Open Access Article
Open Access ArticleAsymmetric synthesis of polysubstituted chiral chromans via an organocatalytic oxa-Michael-nitro-Michael domino reaction†‡
Cheng-Ke Tang ,
Kai-Xiang Feng,
Ai-Bao Xia
,
Kai-Xiang Feng,
Ai-Bao Xia *,
Chen Li,
Ya-Yun Zheng,
Zhen-Yuan Xu and
Dan-Qian Xu*
*,
Chen Li,
Ya-Yun Zheng,
Zhen-Yuan Xu and
Dan-Qian Xu*
Catalytic Hydrogenation Research Centre, State Key Laboratory Breeding Base of Green Chemistry-Synthesis Technology, Zhejiang University of Technology, Hangzhou, 310014, China. E-mail: xiaaibao@zjut.edu.cn; chrc@zjut.edu.cn
First published on 17th January 2018
Abstract
A catalytic asymmetric method for the synthesis of polysubstituted chromans via an oxa-Michael-nitro-Michael reaction has been developed. The squaramide-catalyzed domino reaction of 2-hydroxynitrostyrenes with trans-β-nitroolefins produced chiral chromans with excellent enantioselectivities (up to 99% ee), diastereoselectivities (up to >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr), and moderate to good yields (up to 82%).
1 dr), and moderate to good yields (up to 82%).
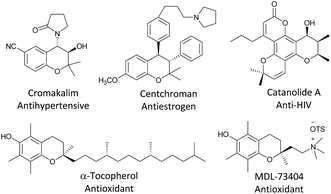
Chromans play an essential role in natural products and pharmaceutical molecules (Fig. 1).1 Given the biological relevance and diverse applications of this indispensable structural motif, numerous valuable methods for building chiral chromans have been developed.2 Rapid, direct, and highly atom-economical asymmetric strategies to construct optically active chromans are the first choice.
In the past several years, considerable effort has been made to build chiral chroman derivatives with multichiral centers via asymmetric domino reactions3 catalyzed by aminocatalysts,4 thiourea organocatalysts5 squaramide organocatalysts,6 and other organocatalysts.7 Among these approaches, the addition to nitroolefins is a simple but highly efficient route to obtain chiral chromans containing nitro-group, and the nitro group can often lead to changes in chemical and physical properties.4d–h,4j,5b–d In 2013, Zhu et al. reported the organocatalytic oxa-Michael-Michael cascade strategy for the construction of spiro [chroman/tetrahydroquinoline-3,3′-oxindole] scaffolds from 2-hydroxynitrostyrenes and N-Boc-protected methyleneindolinones using a squaramide-cinchona bifunctional catalyst.6a Peng et al. disclosed the highly efficient synthesis of polysubstituted 4-amino-3-nitrobenzopyrans from 2-hydroxyaryl-substituted α-amido sulfones and nitroolefins mediated by chiral squaramides.6b Furthermore, Yan and Wang's group developed the squaramide-catalyzed cascade reaction of 2-hydroxychalcones with β-CF3-nitroolefins to yield CF3-containing heterocyclic compounds with a quaternary stereocenter.6c Notably, a general strategy for the synthesis of chiral chromans bearing two nitro moieties was never reported, especially for 2-alkyl-substituted chromans. In 2003, an easy and efficient method for the synthesis of 3-nitrochromans via the reaction of 2-hydroxynitrostyrenes and trans-β-nitroolefins in the presence of DABCO was reported.8 Motivated by our previous work concerning the asymmetric synthesis of chromans,4i,9 this paper presents a highly efficient asymmetric method for the synthesis of polysubstituted chiral chroman derivatives, especially 2-alkyl-substituted chiral types, from 2-hydroxynitrostyrenes and trans-β-nitroolefins using a chiral bifunctional squaramide organocatalyst.10,11
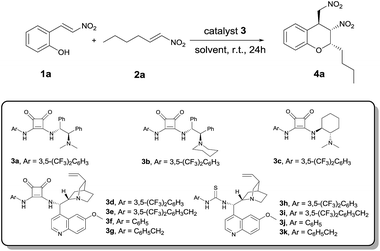
Given these considerations and previous work, the organocatalytic oxa-Michael-nitro-Michael reaction was performed with 2-hydroxynitrostyrene 1a and aliphatic trans-β-nitroolefin 2a as model substrates to examine the feasibility of our approach. Different parameters (Table 1), such as the catalyst and solvent, were studied. Efficient bifunctional squaramide organocatalysts possessing both H-bonding (thiourea, squaramide) and basic/nucleophilic moieties (tertiary amine), which act cooperatively, have been developed by several research groups for a broad range of enantioselective transformations.5c,d An appropriate basic/nucleophilic moiety is critical to this kind of reaction. Under this consideration, chiral 1,2-diphenylethylenediamine, cyclohexanediamine, and quinine were selected as scaffolds. After a preliminary study, the quinine scaffold revealed excellent stereoinduction for the asymmetric synthesis of the chiral chroman 4a when paired with the squaramide unit (Table 1, entries 1–4). Therefore, the influence of the quinine-derived catalysts should be studied systematically. Among the quinine-derived thiourea and squaramide organocatalysts 3d–3k, the squaramide catalyst 3e could promote the efficient formation of 4a with increased enantioselectivities (up to 92% ee) (entries 4–11). A series of solvents was tested, and the performance of dichloromethane was satisfactory to afford 4a in 78% yield, 92% ee, and >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (Table 1, entries 5 and 12–20). Consequently, the best conditions were found with 5 mol% of catalyst 3e loading in CH2Cl2 at room temperature.
1 dr (Table 1, entries 5 and 12–20). Consequently, the best conditions were found with 5 mol% of catalyst 3e loading in CH2Cl2 at room temperature.
| Entry | Catalyst | Solvent | Yieldb (%) | eec (%) | drc |
|---|---|---|---|---|---|
| a All the reactions were conducted with 1a (0.2 mmol), 2a (0.24 mmol), and solvent (2 mL) in the presence of 5 mol% organocatalyst 3 at room temperature with vigorous stirring for 24 h.b Isolated yield of 4a.c Determined by chiral HPLC using an OJ–H column.d N.D. = Not determined. | |||||
| 1 | 3a | CH2Cl2 | 47 | 5 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | 3b | CH2Cl2 | Trace | N.D.d | N.D. |
| 3 | 3c | CH2Cl2 | 42 | −39 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | 3d | CH2Cl2 | 73 | 80 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | 3e | CH2Cl2 | 78 | 92 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | 3f | CH2Cl2 | 65 | 43 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | 3g | CH2Cl2 | 71 | 85 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | 3h | CH2Cl2 | 69 | 75 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | 3i | CH2Cl2 | 75 | 79 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | 3j | CH2Cl2 | 67 | 58 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11 | 3k | CH2Cl2 | 68 | 69 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12 | 3e | CHCl3 | 65 | 93 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 13 | 3e | ClCH2CH2Cl | 77 | 88 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 14 | 3e | EtOAc | 71 | 57 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 15 | 3e | THF | 32 | 87 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 16 | 3e | 1,4-Dioxane | Trace | N.D. | N.D. |
| 17 | 3e | Et2O | Trace | N.D. | N.D. |
| 18 | 3e | CH3CN | 61 | 27 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 19 | 3e | Toluene | 77 | 81 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 20 | 3e | Cyclohexane | 49 | 38 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
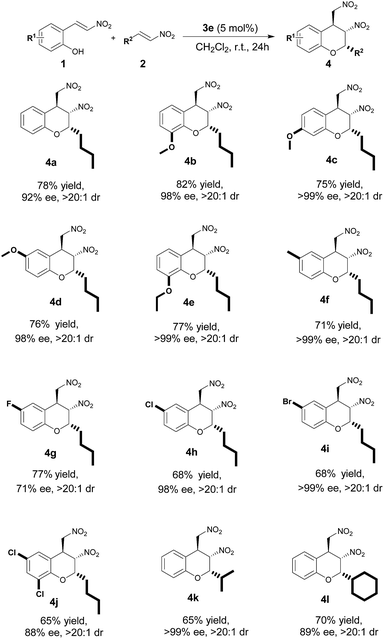
The reaction scope was determined under the optimal conditions. To investigate the versatility of the catalytic system, we first explored the universality of 1-nitro-1-hexene 2a in this squaramide-catalyzed domino reaction. The reaction was tolerant to a range of substituents, such as OMe, OEt, Me, F, Cl, and Br, on the aromatic ring of 2-hydroxynitrostyrenes. The results revealed that the current transformation was a general and efficient strategy for the asymmetric synthesis of n-Bu group-substituted chiral chromans in the 2-position with three contiguous stereogenic centers. More specifically, when the substrates bore electron-donating groups (R1 = OMe, OEt, Me) or electron-withdrawing groups (R1 = F, Cl, Br) at the 6-, 7-, and 8-positions of the benzene ring, the target products achieved 68–82% yields with excellent diastereoselectivities (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr) and enantioselectivities (71–99% ee). The targeted products with electron-donating groups resulted in high yields (Scheme 1, 4b, 4c, 4d, 4e, 4f versus 4g, 4h, 4i, 4j). In particular, other aliphatic nitroolefins substituted by branched chain aliphatic group (R2 = i-Pr) and cycloaliphatic group (R2 = cyclohexyl) were further explored, and these compounds delivered products with good asymmetric inductions (99% and 89% ee, >20
1 dr) and enantioselectivities (71–99% ee). The targeted products with electron-donating groups resulted in high yields (Scheme 1, 4b, 4c, 4d, 4e, 4f versus 4g, 4h, 4i, 4j). In particular, other aliphatic nitroolefins substituted by branched chain aliphatic group (R2 = i-Pr) and cycloaliphatic group (R2 = cyclohexyl) were further explored, and these compounds delivered products with good asymmetric inductions (99% and 89% ee, >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr).
1 dr).
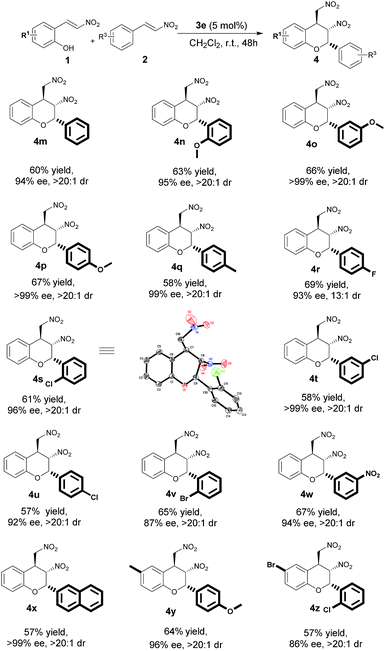
In-depth study of the current situation revealed that the enantioselectivities and diastereoselectivities of the chromans with 2-alkyl substituted groups were satisfactory. Therefore, further exploration of aromatic nitroolefins in the oxa-Michael-nitro-Michael domino reaction was necessary. Scheme 2 shows that nitroolefins incorporating electron-withdrawing groups and electron-donating groups at the aryl substituents in the 2-position could be successfully employed under these conditions, resulting in final adducts with high to excellent enantioselectivities (86–99% ee), high-to-excellent diastereoselectivities (up to >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr), and moderate yields (57–69%). In general, nitroolefins with OMe and Me as substituent R3 groups yielded products in excellent 95–99% ee (Scheme 2, 4n–4q, 4y), which were superior to nitroolefins incorporating electron-withdrawing groups (R3 = F, Cl, Br, NO2). However, product 4t was an interesting special case with 99% ee. The absolute configuration of product 4s was determined to be (2S, 3S, 4S) by single-crystal X-ray diffraction analysis (Scheme 3).12
1 dr), and moderate yields (57–69%). In general, nitroolefins with OMe and Me as substituent R3 groups yielded products in excellent 95–99% ee (Scheme 2, 4n–4q, 4y), which were superior to nitroolefins incorporating electron-withdrawing groups (R3 = F, Cl, Br, NO2). However, product 4t was an interesting special case with 99% ee. The absolute configuration of product 4s was determined to be (2S, 3S, 4S) by single-crystal X-ray diffraction analysis (Scheme 3).12
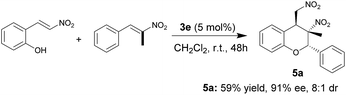
To further demonstrate the synthetic utility of this reaction, we tested trans-α-Me-β-nitroolefin. As listed in Scheme 4, trans-α-Me-β-nitroolefin was suitable for this reaction and afforded the product 5a with an all-carbon quaternary stereocenter in the 3-position in 59% yield, 91% ee, and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr.
1 dr.
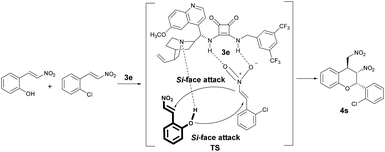
On the basis of the X-ray crystallographic analysis of the absolute configuration of adduct 4s, we proposed a transition state model (Scheme 4). 2-Chloro-nitroolefin was activated well through the hydrogen-bonding interaction between the nitro group of 2-chloro-nitroolefin and the N–H of squaramide catalyst 3e. Meanwhile, 2-hydroxynitrostyrene 1a was activated through a hydrogen-bonding interaction between the hydroxyl group of 1a and the basic/nucleophilic moiety of 3e. Therefore, the hydroxyl group of 1a attacked the β-carbon of the activated 2-chloro-nitroolefin from the Si face under the control of the catalyst 3e. Subsequently, the α-carbon of activated 2-chloro-nitroolefin attacked the β-carbon of 1a from the Si face to yield the major stereoisomer of chiral chroman 4s with the configuration of (2S, 3S, 4S).
In conclusion, the first enantioselective, organocatalytic oxa-Michael-nitro-Michael domino reaction of 2-hydroxynitrostyrenes with trans-β-nitroolefins was successfully demonstrated. The new domino reaction provided an easy and efficient approach to construct 2-alkyl-substituted chiral chroman derivatives bearing three contiguous stereogenic centers with two nitro moieties. This strategy was also suitable for the asymmetric synthesis of 2-aryl-substituted chiral derivatives. Furthermore, this methodology could be used to construct chiral chromans with an all-carbon quaternary stereocenter in the 3-position. Further applications of this organocatalytic system are ongoing in our laboratory.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Zhejiang Natural Science Foundation (LY18B020017), Zhejiang Key Laboratory of Green Pesticides and Cleaner Production Technology.Notes and references
- (a) G. W. Burton and K. U. Ingold, Acc. Chem. Res., 1986, 19, 194 CrossRef CAS; (b) J. Martin Grisar, M. A. Petty, F. N. Bolkenius, J. DOW, J. Wagner, E. R. Wagner, K. D. Haegele and W. De Jong, J. Med. Chem., 1991, 34, 257 CrossRef; (c) K. C. Nicolaou, J. A. Pfefferkorn, A. J. Roecker, G. Q. Cao, S. Barluenga and H. J. Mitchell, J. Am. Chem. Soc., 2000, 122, 9939 CrossRef CAS; (d) J.-M. Zingg and A. Azzi, Curr. Med. Chem., 2004, 11, 1113 CrossRef CAS PubMed; (e) J. Lal, Contraception, 2010, 81, 275 CrossRef CAS PubMed; (f) C. M. Starks, R. B. Williams, V. L. Norman, S. M. Rice, M. O'Neil-Johnson, J. A. Lawrence and G. R. Eldridge, Phytochemistry, 2014, 98, 216 CrossRef CAS PubMed; (g) S. Khan, S. Shukla, S. Sinha, A. D. Lakra, H. K. Bora and S. M. Meeran, Int. J. Biochem. Cell Biol., 2015, 58, 1 CrossRef CAS PubMed; (h) M. Fridén-Saxin, T. Seifert, M. Malo, K. S. Andersson, N. Pemberton, C. Dyrager, A. Friberg, K. Dahlén, E. A. A. Wallén, M. Grøtli and M. Luthman, Eur. J. Med. Chem., 2016, 114, 59 CrossRef PubMed.
- (a) G. Zeni and R. C. Larock, Chem. Rev., 2004, 104, 2285 CrossRef CAS PubMed; (b) H. C. Shen, Tetrahedron, 2009, 65, 3931 CrossRef CAS; (c) T. P. Pathak and M. S. Sigman, J. Org. Chem., 2011, 76, 9210 CrossRef CAS PubMed; (d) W.-J. Bai, J. G. David, F.-Z. Feng, M. G. Weaver, K.-L. Wu and T. R. R. Pettus, Acc. Chem. Res., 2014, 47, 3655 CrossRef CAS PubMed; (e) T. Netscher, Angew. Chem., Int. Ed., 2014, 53, 14313 CrossRef CAS PubMed; (f) N. Majumdar, N. D. Paul, S. Mandal, B. Bruin and W. D. Wulff, ACS Catal., 2015, 5, 2329 CrossRef CAS; (g) N. Hu, K. Li, Z. Wang and W. Tang, Angew. Chem., Int. Ed., 2016, 55, 5044 CrossRef CAS PubMed.
- For selected reviews on organocatalytic domino reactions, see: (a) D. Enders, C. Grondal and M. R. M. Hüttl, Angew. Chem., Int. Ed., 2007, 46, 1570 CrossRef CAS PubMed; (b) X. Yu and W. Wang, Org. Biomol. Chem., 2008, 6, 2037 RSC; (c) L.-Q. Lu, J.-R. Chen and W.-J. Xiao, Acc. Chem. Res., 2012, 45, 1278 CrossRef CAS PubMed; (d) H. Pellissier, Adv. Synth. Catal., 2012, 354, 237 CrossRef CAS; (e) F. Lv, S. Liu and W. Hu, Asian J. Org. Chem., 2013, 2, 824 CrossRef CAS; (f) H. Pellisier, Chem. Rev., 2013, 113, 442 CrossRef PubMed; (g) C. M. R. Volla, I. Atodiresei and M. Rueping, Chem. Rev., 2014, 114, 2390 CrossRef CAS PubMed; (h) Y. Wang, H. Lu and P.-F. Xu, Acc. Chem. Res., 2015, 48, 1832 CrossRef CAS PubMed.
- For selected examples of the synthesis of chiral chromans by aminocatalysis, see: (a) P. Kotame, B.-C. Hong and J.-H. Liao, Tetrahedron Lett., 2009, 50, 704 CrossRef CAS; (b) L. Zu, S. Zhang, H. Xie and W. Wang, Org. Lett., 2009, 7, 1627 CrossRef PubMed; (c) D. B. Ramachary and R. Sakthidevi, Chem.–Eur. J., 2009, 15, 4516 CrossRef CAS PubMed; (d) D. B. Ramachary and R. Sakthidevi, Org. Biomol. Chem., 2010, 8, 4259 RSC; (e) D. Enders, C. Wang, X. Yang and G. Raabe, Adv. Synth. Catal., 2010, 352, 2869 CrossRef CAS; (f) B.-C. Hong, P. Kotame, C.-W. Tsai and J.-H. Liao, Org. Lett., 2010, 12, 776 CrossRef CAS PubMed; (g) C. Wang, X. Yang, G. Raabe and D. Enders, Adv. Synth. Catal., 2012, 354, 2629 CrossRef CAS; (h) Z.-C. Geng, S.-Y. Zhang, N.-K. Li, N. Li, J. Chen, H.-Y. Li and X.-W. Wang, J. Org. Chem., 2014, 79, 10772 CrossRef CAS PubMed; (i) A.-B. Xia, C. Wu, T. Wang, Y.-P. Zhang, X.-H. Du, A.-G. Zhong, D.-Q. Xu and Z.-Y. Xu, Adv. Synth. Catal., 2014, 356, 1753 CrossRef CAS; (j) P. H. Poulsen, K. S. Feu, B. M. Paz, F. Jensen and K. A. Jørgensen, Angew. Chem., Int. Ed., 2015, 54, 8203 CrossRef CAS PubMed.
- For selected examples of the synthesis of chiral chromans by thiourea organocatalysis, see: (a) X.-F. Wang, Q.-L. Hua, Y. Cheng, X.-L. An, Q.-Q. Yang, J.-R. Chen and W.-J. Xiao, Angew. Chem., Int. Ed., 2010, 49, 8379 CrossRef CAS PubMed; (b) D. B. Ramachary, R. Sakthidevi and K. S. Shruthi, Chem.–Eur. J., 2012, 18, 8008 CrossRef CAS PubMed; (c) Z.-X. Jia, Y.-C. Luo, X.-N. Cheng, P.-F. Xu and Y.-C. Gu, J. Org. Chem., 2013, 78, 6488 CrossRef CAS PubMed; (d) P. Saha, A. Biswas, N. Molleti and V. K. Singh, J. Org. Chem., 2015, 80, 11115 CrossRef CAS PubMed; (e) W. Zheng, J. Zhang, S. Liu, C. Yu and Z. Miao, RSC Adv., 2015, 5, 91108 RSC; (f) K. Zhao, Y. Zhi, T. Shu, A. Valkonen, K. Rissanen and D. Enders, Angew. Chem., Int. Ed., 2016, 55, 12104 CrossRef CAS PubMed.
- For selected examples of the synthesis of chiral chromans by squaramide organocatalysis, see: (a) H. Mao, A. Lin, Y. Tang, Y. Shi, H. Hu, Y. Cheng and C. Zhu, Org. Lett., 2013, 15, 4062 CrossRef CAS PubMed; (b) B. Zheng, W. Hou and Y. Peng, ChemCatChem, 2014, 6, 2527 CrossRef CAS; (c) Y. Zhu, X. Li, Q. Chen, J. Su, F. Jia, S. Qiu, M. Ma, Q. Sun, W. Yan, K. Wang and R. Wang, Org. Lett., 2015, 17, 3826 CrossRef CAS PubMed.
- (a) M. Rueping and M.-Y. Lin, Chem.–Eur. J., 2010, 16, 4169 CrossRef CAS PubMed; (b) M. Rueping, U. Uria, M.-Y. Lin and I. Atodiresei, J. Am. Chem. Soc., 2011, 133, 3732 CrossRef CAS PubMed; (c) M. J. Climent, S. Iborra, M. J. Sabater and J. D. VidalI, Appl. Catal., A, 2014, 481, 27 CrossRef CAS.
- C.-F. Yao, Y.-J. Jang and M.-C. Yan, Tetrahedron Lett., 2003, 44, 3813 CrossRef CAS.
- (a) D.-Q. Xu, Y.-F. Wang, S.-P. Luo, S. Zhang, A.-G. Zhong, H. Chen and Z.-Y. Xu, Adv. Synth. Catal., 2008, 350, 2610 CrossRef CAS; (b) S.-P. Luo, Z.-B. Li, L.-P. Wang, Y. Guo, A.-B. Xia and D.-Q. Xu, Org. Biomol. Chem., 2009, 7, 4539 RSC; (c) A.-B. Xia, D.-Q. Xu, S.-P. Luo, J.-R. Jiang, J. Tang, Y.-F. Wang and Z.-Y. Xu, Chem.–Eur. J., 2010, 16, 801 CrossRef CAS PubMed; (d) A.-B. Xia, G.-J. Pan, C. Wu, X.-L. Liu, X.-L. Zhang, Z.-B. Li, X.-H. Du and D.-Q. Xu, Adv. Synth. Catal., 2016, 358, 3155 CrossRef CAS.
- For selected reviews on the hydrogen bonding organocatalysis, see: (a) R. Ian Storer, C. Aciroa and L. H. Jones, Chem. Soc. Rev., 2011, 40, 2330 RSC; (b) J. Alemán, A. Parra, H. Jiang and K. A. Jørgensen, Chem.–Eur. J., 2011, 17, 6890 CrossRef PubMed; (c) P. Chauhan, S. Mahajan, U. Kaya, D. Hack and D. Enders, Adv. Synth. Catal., 2015, 357, 253 CrossRef CAS; (d) F. E. Held and S. B. Tsogoeva, Catal. Sci. Technol., 2016, 6, 645 RSC.
- For selected examples catalyzed by hydrogen bonding, see: (a) J. P. Malerich, K. Hagihara and V. H. Rawal, J. Am. Chem. Soc., 2008, 130, 14416 CrossRef CAS PubMed; (b) Y. Zhu, J. P. Malerich and V. H. Rawal, Angew. Chem., Int. Ed., 2010, 49, 153 CrossRef CAS PubMed; (c) M. Rombola, C. S. Sumaria, T. D. Montgomery and V. H. Rawal, J. Am. Chem. Soc., 2017, 139, 5297 CrossRef CAS PubMed.
- CCDC 1031452 contains the supplementary crystallographic data for the compound 4s.‡.
Footnotes |
| † Dedicated to Professor Zhen-Yuan Xu on the occasion of his 80th birthday. |
| ‡ Electronic supplementary information (ESI) available. CCDC 1031452. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra13525d |
| This journal is © The Royal Society of Chemistry 2018 |