Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nano-γ-Al2O3/SbCl5: an efficient catalyst for the synthesis of 2,3-dihydroperimidines†
Abdolhamid Bamoniri *a,
Bi Bi Fatemeh Mirjalili
*a,
Bi Bi Fatemeh Mirjalili b and
Sepideh Saleha
b and
Sepideh Saleha
aDepartement of Org. Chem., Faculty of Chem., University of Kashan, Kashan, I. R. Iran. E-mail: bamoniri@kashanu.ac.ir
bDepartment of Chem., College of Sci., Yazd University, Yazd, I. R. Iran
First published on 7th February 2018
Abstract
Nano-γ-Al2O3/SbCl5 as a new Lewis acid nano catalyst was synthesized and characterized by FTIR, XRD, FESEM, TEM, EDS, BET and TGA techniques. Nano-γ-Al2O3/SbCl5 has been employed for synthesis of 2-substituted perimidines via reaction of naphthalene-1,8-diamine with various aldehydes at room temperature under solvent-free conditions. This protocol proffers several benefits including high yields, easy workup, short reaction times and simple reaction conditions.
1. Introduction
Perimidines exhibit a diverse range of biological activities; such as antibacterial, antifungal, anti-inflammatory and antitumor.1–6 It was of interest to explore the suitability of some perimidines derivatives as potential DNA-intercalating ligands.7 A synthetic method for the preparation of perimidines is the condensation reaction of 1,8-diaminonaphthalene with various carbonyl groups.8–10 However, most of these methods suffers of significant side reaction, low yield and have cumbersome work-up procedures.Some catalysts are reported for perimidine synthesis such as zeolite,11 CMK-5-SO3H,12 BiCl3,13 BF3·H2O,14 Yb(OTf)3,15 Cu(NO3)2·6H2O,16 FePO4,17 Fe3O4/SiO2/(CH2)3N+Me3Br3−,18 [BTBA]Cl–FeCl3,19 nano-silica sulfuric acid,20 amberlyst 15 (ref. 21) and molecular iodine.22
Antimony pentachloride (SbCl5), a thin and fuming liquid, is applied in industry and organic synthesis. Where of, antimony pentachloride is a liquid with a great specific gravity that fumigates in air and reacts with the humidity to form HCl, the tactility and the usability of SbCl5 as a liquid form is arduous and the supported form is really preferable. It has been acclaimed that the supported SbCl5 is a solid superacid. SbCl5 is used immensely in organic synthesis as a Lewis acid for elevating a variety of organic reactions.23 Solid-acid catalysts are commonly classified by their Brønsted and/or Lewis acidity, the intensity and number of these positions, and the morphology of the support. The synthesis of net Brønsted and net Lewis acid catalysts attracts a major degree of academic concern.24 Alumina![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Al2O3)
(Al2O3)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) is
is![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) applied
applied![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) both
both![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) as
as![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst
catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) for divers types of reaction and as a support for metals. Al2O3 is very repeatedly applied as a support of industrial divers' types of reaction and as a support for
for divers types of reaction and as a support for metals. Al2O3 is very repeatedly applied as a support of industrial divers' types of reaction and as a support for![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) metals.
metals.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al2O3
Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) is
is![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) very
very![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) repeatedly applied as
repeatedly applied as![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) support
support![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) of catalysts
of catalysts![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) for
for![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) its
its![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mechanical
mechanical![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) intensity also its
intensity also its![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) potent interaction
potent interaction![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) with
with![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) metals
metals![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) and
and![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) metal
metal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) oxides that provides high propagation of
oxides that provides high propagation of![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) the
the![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) supported
supported![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) compounds. As for the surface properties,
compounds. As for the surface properties,![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) alumina
alumina![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) is
is![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) commonly considered as acidic rather than basic,
commonly considered as acidic rather than basic,![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) but
but![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) basic
basic![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) positions coexist.25 Alumina is a main material for usages in ultrafiltration of salts, as an automobile exhaust catalyst, and in petroleum purification. Porous γ-alumina with equal channels, high surface area, and slender pore-size repartition possesses conjunction better physicochemical properties. However, the manufacturing of ordered and thermally constant porous alumina is demonstrated due to its susceptibility for hydrolysis and phase transition-induced demolition of ordered pore structure.26
positions coexist.25 Alumina is a main material for usages in ultrafiltration of salts, as an automobile exhaust catalyst, and in petroleum purification. Porous γ-alumina with equal channels, high surface area, and slender pore-size repartition possesses conjunction better physicochemical properties. However, the manufacturing of ordered and thermally constant porous alumina is demonstrated due to its susceptibility for hydrolysis and phase transition-induced demolition of ordered pore structure.26
Here in, we wish to report a simple method for the synthesis of nano-γ-Al2O3/SbCl5 and its usage in the synthesis of 2,3-dihydroperimidines under solvent-free grinding condition at room temperature.
2. Experimental
2.1 Material and methods
All compounds were purchased from Fluka and Merck chemical company and used without any additional purification. Fourier transform infrared (FT-IR) spectra were run on a Nicolet Magna 550 spectrometer. A Bruker (DRX-400 Avance) NMR was used to record the 1H-NMR and 13C-NMR spectra. XRD pattern using Philips Xpert MP diffractometer (Cu Kα, radiation, k = 0.154056 nm) was achieved. FE-SEM was obtained on a Mira Tescan. Transmission electron microscope (TEM) was recorded on a Philips-CM 120-with LaB6 cathode instrument on an accelerating voltage of 120 kV. The thermal gravimetric analysis (TGA) was done with “STA 504” instrument. Energy-dispersive X-ray spectroscopy (EDS) of SbCl5/nano-γ-Al2O3 was measured by EDS instrument, Phenom pro X. BET surface area analysis of catalyst was done with Micrometrics, Tristar II 3020 analyser.2.2 Preparation of nano-γ-Al2O3
NaOH (600 ml, 1 M), was added drop-wise to a slurry containing Al2(SO4)3·18H2O (66 g). The mixture was stirred at room temperature. The resulted suspension was filtered to obtain the white solid Al(OH)3. Then solid were washed with distilled water until no more sulfate ions were detected in the washings. Following the aging step, NaOH (100 ml, 1 M) was added to a beaker containing Al(OH)3 (20 g) to produce NaAl(OH)4. Then PEG 4000 (0.3%) was added to solution and it was neutralized with HCl (0.1 M), to pH 8 until Al(OH)3 produced again.The obtained precipitate filtered and washed with distilled water. The as-dried solid was calcined in the furnace at 800 °C for 3 hours through atmospheric air to produce nano-γ-Al2O3 powder.
2.3 Preparation of nano-γ-Al2O3/SbCl5
To a mixture of nano-γ-Al2O3 (1 g) and CH2Cl2 (10 ml), SbCl5 (0.5 ml) was added drop wise in the well ventilated hood. The resulting suspension was stirred for 1 hour at room temperature, filtered, washed with CH2Cl2, and dried at room temperature.2.4 General procedure for the preparation of 2,3-dihydroperimidines
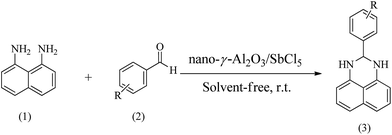
Naphthalene-1,8-diamine (1 mmol), aromatic aldehydes (1 mmol) and nano-γ-Al2O3/SbCl5 (0.1 g) were grounded in a mortar with a pestle for a few minutes to obtain a homogeneous mixture. After completed conversion as indicated by TLC, 10 ml of ethanol was added then the heterogeneous catalyst was filtered. By adding crushed ice to filtrate, the pure products were obtained as white solids.3. Results and discussion
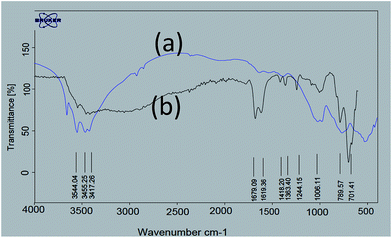
In continuation of our investigation on the utilization of solid acids in organic synthesis, we have synthesized nano-γ-Al2O3/SbCl5 as a new nano catalyst and studied its efficiency in the synthesis of 2,3-dihydroperimidines at room temperature under grinding conditions.For exploration of the structure of nano-γ-Al2O3/SbCl5, we have studied FT-IR spectra of nano-γ-Al2O3 and nano-γ-Al2O3/SbCl5 (Fig. 1). In nano-γ-Al2O3 FT-IR spectrum, the band in the region of 500–1000 cm−1 is attributed to the stretching vibrations of the (Al–O) bond in γ-Al2O3 (Fig. 1). In nano-γ-Al2O3/SbCl5 spectrum, in addition to γ-Al2O3 signal, two additional band at 701 show binding of SbCl5 to γ-Al2O3.
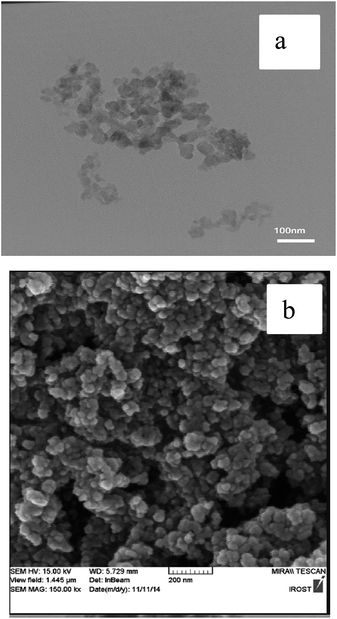
The FESEM and TEM images of the nano-γ-Al2O3/SbCl5 are demonstrated in Fig. 2. They exhibit disordered spherical shape for nano particles below 50 nm.
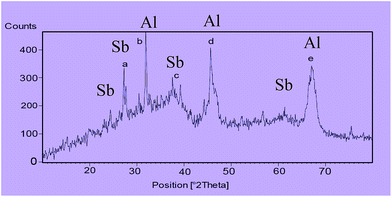
The X-ray diffraction (XRD) pattern of nano-γ-Al2O3/SbCl5 is exhibited in (Fig. 3). The signals at 2θ equal to 37 (c), 45 (d) and 67 (e) are displayed nano-γ-Al2O3 structure. According to XRD pattern, the two additional signals at 2θ equal to 28 (a) and 32 (b) respectively, are shown the presentment of bonded Sb to nano-γ-Al2O3 (Fig. 3).
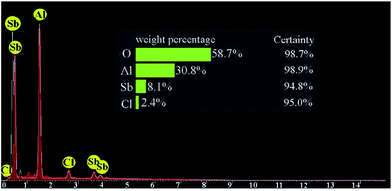
The energy-dispersive X-ray spectroscopy (EDS) of the synthesized catalyst is displayed in Fig. 4. EDX pattern obviously approbates the presence of the anticipated elements in the construction of this catalyst and corroborated supporting of SbCl5 on nano-γ-Al2O3. The elemental compositions of nano-γ-Al2O3/SbCl5 were found to be 58.7, 30.8 and 8.1% for O, Al and Sb, respectively.
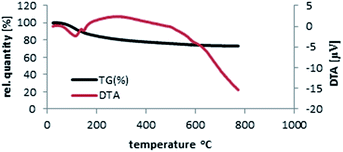
Thermal gravimetric analysis (TG-DTA) template of SbCl5/nano-γ-Al2O3 was discovered by heating from 20 °C to 780 °C and then cooling until 165 °C (Fig. 5). The catalyst is stable until 390 °C and only 10.5% of its weight was reduce due to the removal of catalyst humidity. The char yield of the catalyst in 390 °C is 89.5%. According to the TG-DTA pattern of nano-γ-Al2O3/SbCl5 and our discussion, it was disclosed that this catalyst is appropriate for the advancement of organic reactions until 400 °C.
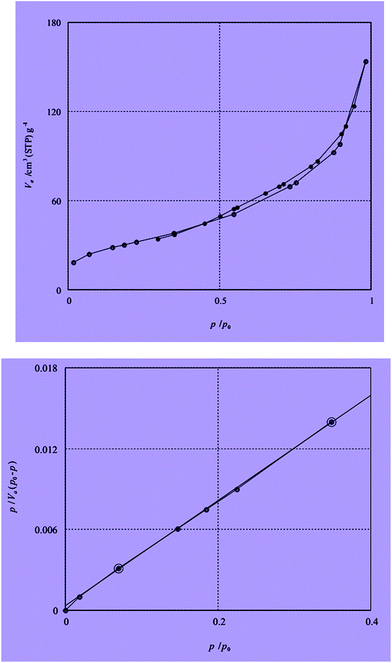
The BET N2 adsorption method is applied to measure the surface area. The BET surface areas is assigned as 92.503 m2 g−1. The N2 adsorption isotherm of catalyst is described in Fig. 6. Inductive coupled plasma (ICP) analysis have determined the existence 200 mg of Sb in 1 g of catalyst.
After characterization of catalyst, we have investigated catalytic activity of nano-γ-Al2O3/SbCl5 for the synthesis of 2,3-dihydroperimidines derivatives. For optimization of the reaction reservations, 1,8-diaminonaphthalene (1 mmol), and 4-chlorobenzaldehyde (1 mmol) were used as model reactants under solvent-free conditions (Table 1). The best resultant based on yield and time of the reaction was afforded with 0.16 g of nano-γ-Al2O3/SbCl5. At first, in order to show the unrivalled catalytic behaviour of nano-γ-Al2O3/SbCl5 and to contrast its activity with other catalysts. Also, Table 1, shows the performance of our nano-catalyst in the preparation of 2,3-dihydroperimidines contrast to that of other reported methods.
| Entry | Catalyst | Solvent | Temp (°C) | Time (min) | Yield% |
|---|---|---|---|---|---|
| a 1,8-Diaminonaphthalene (1 mmol), and 4-chlorobenzaldehyde (1 mmol) were used. | |||||
| 1 | Zeolite | Ethanol | r. t. | 2700 | 40 (ref. 11) |
| 2 | Fe3O4/SiO2/(CH2)3N+Me3Br3− | — | 80 | 15 | 95 (ref. 18) |
| 3 | FePO4 | Ethanol | r. t. | 420 | 90 (ref. 17) |
| 4 | Nano-γ-Al2O3/SbCl5 (0.005 g) | — | r. t. | 60 | 20 |
| 5 | Nano-γ-Al2O3/SbCl5 (0.008 g) | — | r. t. | 60 | 30 |
| 6 | Nano-γ-Al2O3/SbCl5 (0.01 g) | — | r. t. | 60 | 35 |
| 7 | Nano-γ-Al2O3/SbCl5 (0.08 g) | — | r. t. | 30 | 50 |
| 8 | Nano-γ-Al2O3/SbCl5 (0.1 g) | — | r. t. | 15 | 70 |
| 9 | Nano-γ-Al2O3/SbCl5 (0.14 g) | — | r. t. | 15 | 80 |
| 10 | Nano-γ-Al2O3/SbCl5 (0.16 g) | — | r. t. | 15 | 95 |
| 11 | Nano-γ-Al2O3/SbCl5 (0.20 g) | — | r. t. | 15 | 95 |
| 12 | Nano-γ-Al2O3/SbCl5 (0.25 g) | — | r. t. | 15 | 95 |
Using the optimized reaction provisions, the reactions of various substituted benzaldehydes with naphthalene-1,8-diamine were studied (Scheme 1, Table 2).
| Entry | R | Product | Time (min) | Yieldb (%) |
|---|---|---|---|---|
| a 1,8-Diaminonaphthalene (1 mmol), aldehyde (1 mmol) and nano-γ-Al2O3/SbCl5 (0.16 g) were used.b Isolated yield. | ||||
| 1 | 4-Cl | 3a | 14 | 95 |
| 2 | 2-NO2 | 3b | 15 | 90 |
| 3 | 3-NO2 | 3c | 13 | 95 |
| 4 | 4-NO2 | 3d | 15 | 93 |
| 5 | 4-COOH | 3e | 20 | 80 |
| 6 | 4-NMe2 | 3f | 20 | 90 |
| 7 | 4-OMe | 3g | 15 | 85 |
| 8 | 2,4-OMe2 | 3h | 16 | 80 |
| 9 | 2,3-Cl2 | 3i | 14 | 85 |
| 10 | 2,3-OMe2 | 3j | 15 | 80 |
| 11 | 3,4-OMe2 | 3k | 13 | 85 |
As displayed in Table 2, a number of aromatic aldehydes bearing electron withdrawing groups and electron-donating groups were further subjected to reaction employing a catalytic amount of nano-γ-Al2O3/SbCl5. In general, with electron-drawing substituents in the aromatic benzaldehydes, increased yields of products were generated, whereas the affect is reversed with electron donating substituents. However, the variations in the yields were little.
A plausible pathway for the preparation of 2,3-dihydroperimidines in the presence of nano-γ-Al2O3/SbCl5 is revealed in Scheme 2. Nucleophilic attack of 1,8-diamino naphthalene 2 to SbCl5-activated aldehyde 1 generated intermediate 3. In situ dehydration of compound 4 and nucleophilic attack of the second amino group to SbCl5-activated imine intermediate 5 afforded intermediate 6 to produce the compound 7.
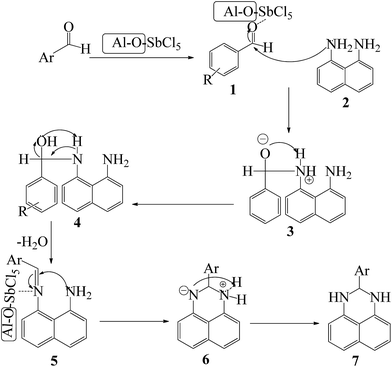 | ||
| Scheme 2 Proposed mechanism of the SbCl5/nano-γ-Al2O3-catalysed synthesis of 2,3-dihydroperimidines. | ||
4. Conclusions
In conclusion, nano-γ-Al2O3/SbCl5 was successfully synthesized, characterized and applied for the synthesis of 2,3-dihydroperimidine derivatives. Short reaction times, high conversions, clean reaction profiles, simple work-up, availability and high activity of catalyst, make this method suitable for many acid catalysed organic reactions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to University of Kashan for supporting this work by Grant No. (159189/50).References
- P. Sharma, N. Rane and V. K. Gurram, Bioorg. Med. Chem. Lett., 2004, 14, 4185–4190 CrossRef CAS PubMed.
