q. Z. Du and N. Wang, J. Asian Nat. Prod. Res., 2011, 13, 260–264 CrossRef CAS PubMed.
- W. X. Wang, J. J. Zhu, Y. K. Zou, Z. L. Hong, S. T. Liu, M. Li, Y. Huang, J. Xiong, Y. Zhao, G. X. Yang, G. Xia and J. F. Hu, Tetrahedron Lett., 2013, 54, 2549–2552 CrossRef CAS.
- Q. Liu, H. J. Hu, P. F. Li, Y. B. Yang, L. H. Wu, G. X. Chou and Z. T. Wang, Phytochemistry, 2014, 103, 196–202 CrossRef CAS PubMed.
- X. Tian, Z. Min, N. Xie, Y. Lei, Z. Tian, Q. Zheng, R. Xu, T. Tanaka, M. Iinuma and M. Mizuno, Chem. Pharm. Bull., 1993, 41, 1415–1417 CrossRef CAS.
- M. F. Xu, L. Q. Shen, K. W. Wang, Q. Z. Du and N. Wang, Nat. Prod. Commun., 2011, 6, 3–5 CAS.
- A. Ulubelen, G. Topcu and S. Olcal, Phytochemistry, 1994, 37, 1371–1375 CrossRef CAS.
- W. X. Wang, J. Xiong, Y. Tang, J. J. Zhu, M. Li, Y. Zhao, G. X. Yang, G. Xia and J. F. Hu, Phytochemistry, 2013, 89, 89–95 CrossRef CAS PubMed.
- H. D. Flack, Acta Crystallogr., 1983, 39, 876–881 CrossRef.
- R. W. W. Hooft, L. H. Straver and A. L. Spek, J. Appl. Crystallogr., 2008, 41, 96–103 CrossRef CAS PubMed.
- M. A. Gonzalez, Nat. Prod. Rep., 2015, 32, 684–704 RSC.
- T. Murata, Y. Ishikawa, E. Saruul, E. Selenge, K. Sasaki, K. Umehara, F. Yoshizaki and J. Batkhuu, Phytochemistry, 2016, 130, 152–158 CrossRef CAS PubMed.
- Y. W. Chin, W. P. Jones, Q. Mi, I. Rachman, S. Riswan, L. B. Kardono, H. B. Chai, N. R. Farnsworth, G. A. Cordell, S. M. Swanson, J. M. Cassady and A. D. Kinghorn, Phytochemistry, 2006, 67, 1243–1248 CrossRef CAS PubMed.
- A. Hymavathi, K. Suresh Babu, V. G. Naidu, S. Rama Krishna, P. V. Diwan and J. Madhusudana Rao, Bioorg. Med. Chem. Lett., 2009, 19, 5727–5731 CrossRef CAS PubMed.
- G. Suresh, K. S. Babu, M. S. A. Rao, V. R. S. Rao, P. A. Yadav, V. L. Nayak and S. Ramakrishna, Tetrahedron Lett., 2011, 52, 5016–5019 CrossRef CAS.
- A. W. Salae and N. Boonnak, Tetrahedron Lett., 2013, 54, 1356–1359 CrossRef CAS.
- T. Kallio, J. Kallio, M. Jaakkola, M. Maki, P. Kilpelainen and V. Virtanen, J. Agric. Food Chem., 2013, 61, 10720–10729 CrossRef CAS PubMed.
- H. M. Ge, W. H. Yang, J. Zhang and R. X. Tan, J. Agric. Food Chem., 2009, 57, 5756–5761 CrossRef CAS PubMed.
- Y. Yao, W. Sang, M. J. Zhou and G. X. Ren, J. Agric. Food Chem., 2010, 58, 770–774 CrossRef CAS PubMed.
- G. M. Sheldrick and T. R. Schneider, Methods Enzymol., 1997, 277, 319–343 CAS.
- A. L. Spek, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 9–18 CAS.
- H. D. Flack and G. Bernardinelli, Chirality, 2008, 20, 681 CrossRef CAS PubMed.
- P. Y. Liao, D. Wang, Y. J. Zhang and C. R. Yang, Dammara-ne-type glycosides from steamed notoginseng, J. Agric. Food Chem., 2008, 56, 1751–1756 CrossRef CAS PubMed.
- S. B. Wu, J. Wu, Z. Yin, J. Zhang, C. Long, E. J. Kennelly and S. Zheng, J. Agric. Food Chem., 2013, 61, 4035–4043 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed 1D and 2D NMR, HRESIMS, IR, UV and X-ray crystallographic data. CCDC 1554050–1554052. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra13309j |
| ‡ D.-B. Pu and T. Wang contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |