Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis, characterization and olefin polymerization behaviors of phenylene-bridged bis-β-carbonylenamine binuclear titanium complexes†
Derong Luo‡
a,
Yi Zeng‡a,
Xiong Chena,
Ping Xiaa,
Guangyong Xie *a,
Qingliang Youb,
Li Zhanga,
Tingcheng Lia,
Xiangdan Lia and
Aiqing Zhanga
*a,
Qingliang Youb,
Li Zhanga,
Tingcheng Lia,
Xiangdan Lia and
Aiqing Zhanga
aKey Laboratory of Catalysis and Materials Science of the State Ethnic Affairs Commission, Ministry of Education Hubei Province, South-Central University for Nationalities, Wuhan 430074, China. E-mail: xiegy@scuec.edu.cn
bKey Laboratory of Optoelectronic Chemical Materials and Devices, Ministry of Education, School of Chemical and Environmental Engineering, Jianghan University, Wuhan 430056, China
First published on 13th February 2018
Abstract
Binuclear and multinuclear complexes have attracted much attention due to their unique catalytic performances for olefin polymerization compared with their mononuclear counterparts. In this work, a series of phenyl-bridged bis-β-carbonylenamine [O−NSR] (R = alkyl or phenyl) tridentate ligands and their binuclear titanium complexes (Ti2L1–Ti2L5) were synthesized and characterized by 1H NMR, 13C NMR, FTIR and elemental analysis. The molecular structure of ligand L2 (R = nPr) and its corresponding Ti complex Ti2L2 were further investigated by single-crystal X-ray diffraction, which showed that each titanium coordinated with six atoms to form a distorted octahedral configuration along with the conversion of the ligand from β-carbonylenamine to β-imino enol form. Under the activation of MMAO, these complexes catalyzed ethylene polymerization and ethylene/α-olefin copolymerization with extremely high activity (over 106 g mol (Ti)−1 h−1 atm−1) to produce high molecular weight polyethylene. At the same time, wider polydispersity as compared with the mononuclear counterpart TiL6 was observed, indicating that two active catalytic centers may be present, consistent with the asymmetrical crystal structure of the binuclear titanium complex. Furthermore, these complexes possessed better thermal stability than their mononuclear analogues. Compared with the complexes bearing alkylthio sidearms, the complex Ti2L5 bearing a phenylthio sidearm exhibited higher catalytic activity towards ethylene polymerization and produced polyethylene with much higher molecular weight, but with an appreciably lower 1-hexene incorporation ratio. Nevertheless, these bis-β-carbonylenamine-derived binuclear titanium complexes showed much higher ethylene/1-hexene copolymerization activity and 1-hexene incorporation ratios as compared with the methylene-bridged bis-salicylaldiminato binuclear titanium complexes, and the molecular weight and 1-hexene incorporation ratio could be flexibly tuned by the initial feed of α-olefin commoners and catalyst structures.
1 Introduction
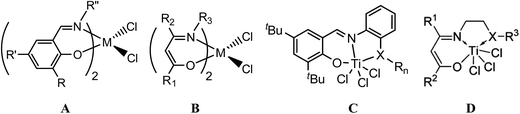
Polyolefins are by far the most important and most produced synthetic polymers today, and the design and synthesis of effective catalysts for olefin polymerization and copolymerization is of great interest in both academic research and industrial applications. The discovery of single-site group 4 metallocene catalysts is considered one of the most significant breakthroughs after the discovery of Ziegler-Natta catalysts.1 Thereafter, single-site non-metallocene catalytic systems, including early and late transition metals catalysts, are thought of as another significant breakthrough, since they can provide novel olefin-based materials with superior activity and greater control over polymer microstructures.2,3Of the non-metallocene candidates, the group 4 non-metallocene complexes with bidentate anionic [N, O] chelate ligands, which was first reported in 1995,4 have been the focus of attention. A great variety of [N, O] chelate complexes have been reported, among which the most prominent were the group 4 bis(phenoxyimine) ligated complexes (A, Chart 1) reported firstly by Floriani et al. in 1995.5 Fujita et al.6 and Coates et al.7 further developed these ligands and reported some new complexes that are excellent for olefin polymerization including ethylene living polymerization, highly syndiospecific propylene living polymerization, living copolymerization of ethylene with α-olefin, and the synthesis of functional and block copolymers of propylene. However, very few successful phenoxyimine catalysts have been reported that effectively catalyze random copolymerization of ethylene and other olefins due to the low comonomer incorporation ratio. The group 4 transition metal complexes based on bis(β-carbonylenamine) ligands (B, Chart 1) were another group of widely-researched ethylene (co)polymerization precatalysts containing [N, O] chelate ligands.4,8
To further improve the catalytic performances of the bidentate anionic [N, O] chelated complexes towards ethylene (co)polymerization, Tang and coworkers have developed a series of mono-ligated tridentate [ONX]TiCl3 complexes (X = O, S, Se, and P) based on either salicylaldiminato or β-carbonylenamine backbone by introducing some sidearms with pendant-coordination heteroatom groups (C and D, Chart 1),9 which exhibited better catalytic performances due to the tuning of the electronic and steric properties of the active species by the sidearm. These complexes were especially effective for ethylene copolymerization with α-olefins, cycloolefins or polar monomers, due partially to the less crowded coordination sphere. Furthermore, they could be prepared in one step by simply mixing the tridentate ligands and titanium tetrachloride, without the need to deprotonate the ligands in advance.
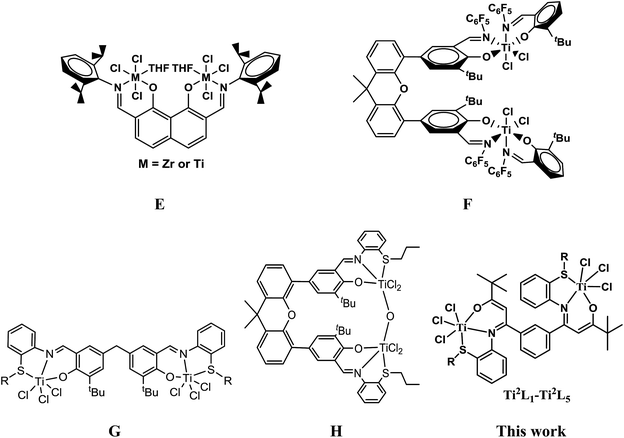
More recently, there have also been growing interests in bi- and multi-nuclear olefin polymerization catalysts,3a–d,10–13 which showed that introducing a proximate metal center could significantly enhance catalytic properties as compared with the mononuclear analogue due to the creation of high local reagent concentrations, conformationally advantageous active-site-substrate proximities, as well as multicenter directed covalent and noncovalent interactions. Most of these works were focused on metallocene and late transition metal complexes, while early transition non-metallocene binuclear complexes have rarely been reported. Marks' group reported a class of naphthoylimine-ligated early transition bimetallic catalysts (E, Chart 2) with moderate activity (∼104 g mol−1 h−1 atm−1) and higher comonomer incorporation ratios compared to the mononuclear analogue due to nuclearity and cooperativity effects in binuclear catalysts.10c,d Ma and coworkers lately described a bidentate salicylaldimine heteroligated binuclear titanium catalyst (F, Chart 2) with high activity and higher ethylene/1,5-hexadiene copolymerization capability than that of its mononuclear counterpart.13
Recently we have been committed to developing some novel early and late transition non-metallocene catalysts based on tuning the coordination environment of the active species with electronic and/or steric effects of the substituents.14 In view of the advantage of the less crowded coordination sphere of tridentate ligands and the cooperative effect of binuclear complexes, we have designed a number of binuclear titanium catalysts with methylene- or xanthene-bridged bis(salicylaldiminato) tridentate ligands (G and H, Chart 2) and investigated their catalytic behaviors for ethylene homo- and copolymerization.15 Here we describe the synthesis, structure and ethylene (co)polymerization behaviors of a series of novel phenyl-bridged bis-β-carbonylenamine [O−NSR] (R = alkyl or phenyl) tridentate binuclear titanium complexes Ti2L1–Ti2L5 (Chart 2).
2 Results and discussion
2.1. Synthesis and structure of ligands and binuclear Ti complexes
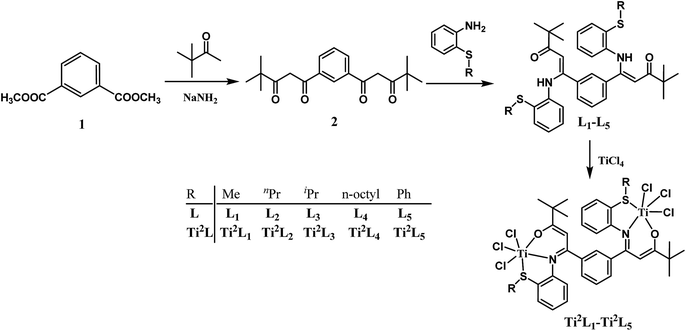
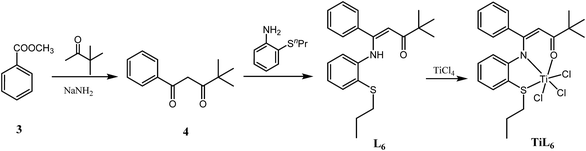
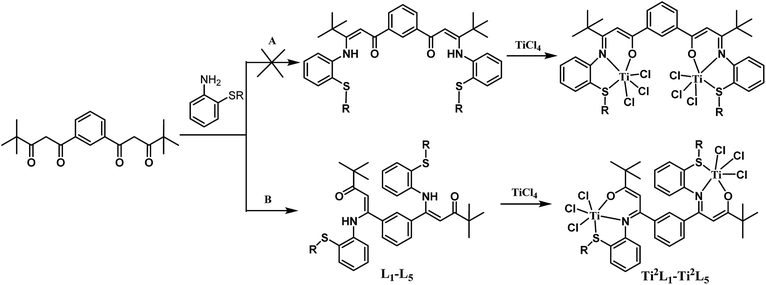
The synthetic routes for the ligands L1–L6 and the corresponding complexes Ti2L1–Ti2L5 and TiL6 were shown in Scheme 1 and 2.Firstly, 1,1′-(1,3-phenylene)-bis(4,4-dimethylpentane-1,3-dione) (2) and 4,4-dimethyl-1-phenylpentane-1,3-dione (4) were synthesized according to the work of L. F. Lindoy's group16 through Claisen condensation of dimethyl isophthalate or methyl benzoate with pinacolone deprotonated by sodium amide, which then reacted with alkylthio anilines to obtain bis- and mono-β-carbonylenamine [ONS] tridentate ligands L1–L6 in 68–83% yields. Finally, binuclear Ti complexes Ti2L1–Ti2L5 and mononuclear counterpart TiL6 were prepared by reacting the ligands L1–L6 directly with excess TiCl4 according to Tang's9 and our previous works.15 The structures of the free ligands and the corresponding bi- and mono-nuclear Ti complexes were characterized by 1H NMR, 13C NMR, FT IR and elemental analysis. Notable changes of the 1H NMR spectra were that the NH resonances of the ligands at δ 12.05–12.22 ppm disappeared upon forming the Ti complexes.
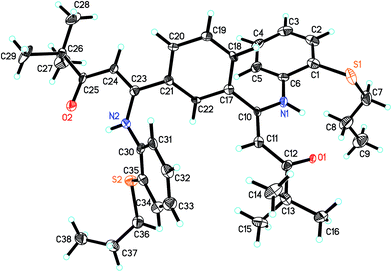
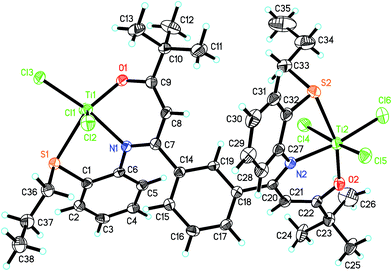
The molecular structures of ligand L2 and its corresponding Ti complex Ti2L2 were further confirmed by single-crystal X-ray diffraction, as shown in Fig. 1 and 2. The crystal data and details of data collection and refinement are summarized in Table 1, and selected bond lengths and angles are listed in Table 2.
| L2 | Ti2L2 | |
|---|---|---|
| Empirical formula | C38H48N2O2S2 | C38H46Cl6N2O2S2Ti2 |
| Formula weight | 628.90 | 935.39 |
| Crystal size (mm3) | 0.08 × 0.05 × 0.03 | 0.211 × 0.165 × 0.112 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P121/c1 | P21/c |
| a (Å) | 14.149(5) | 18.572(3) |
| b (Å) | 10.258(3) | 14.833(3) |
| c (Å) | 24.194(8) | 18.032(3) |
| α (°) | 90° | 90° |
| β (°) | 96.710(6)° | 108.118(3)° |
| γ (°) | 90° | 90° |
| V (Å3) | 3487.7(19) | 4721.4(14) |
| Z | 4 | 4 |
| Density (Mg m−3) | 1.198 | 1.316 |
| Absorption coefficient (mm−1) | 0.188 | 0.798 |
| θmax/° | 25.248° | 25.5° |
| Reflections collected/unique | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 094/6060 [R(int) = 0.1731] 094/6060 [R(int) = 0.1731] |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 859/8776 [R(int) = 0.0680] 859/8776 [R(int) = 0.0680] |
| Goodness-of-fit on F2 | 0.963 | 1.002 |
| Final R indices [I > 2σ(I)] | R1 = 0.0916, wR2 = 0.2043 | R1 = 0.0705, wR2 = 0.1858 |
| L2 | Ti2L2 | ||||
|---|---|---|---|---|---|
| S(1)–C(1) | 1.769(7) | S(1)–C(1) | 1.780(5) | O(1)–Ti(1)–N(1) | 84.26(15) |
| S(1)–C(7) | 1.845(8) | S(1)–C(36) | 1.819(6) | O(1)–Ti(1)–Cl(3) | 104.94(12) |
| S(2)–C(35) | 1.772(6) | S(2)–C(32) | 1.755(6) | N(1)–Ti(1)–Cl(3) | 170.77(12) |
| S(2)–C(36) | 1.831(7) | S(2)–C(33) | 1.826(6) | O(1)–Ti(1)–Cl(2) | 92.15(13) |
| O(1)–C(12) | 1.251(7) | O(1)–C(9) | 1.338(6) | N(1)–Ti(1)–Cl(2) | 86.38(12) |
| O(2)–C(25) | 1.225(7) | O(2)–C(22) | 1.322(6) | Cl(3)–Ti(1)–Cl(2) | 92.34(6) |
| N(1)–C(10) | 1.376(7) | N(1)–C(7) | 1.315(6) | O(1)–Ti(1)–Cl(1) | 96.64(13) |
| N(2)–C(23) | 1.370(7) | N(2)–C(20) | 1.302(6) | N(1)–Ti(1)–Cl(1) | 86.44(12) |
| C(10)–C(11) | 1.400(8) | C(7)–C(8) | 1.447(7) | Cl(3)–Ti(1)–Cl(1) | 93.22(6) |
| C(11)–C(12) | 1.444(8) | C(8)–C(9) | 1.351(7) | Cl(2)–Ti(1)–Cl(1) | 168.04(7) |
| C(23)–C(24) | 1.379(8) | C(20)–C(21) | 1.447(7) | O(1)–Ti(1)–S(1) | 161.35(12) |
| C(24)–C(25) | 1.441(8) | C(21)–C(22) | 1.367(7) | N(1)–Ti(1)–S(1) | 77.11(11) |
| Ti(1)–O(1) | 1.825(3) | Cl(3)–Ti(1)–S(1) | 93.71(6) | ||
| Ti(1)–N(1) | 2.172(4) | Cl(2)–Ti(1)–S(1) | 87.51(6) | ||
| Ti(1)–Cl(3) | 2.2599(16) | Cl(1)–Ti(1)–S(1) | 81.57(6) | ||
| Ti(1)–Cl(2) | 2.2941(17) | O(2)–Ti(2)–N(2) | 84.69(16) | ||
| Ti(1)–Cl(1) | 2.2997(16) | O(2)–Ti(2)–Cl(6) | 105.11(12) | ||
| Ti(1)–S(1) | 2.5642(16) | N(2)–Ti(2)–Cl(6) | 170.20(13) | ||
| O(2)–Ti(2)–Cl(5) | 99.81(13) | ||||
| N(2)–Ti(2)–Cl(5) | 87.78(12) | ||||
| Cl(6)–Ti(2)–Cl(5) | 90.35(7) | ||||
| O(2)–Ti(2)–Cl(4) | 91.01(13) | ||||
| N(2)–Ti(2)–Cl(4) | 86.13(12) | ||||
| Cl(6)–Ti(2)–Cl(4) | 93.72(7) | ||||
| Cl(5)–Ti(2)–Cl(4) | 167.04(7) | ||||
| O(2)–Ti(2)–S(2) | 161.71(12) | ||||
| N(2)–Ti(2)–S(2) | 77.18(12) | ||||
| Cl(6)–Ti(2)–S(2) | 93.03(6) | ||||
| Cl(5)–Ti(2)–S(2) | 82.01(6) | ||||
| Cl(4)–Ti(2)–S(2) | 85.48(7) | ||||
Two possible pathways existed for the synthesis of ligands L1–L5 and complexes Ti2L1–Ti2L5 due to the presence of two different carbonyl groups in phenylene-bridged β-dione (2), as shown in Scheme 3. In the case that 1-phenylbutane-1,3-dione was employed for the preparation of enamine, X-ray crystallographic analysis showed that the acetyl group of 1-phenylbutane-1,3-dione reacted with amine (L7, Fig. 3).9c However, in the case of our bis-β-carbonylenamine ligands L1–L5, the single-crystal XRD proved that the alkylthio anilines reacted with the carbonyl group adjacent to phenylene group (Path B), not the one next to the tbutyl group (Path A), which resulted in far-separated and relatively independent titanium centers in complexes Ti2L1–Ti2L5 and would profoundly influence their catalytic performances for ethylene (co)polymerization.
From Fig. 1, it can be seen that in L2, the N1–C10–C11–C12–O1 and N2–C23–C24–C25–O2 are almost coplanar, which further formed two six-member rings via intramolecular hydrogen bonds between H and O1 or O2. The O1–C12 and O2–C25 bond lengths are 1.251 and 1.255 Å, respectively, a little longer than the typical C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond but much shorter than C–O single bond. The C10–N1 and C23–N2 bond distances are 1.376(7) and 1.370(7) Å, respectively, showing clearly that the C–N bonds are single bonds. Thus, the ligand L2 exists in β-carbonylenamine form. The C11–C12 and C24–C25 bond lengths are 1.444(8) and 1.441(8) Å, respectively, and the C10–C11 and C23–C24 bond lengths are 1.400(8) and 1.379(8) Å, respectively, which are all between C–C single bond (1.54 Å) and C
O double bond but much shorter than C–O single bond. The C10–N1 and C23–N2 bond distances are 1.376(7) and 1.370(7) Å, respectively, showing clearly that the C–N bonds are single bonds. Thus, the ligand L2 exists in β-carbonylenamine form. The C11–C12 and C24–C25 bond lengths are 1.444(8) and 1.441(8) Å, respectively, and the C10–C11 and C23–C24 bond lengths are 1.400(8) and 1.379(8) Å, respectively, which are all between C–C single bond (1.54 Å) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond (1.34 Å) and show a certain extent of delocalization of the double bonds. Furthermore, the distances of the corresponding bonds in two β-carbonylenamine units are a little different, which would provide different coordination environments for the two titanium metal centers.
C double bond (1.34 Å) and show a certain extent of delocalization of the double bonds. Furthermore, the distances of the corresponding bonds in two β-carbonylenamine units are a little different, which would provide different coordination environments for the two titanium metal centers.
As revealed by XRD analysis, complex Ti2L2 adopted a distorted octahedral configuration with each titanium coordinated with an oxygen, a nitrogen, a sulfur and three chlorine atoms. The O1–C9 and O2–C22 bond lengths were 1.338(6) and 1.322(6) Å, respectively, which were appreciably longer than the corresponding O1–C12 and O2–C25 bonds in the ligand. Compared with the C11–C12 and C24–C25 bonds in ligand, the corresponding C9–C8 and C22–C21 bond lengths (1.351(7), 1.367(7) Å) were significantly shorter and clearly showed some double bond characteristic. Furthermore, compared with the C–C and C–N bonds in ligand, the corresponding C7–C8 and C20–C21 bonds were elongated, and the C7–N1 and C20–N2 were shortened, all of which demonstrated that the ligand has converted to β-imino enol form after coordinating with titanium.
The bond angle sum of O1–Ti1–N1 (84.26°), N1–Ti1–S1 (77.11°), S1–Ti1–Cl3 (93.71°) and Cl3–Ti1–O1 (104.94°) is nearly 360°, so the N1, O1, S1, Cl3 and Ti1 are almost coplanar. The same is true for N2, O2, S2, Cl6 and Ti2. The three chlorine atoms on Ti1 are in a mer disposition with the bond angles of Cl1–Ti–Cl3, Cl2–Ti–Cl3 and Cl1–Ti–Cl2 of 93.22(6), 92.34(6) and 168.04(7)°, respectively, which is favorable for the olefin coordination and insertion. The Ti1–O1, Ti1–N1 and Ti1–S1 distances and the average Ti–Cl distance are close to the mononuclear β-carbonylenamine-derived [O−NS]TiCl3 complexes reported by Tang's group. The bond angle sum of C1–S1–C36 (103.7(3)°), C1–S1–Ti1 (95.87(17)°), and C36–S1–Ti1 (112.2(2)°) is 311.77°, suggesting that the S atom in Ti2L2 is sp3-hybridized. The dihedral angel between N1–C7–C8–C9–O1 and N1–Ti1–O1 or the C14–C15–C16–C17–C18–C19 ring are 32.98° and 42.35°, and the dihedral angel between N2–C20–C21–C22–O2 and N2–Ti2–O2 or the C14–C15–C16–C17–C18–C19 ring are 29.47° and 59.08°, respectively.
2.2. Ethylene polymerization
We investigated the catalytic performances of binuclear complexes Ti2L1–Ti2L5 towards ethylene polymerization under activation of MMAO, with the mononuclear analogue TiL6 for comparison, and the results were listed in Table 3.| Entry | Cat. | Al/Ti | Temp (°C) | PE (g) | Act.b | Mwc | Mw/Mn |
|---|---|---|---|---|---|---|---|
| a Toluene 30 ml, 2 μmol of catalyst, 1 atm ethylene pressure, reaction time 5 min.b Activity, 106 g mol (Ti)−1 h−1 atm−1.c 104 g mol−1, determined by GPC using polystyrene standard.d 4 μmol of catalyst. | |||||||
| 1 | Ti2L2 | 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
30 | 0.3200 | 0.96 | ||
| 2 | Ti2L2 | 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50 | 0.5608 | 1.68 | 3.62 | 3.65 |
| 3 | Ti2L2 | 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
70 | 0.3029 | 0.91 | ||
| 4 | Ti2L2 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50 | 0.3578 | 1.07 | ||
| 5 | Ti2L2 | 1500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50 | 0.3813 | 1.14 | ||
| 6 | Ti2L2 | 2000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50 | 0.3342 | 1.00 | ||
| 7 | Ti2L1 | 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50 | 0.4421 | 1.33 | 4.53 | 2.82 |
| 8 | Ti2L3 | 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50 | 0.3634 | 1.09 | 4.95 | 4.30 |
| 9 | Ti2L4 | 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50 | 0.2736 | 0.82 | 4.47 | 4.08 |
| 10 | Ti2L5 | 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50 | 0.6451 | 1.94 | 14.82 | 2.65 |
| 11d | TiL6 | 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50 | 0.5159 | 1.55 | 5.24 | 2.59 |
| 12d | TiL6 | 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
70 | 0.1415 | 0.42 | ||
In general, these binuclear complexes exhibited very high activity (over 106 g mol−1 h−1 atm−1) under suitable conditions, producing typical high-density polyethylene. The polymerization conditions such as reaction temperatures and Al/Ti molar ratios exerted great influence upon catalytic activity and polymer properties.
Firstly, we used Ti2L2 as catalyst precursor and explored the influence of polymerization temperature at 1 atm ethylene pressure with Al/Ti ratio fixed at 1000. When the reaction temperature was increased from 30 to 70 °C, the activity increased gradually to a maximum at 50 °C and then decreased slightly. The highest activity reached 1.68 × 106 g mol (Ti)−1 h−1 atm−1 at 50 °C (entry 2, Table 3), which was similar to that catalyzed by the mononuclear analogue TiL6/MMAO (1.55 × 106 g mol (Ti)−1 h−1 atm−1, entry 10 in Table 3). However, the binuclear complex appeared more stable at elevated temperature compared with the mononuclear TiL6. At 70 °C, the activity of Ti2L2/MMAO was still of 9.1 × 105 g mol (Ti)−1 h−1 atm−1, which was more than twice that of TiL6/MMAO at the same temperature.
Both bi- and mono-nuclear titanium catalysts catalyzed ethylene polymerization to produce polyethylene with over 104 g mol−1 of molecular weight (Mw). The molecular weight distribution (Mw/Mn) of polyethylene produced by TiL6/MMAO was only 2.59, which was typical of single active center; however the polymer obtained with Ti2L2/MMAO exhibited much wider polydispersity (3.65), indicating that two active centers may have formed, consistent with the asymmetrical crystal structure of the binuclear complex.
The catalytic activity of the binuclear catalyst was less sensitive to the Al/Ti molar ratio. The catalyst exhibited high activity of over 106 g mol (Ti)−1 h−1 atm−1 even at a low Al/Ti ratio of 500, and with Al/Ti ratio increased from 500 to 2000, the activity increased slightly to a maximum at an Al/Ti ratio of 1000 and then slowly decreased.
The catalytic performances of the binuclear complexes bearing different alkylthio and phenylthio sidearms were also compared. The steric hindrance of substituents on sulfur atom influenced both the catalytic activity and molecular weight of the resulting polyethylene. Take Ti2L2 and Ti2L3 for example (entry 2 vs. 8, Table 3), as the sidearm n-propylthio changed to bulkier iso-propylthio, the catalytic activity decreased from 1.68 to 1.09 × 106 g mol (Ti)−1 h−1 atm−1, while the molecular weight increased from 3.62 to 4.95 × 104 g mol−1. The complex Ti2L5 which bears the bulkier phenylthio sidearm produced polyethylene with still-higher molecular weight than those with the alkylthio sidearms (entry 10 vs. 2, 7–9, Table 3). Similar influences of steric hindrance have also been observed in mononuclear titanium complexes.9b
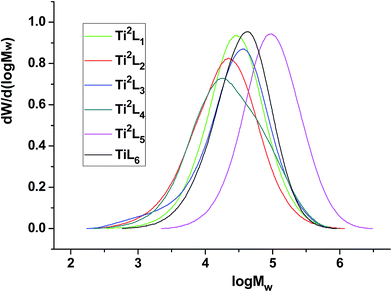
The influence of substituents was also investigated by varying the length of the linear alkylthio sidearms. Unlike the mononuclear analogues reported by Tang's group9c and the methylene-bridged salicylaldiminato binuclear titanium complexes reported by us15a previously, the catalytic activity decreased from 1.68 to 0.82 × 106 g mol (Ti)−1 h−1 atm−1 (entry 2 vs. 9, Table 3) when the substituent on sulfur atom was changed from n-propyl group to n-octyl group. However, replacement of n-propyl group with methyl group also decreased the activity slightly, due probably to the weaker solubility of Ti2L1 (entry 2 vs. 7, Table 3). With the increase of the alkyl chain length of the side group on sulfur atom, the molecular weight distribution of obtained PE increased gradually, while the molecular weight remained almost unchanged. The GPC curves for the PE samples were shown in Fig. 4.
2.3. Ethylene copolymerization with α-olefins
We also explored the catalytic behaviors of these binuclear complexes towards ethylene copolymerization with α-olefins, and the results were shown in Table 4.| Entry | Cat. | Comonomer (mmol) | Polymer (g) | Act. b | Tmc (°C) | Mwd | Mw/Mn | Incorpe (mol%) |
|---|---|---|---|---|---|---|---|---|
| a Toluene 30 ml, 2 μmol of catalyst, 1 atm ethylene pressure, 1000 Al/Ti molar ratio, polymerization temperature 30 °C, reaction time 5 min.b Activity, 106 g mol (Ti)−1 h−1 atm−1.c Melting temperature determined by DSC.d 104 g mol−1, determined by GPC using polystyrene standard.e Determined by high temperature 13C NMR.f 4 μmol of catalyst. | ||||||||
| 1 | Ti2L2 | C6(6) | 0.6807 | 2.04 | 107.0 | 5.1 | ||
| 2 | Ti2L2 | C6(12) | 1.4062 | 4.22 | 94.6 | 8.42 | 3.89 | 11.3 |
| 3 | Ti2L2 | C6(24) | 1.8634 | 5.59 | 84.5 | 18.7 | ||
| 4 | Ti2L2 | C6(36) | 1.4110 | 4.23 | — | 19.1 | ||
| 5 | Ti2L1 | C6(12) | 0.6548 | 1.96 | 99.2 | 3.42 | 3.20 | 18.3 |
| 6 | Ti2L3 | C6(12) | 1.2367 | 3.71 | 104.2 | 15.12 | 2.82 | 9.6 |
| 7 | Ti2L4 | C6(12) | 0.9662 | 2.90 | 88.9 | 14.51 | 2.75 | 7.7 |
| 8 | Ti2L5 | C6(12) | 1.0759 | 3.23 | 93.3 | 12.93 | 2.39 | 6.3 |
| 9f | TiL6 | C6(12) | 1.8898 | 5.67 | 106.7 | 3.36 | 3.04 | 17.2 |
| 10 | Ti2L2 | C8(12) | 1.5343 | 4.60 | 97.1 | 5.5 | ||
| 11 | Ti2L2 | C10(12) | 1.7985 | 5.40 | 96.9 | 8.4 | ||
All of these complexes showed extremely high activity for the copolymerization of ethylene and α-olefins, which were 2–5 times higher than the homopolymerization activity (Table 3). The products were branched polyethylene as revealed by their much reduced melting points and the high temperature 13C NMR spectra. The 1-hexene incorporation ratio in the copolymer could be flexibly tuned by the initial feed of α-olefin commoners and catalyst structures. It should be noted that these bis-β-carbonylenamine-derived binuclear titanium complexes showed much higher copolymerization activity and α-olefin incorporation ratio compared with the methylene-bridged bis-salicylaldiminato binuclear titanium complexes reported by us before under similar conditions.15a
The influences of 1-hexene feeds upon catalytic performances were investigated with Ti2L2/MMAO as a representative. As the feed of 1-hexene was increased from 6 to 36 mmol, the 1-hexene incorporation ratio increased sharply from 5.1 to 19.1 mol% (calculated from the 13C NMR spectra, entry 1–4, Table 4), while the copolymerization activity increased from 2.04 × 106 g mol (Ti)−1 h−1 atm−1 to a maximum of 5.59 × 106 g mol (Ti)−1 h−1 atm−1 at 24 mmol of 1-hexene, and then decreased slightly to 4.23 × 106 g mol (Ti)−1 h−1 atm−1 at 36 mmol. It appeared that within a certain range the activity of the binuclear Ti complex increased apparently with the increase of 1-hexene concentration, showing positive “comonomer effect”.
The structure of binuclear titanium complexes also affected their catalytic performances for ethylene/1-hexene copolymerization. Under the same conditions, the increase of steric hindrance of the substituents on sulfur atom reduced the copolymerization activity and 1-hexene incorporation ratio, but increased the molecular weight of obtained copolymers (entry 2 vs. 6, n-propyl vs. iso-propyl, entry 2 vs. 7, n-propyl vs. n-octyl, Table 4). Furthermore, replacement of n-propyl group with smaller sized methyl group enhanced significantly the 1-hexene incorporation ratio from 11.3 to 18.3 mol% and decreased the molecular weight from 8.42 to 3.42 × 104 g mol−1 (entry 2 vs. 5, Table 4). However, replacement of alkyl group with phenyl group on sulfur atom lowered the 1-hexene incorporation ratio (entry 2, 5–7 vs. 8, Table 4), which was in good accord with the salicylaldiminato mononuclear titanium complexes reported by Tang.9
The high temperature 13C NMR spectra of copolymers produced by Ti2L1, Ti2L2, Ti2L4 and Ti2L5 were shown in Fig. 5, with the corresponding carbon units marked for different peaks. Variation of branch density can be clearly observed, as demonstrated by the relative peak heights. The GPC curves for the ethylene/1-hexene copolymers obtained with bi- and mono-nuclear Ti complexes were shown in Fig. 6.
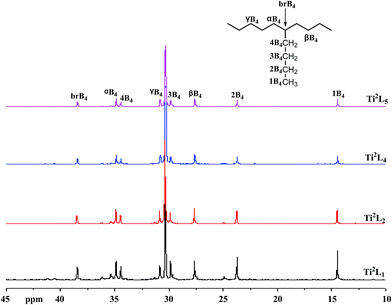 | ||
| Fig. 5 13C NMR spectra of PE samples from entries 2, 5, 7 and 8 in Table 4. | ||
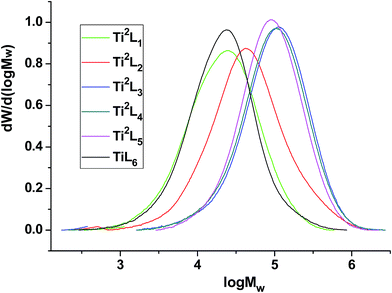 | ||
| Fig. 6 GPC curves of the ethylene/1-hexene copolymers obtained with bi- and mono-nuclear Ti complexes. | ||
Under the same conditions, complex Ti2L2 demonstrated lower copolymerization activity and 1-hexene incorporation ratio than those of its mononuclear counterpart TiL6 (entry 2 vs. 9, Table 4), probably due to the steric and electronic effects of the altered coordination environment. This type of binuclear titanium complexes showed negligible bimetallic cooperative effects due to the far-separation of the two titanium centers.
The binuclear titanium complex Ti2L2 could also efficiently catalyze ethylene copolymerization with 1-octene or 1-decene, with higher activity but lower comonomer incorporation ratio (entry 10 and 11, Table 4) compared with ethylene/1-hexene copolymerization.
3 Conclusions
A series of phenyl-bridged bis-β-carbonylenamine [ONSR] (R = alkyl or phenyl) tridentate ligands L1–L5 and their binuclear titanium complexes Ti2L1–Ti2L5 were synthesized and characterized. The molecular structures of ligand L2 (R = nPr) and its corresponding Ti complex Ti2L2 as studied by single-crystal X-ray diffraction revealed that each titanium coordinated with an oxygen, a nitrogen, a sulfur and three chlorine atoms to form a distorted octahedral configuration. Furthermore, the alkylthio or phenylthio anilines reacted with the carbonyl groups adjacent to phenylene group, resulting in isolated and relatively independent titanium centers in the complex. Compared with the mononuclear analogue TiL6, these complexes exhibited better thermal stability for ethylene polymerization and produced PE with higher molecular weight and wider polydispersity, suggesting that two active centers were formed. The molecular weight and α-olefin incorporation ratio can be flexibly tuned by the catalyst structure. The complex Ti2L5 which bears phenylthio sidearm exhibited higher activity towards ethylene polymerization and produced polyethylene with much higher molecular weight compared with the complexes bearing alkylthio sidearms, but resulted in lower 1-hexene incorporation ratio. Meanwhile, the 1-hexene incorporation ratio could also be tuned by the initial feed of the α-olefin commoner. However, this type of binuclear titanium complexes showed weak or negligible bimetallic cooperative effects due to the far separation of the titanium centers.4 Experimental section
4.1. General procedures
All manipulations involving air- and/or moisture-sensitive compounds were performed under dry nitrogen using standard Schlenk-line and glovebox. Toluene and hexane were purified by distillation over sodium/benzophenone ketyl, while CH2Cl2 was refluxed over CaH2. Gases and other solvents were purified by standard techniques. Modified methylaluminoxane (MMAO) was purchased from Akzo Chemical as a 7 wt% solution in heptane. All other chemical reagents were used as received unless noted otherwise.1H and 13C NMR spectra of ligands and complexes were recorded on a Bruker Avance III 400 MHz spectrometer with tetramethylsilane as an internal standard. Elemental analyses were carried out using Vario EL 111. 13C NMR spectra of polymers were obtained on a Varian XL 300 MHz spectrometer at 120 °C with o-C6D4Cl2 as the solvent. IR spectra were collected with a Nicolet Nexus 470 Fourier transform infrared (FTIR) spectrometer. DSC measurements were performed on a Netzsch DSC200 F3 instrument at a heating rate of 10 °C min−1 from 20 to 160 °C, with the melting points obtained from the endothermic peak of the second heating scan. The Mn and Mw/Mn of the polymers were determined at 150 °C with a Viscotek 350A HT-GPC System using a polystyrene calibration. 1,2,4-Trichlorobenzene was employed as the solvent at a flow rate of 1.0 mL min−1.
4.2. Synthesis of the ligands and binuclear titanium complexes
L1. To a 100 ml 3-neck flask were added 0.6837 g of 2, 0.6791 g of methylthio aniline, 40 ml of toluene, and 0.1 g of p-toluene sulfonic acid (TsOH) as catalyst, and heated to reflux for 24 h. The mixture was then vacuum dried, and recrystallized with ethanol to afford L1 as a yellow solid with 78% yield. 1H NMR (400 MHz, CDCl3): δ 12.05 (s, 1H), 7.30 (s, 1H), 7.25 (s, 1H), 7.23 (d, J = 5.0 Hz, 1H), 7.21–7.13 (m, 1H), 7.00 (t, J = 7.7 Hz, 1H), 6.79 (t, J = 7.6 Hz, 1H), 6.19 (d, J = 7.7 Hz, 1H), 5.45 (s, 1H), 2.51 (s, 3H), 1.24 (d, J = 21.4 Hz, 9H). 13C NMR (101 MHz, CDCl3): δ 206.30 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 158.87 (Ar-NH–C), 137.91, 136.55, 132.38, 129.14, 128.52, 127.94, 126.51, 125.14, 124.75, 124.71 (Ar-C), 97.14, 42.56, 27.60, 15.53. Anal. calcd for C34H40N2O2S2: C, 71.29; H, 7.04; N, 4.89%. Found: C, 71.38; H, 6.92; N, 4.75%. IR (KBr, cm−1): 3452, 3070, 2964, 1608, 1501, 1475, 1441, 1325, 1219, 1122, 1087, 751.
O), 158.87 (Ar-NH–C), 137.91, 136.55, 132.38, 129.14, 128.52, 127.94, 126.51, 125.14, 124.75, 124.71 (Ar-C), 97.14, 42.56, 27.60, 15.53. Anal. calcd for C34H40N2O2S2: C, 71.29; H, 7.04; N, 4.89%. Found: C, 71.38; H, 6.92; N, 4.75%. IR (KBr, cm−1): 3452, 3070, 2964, 1608, 1501, 1475, 1441, 1325, 1219, 1122, 1087, 751.Ligands L2–L6 were synthesized with a similar procedure as for L1.
L2. Yield: 80%. 1H NMR (400 MHz, CDCl3): δ 12.06 (s, 2H), 7.31 (s, 1H), 7.30 (s, 2H), 7.24 (d, J = 7.1 Hz, 2H), 7.19 (d, J = 8.6 Hz, 1H), 6.95 (t, J = 7.3 Hz, 2H), 6.81 (t, J = 7.3 Hz, 2H), 6.20 (d, J = 7.9 Hz, 2H), 2.93 (t, J = 7.3 Hz, 4H), 1.81–1.67 (m, 4H), 1.21 (s, 18H), 1.07 (t, J = 7.4 Hz, 6H). 13C NMR (101 MHz, CDCl3): δ 206.13 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 158.43 (Ar-NH–C), 139.19, 136.73, 130.21, 129.50, 129.11, 126.74, 125.82, 125.54, 124.97, 124.31 (Ar-C), 97.35, 42.56, 34.97, 27.61, 22.50, 13.62. Anal. calcd for C38H48N2O2S2: C, 72.57; H, 7.69; N, 4.45%. Found: C, 72.83; H, 7.88; N, 4.16%. IR (KBr, cm−1): 3446, 3063, 2959, 2868, 1615, 1589, 1571, 1533, 1497, 1474, 1451, 1325, 1286, 1123, 1088, 751.
O), 158.43 (Ar-NH–C), 139.19, 136.73, 130.21, 129.50, 129.11, 126.74, 125.82, 125.54, 124.97, 124.31 (Ar-C), 97.35, 42.56, 34.97, 27.61, 22.50, 13.62. Anal. calcd for C38H48N2O2S2: C, 72.57; H, 7.69; N, 4.45%. Found: C, 72.83; H, 7.88; N, 4.16%. IR (KBr, cm−1): 3446, 3063, 2959, 2868, 1615, 1589, 1571, 1533, 1497, 1474, 1451, 1325, 1286, 1123, 1088, 751.
L3. Yield: 68%. 1H NMR (400 MHz, CDCl3): δ 12.14 (s, 1H), 7.39 (d, J = 6.6 Hz, 1H), 7.29 (s, 1H), 7.25 (s, 1H), 6.90 (d, J = 21.1 Hz, 2H), 6.22 (s, 1H), 5.45 (s, 1H), 3.48 (s, 1H), 1.38 (s, 5H), 1.21 (s, 9H). 13C NMR (101 MHz, CDCl3): δ 203.30 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 183.66 (Ar-NH–C), 150.68, 138.85, 137.37, 136.14, 131.15, 130.33, 128.94, 125.87, 125.48, 117.36 (Ar-C), 92.40, 39.97, 36.12, 27.40, 23.02. Anal. calcd for C38H48N2O2S2: C, 72.57; H, 7.69; N, 4.45%. Found: C, 72.29; H, 7.35; N, 4.68%. IR (KBr, cm−1): 3451, 3061, 2966, 1623, 1386, 1316, 1189, 1088, 569, 461.
O), 183.66 (Ar-NH–C), 150.68, 138.85, 137.37, 136.14, 131.15, 130.33, 128.94, 125.87, 125.48, 117.36 (Ar-C), 92.40, 39.97, 36.12, 27.40, 23.02. Anal. calcd for C38H48N2O2S2: C, 72.57; H, 7.69; N, 4.45%. Found: C, 72.29; H, 7.35; N, 4.68%. IR (KBr, cm−1): 3451, 3061, 2966, 1623, 1386, 1316, 1189, 1088, 569, 461.
L4. Yield: 78%. 1H NMR (400 MHz, CDCl3): δ 12.08 (d, J = 20.4 Hz, 2H), 7.30 (d, J = 4.1 Hz, 2H), 7.29 (s, 1H), 7.27–7.22 (m, 2H), 7.22–7.14 (m, 1H), 6.95 (t, J = 7.7 Hz, 2H), 6.80 (t, J = 7.5 Hz, 2H), 6.20 (d, J = 8.2 Hz, 2H), 5.44 (s, 2H), 2.94 (t, J = 7.4 Hz, 4H), 1.77–1.67 (m, 4H), 1.46 (dd, J = 13.4, 6.5 Hz, 4H), 1.29 (dd, J = 9.8, 4.0 Hz, 16H), 1.21 (s, 18H), 0.89 (t, J = 6.5 Hz, 6H). 13C NMR (101 MHz, CDCl3): δ 206.02 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 158.39 (Ar-NH–C), 139.26, 136.78, 130.38, 129.56, 129.09, 128.47, 127.93, 125.53, 124.93, 124.28 (Ar-C), 97.37, 42.54, 39.24, 33.16, 31.82, 29.19, 28.97, 28.70, 27.61, 22.65, 14.10. Anal. calcd for C48H68N2O2S2: C, 74.95; H, 8.91; N, 3.64%. Found: C, 74.59; H, 8.62; N, 3.91%. IR (KBr, cm−1): 3448, 3061, 2926, 2858, 1608, 1564, 1466, 1315, 1220, 1188, 1100, 788.
O), 158.39 (Ar-NH–C), 139.26, 136.78, 130.38, 129.56, 129.09, 128.47, 127.93, 125.53, 124.93, 124.28 (Ar-C), 97.37, 42.54, 39.24, 33.16, 31.82, 29.19, 28.97, 28.70, 27.61, 22.65, 14.10. Anal. calcd for C48H68N2O2S2: C, 74.95; H, 8.91; N, 3.64%. Found: C, 74.59; H, 8.62; N, 3.91%. IR (KBr, cm−1): 3448, 3061, 2926, 2858, 1608, 1564, 1466, 1315, 1220, 1188, 1100, 788.