- N. Ingarsal, G. Saravanan, P. Amutha and S. Nagarajan, Eur. J. Med. Chem., 2007, 42, 517–520 CrossRef CAS PubMed.
- S. M. Sondhi, N. Singh, M. Johar and A. Kumar, Bioorg. Med. Chem., 2005, 13, 6158–6166 CrossRef CAS PubMed.
- M. Dzieduszycka, S. Martelli, M. Arciemiuk, M. M. Bontemps-Gracz, A. Kupiec and E. Borowsk, Bioorg. Med. Chem., 2002, 10, 1025–1035 CrossRef CAS PubMed.
- T. A. Farghaly and H. K. Mahmoud, Arch. Pharm. Chem. Life Sci., 2013, 346, 392–402 CrossRef CAS PubMed.
- T. A. Farghaly, E. M. H. Abbas, K. M. Dawood and T. B. A. El-Naggar, Molecules, 2014, 19, 740–755 CrossRef PubMed.
- J. M. Herbert, P. D. Woodgate and W. A. Denny, J. Med. Chem., 1987, 30, 2081–2086 CrossRef CAS PubMed.
- I. Yavari, M. Adib, F. Jahani-Moghaddam and H. R. Bijanzadeh, Tetrahedron, 2002, 58, 6901–6908 CrossRef CAS.
- A. Mobinikhaledi, M. A. Amrollahi, N. Foroughifar and H. F. Jirandehi, Asian J. Chem., 2005, 17, 2411–2414 CAS.
- A. Mobinikhaledi, N. Foroughifar and R. Goli, Phosphorus, Sulfur, Silicon Relat. Elem., 2005, 180, 2549–2554 CrossRef CAS.
- A. Mobinikhaledi, N. Foroughifar and N. Basaki, Turk. J. Chem., 2002, 33, 555–560 Search PubMed.
- H. Alinezhad and M. Zare, J. Chil. Chem. Soc., 2013, 58, 1840–1841 CrossRef CAS.
- J. Zhang and S. Zhang, Synth. Commun., 2007, 37, 2615–2624 CrossRef CAS.
- G. K. S. Prakash, F. Paknia, A. Narayan, T. Mathew and G. A. Olah, J. Fluorine Chem., 2013, 152, 99–105 CrossRef CAS.
- S. l. Zhang and J. M. Zhang, Chin. J. Chem., 2008, 26, 185–189 CrossRef CAS.
- A. Mobinikhaledi and P. J. Steel, Synth. React. Inorg., Met.-Org., Nano-Met. Chem., 2009, 39, 133–135 CAS.
- F. K. Behbahani and F. M. Golchin, Journal of Taibah University for Science, 2016, 11, 85–89 CrossRef.
- A. Farrokhi, K. Ghodrati and I. Yavari, Catal. Commun., 2015, 63, 41–46 CrossRef CAS.
- K. Bahrami and S. Saleh, Synth. React. Inorg., Met.-Org., Nano-Met. Chem., 2016, 46, 852–856 CrossRef CAS.
- A. Mobinikhaledi, H. Moghanian and F. Sasani, Int. J. Green Nanotechnol., 2010, 2, 47–52 CrossRef.
- V. V. Patil and G. S. Shankarling, Catal. Commun., 2014, 57, 138–142 CrossRef.
- A. Mobinikhaledi, M. A. Bodaghi Fard, F. Sasani and M. A. Amrollahi, Bulg. Chem. Commun., 2013, 45, 353–356 CAS.
- B. Sadeghi and M. Baradaran, Iran. J. Org. Chem., 2010, 2, 431–435 Search PubMed.
- K. Wilson and J. H. Clark, Pure Appl. Chem., 2000, 72, 1313–1319 CrossRef CAS.
- H. Hattoni and Y. One, Solid acid catalysis from fundamentals to applications, Pan Stanford Publishing, Singapore, 2015 Search PubMed.
- H. Li, L. Zhang, H. Dai and H. He, Inorg. Chem., 2009, 48, 4421–4434 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra13593a |
| This journal is © The Royal Society of Chemistry 2018 |