L5. Yield: 83%. 1H NMR (400 MHz, CDCl3): δ 12.29 (s, 2H), 7.43–6.83 (m, 22H), 6.20 (d, J = 7.7 Hz, 2H), 1.21 (s, 18H). 13C NMR (101 MHz, CDCl3): δ 206.11 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 158.26 (Ar-NH–C), 139.83, 136.59, 134.33, 132.50, 132.19, 129.48, 129.19, 128.94, 128.44, 127.73, 127.54, 127.16, 125.01, 124.37 (Ar-C), 97.24, 42.53, 27.61. Anal. calcd for C44H44N2O2S2: C, 75.83; H, 6.36; N, 4.02%. Found: C, 75.62; H, 6.53; N, 4.34%. IR (KBr, cm−1): 3447, 3059, 2964, 2865, 2360, 2342, 1616, 1571, 1558, 1497, 1475, 1439, 1289, 1120, 1089, 1023, 792, 750.
O), 158.26 (Ar-NH–C), 139.83, 136.59, 134.33, 132.50, 132.19, 129.48, 129.19, 128.94, 128.44, 127.73, 127.54, 127.16, 125.01, 124.37 (Ar-C), 97.24, 42.53, 27.61. Anal. calcd for C44H44N2O2S2: C, 75.83; H, 6.36; N, 4.02%. Found: C, 75.62; H, 6.53; N, 4.34%. IR (KBr, cm−1): 3447, 3059, 2964, 2865, 2360, 2342, 1616, 1571, 1558, 1497, 1475, 1439, 1289, 1120, 1089, 1023, 792, 750.
L6. Yield: 81%. 1H NMR (400 MHz, CDCl3): δ 12.22 (s, 1H), 7.30 (d, J = 11.2 Hz, 6H), 6.85 (d, J = 47.5 Hz, 2H), 6.34 (s, 1H), 5.66 (s, 1H), 2.95 (d, J = 4.7 Hz, 2H), 1.75 (s, 2H), 1.26 (s, 9H), 1.09 (d, J = 8.5 Hz, 3H). 13C NMR (101 MHz, CDCl3): δ 205.91 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 159.32 (Ar-NH–C), 139.77, 136.51, 132.89, 130.15, 129.56, 129.41, 128.44, 128.35, 128.14, 125.88, 124.48, 123.90 (Ar-C), 97.37, 52.07, 35.31, 27.71, 22.58, 13.56. Anal. calcd for C22H27NOS: C, 74.75; H, 7.70; N, 3.96%. Found: C, 74.39; H, 7.57; N, 3.73%. IR (KBr, cm−1): 3449, 2963, 1617, 1562, 1507, 1387, 1299, 1218, 1186, 1086, 802, 766.
O), 159.32 (Ar-NH–C), 139.77, 136.51, 132.89, 130.15, 129.56, 129.41, 128.44, 128.35, 128.14, 125.88, 124.48, 123.90 (Ar-C), 97.37, 52.07, 35.31, 27.71, 22.58, 13.56. Anal. calcd for C22H27NOS: C, 74.75; H, 7.70; N, 3.96%. Found: C, 74.39; H, 7.57; N, 3.73%. IR (KBr, cm−1): 3449, 2963, 1617, 1562, 1507, 1387, 1299, 1218, 1186, 1086, 802, 766.
4.3. Binuclear titanium complexes (Ti2L1–Ti2L4)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O), 172.20 (Ar-N
C–O), 172.20 (Ar-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 166.35, 151.34, 130.31, 130.02, 128.90, 128.08, 128.04, 125.46, 117.14, 111.60 (Ar-C), 92.59, 39.53, 27.79, 14.11. Anal. calcd for C34H38Cl6N2O2S2Ti2: C, 46.45; H, 4.36; N, 3.19%. Found: C, 46.82; H, 4.73; N, 3.31%. IR (KBr, cm−1): 3070, 2962, 2931, 2875, 1706, 1609, 1558, 1458, 1318, 1275, 1211, 1125, 1091, 765.
C), 166.35, 151.34, 130.31, 130.02, 128.90, 128.08, 128.04, 125.46, 117.14, 111.60 (Ar-C), 92.59, 39.53, 27.79, 14.11. Anal. calcd for C34H38Cl6N2O2S2Ti2: C, 46.45; H, 4.36; N, 3.19%. Found: C, 46.82; H, 4.73; N, 3.31%. IR (KBr, cm−1): 3070, 2962, 2931, 2875, 1706, 1609, 1558, 1458, 1318, 1275, 1211, 1125, 1091, 765.Complexes Ti2L2–Ti2L5 were prepared using the same procedure as for Ti2L1.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O), 165.78 (Ar-N
C–O), 165.78 (Ar-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 149.71, 141.36, 139.37, 136.75, 129.63, 126.57, 125.01, 117.34, 115.96, 113.45, 113.20, 111.53 (Ar-C), 98.55, 31.58, 27.55, 22.64, 14.10, 13.56. Anal. calcd for C38H46Cl6N2O2S2Ti2: C, 48.80; H, 4.96; N, 3.00%. Found: C, 49.25; H, 4.64; N, 3.43%. IR (KBr, cm−1): 2965, 1620, 1563, 1460, 1289, 1208, 1091, 838, 756.
C), 149.71, 141.36, 139.37, 136.75, 129.63, 126.57, 125.01, 117.34, 115.96, 113.45, 113.20, 111.53 (Ar-C), 98.55, 31.58, 27.55, 22.64, 14.10, 13.56. Anal. calcd for C38H46Cl6N2O2S2Ti2: C, 48.80; H, 4.96; N, 3.00%. Found: C, 49.25; H, 4.64; N, 3.43%. IR (KBr, cm−1): 2965, 1620, 1563, 1460, 1289, 1208, 1091, 838, 756.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O), 181.31 (Ar-N
C–O), 181.31 (Ar-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 160.32, 139.43, 136.34, 131.05, 129.67, 129.26, 128.02, 124.41, 112.45, 103.22 (Ar-C), 93.69, 31.58, 27.82, 27.54, 23.08. Anal. calcd for C38H46Cl6N2O2S2Ti2: C, 48.80; H, 4.96; N, 3.00%. Found: C, 48.51; H, 5.30; N, 2.76%. IR (KBr, cm−1): 2964, 1617, 1558, 1497, 1475, 1290, 1120, 1088, 884, 791, 755.
C), 160.32, 139.43, 136.34, 131.05, 129.67, 129.26, 128.02, 124.41, 112.45, 103.22 (Ar-C), 93.69, 31.58, 27.82, 27.54, 23.08. Anal. calcd for C38H46Cl6N2O2S2Ti2: C, 48.80; H, 4.96; N, 3.00%. Found: C, 48.51; H, 5.30; N, 2.76%. IR (KBr, cm−1): 2964, 1617, 1558, 1497, 1475, 1290, 1120, 1088, 884, 791, 755.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O), 157.75 (Ar-N
C–O), 157.75 (Ar-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 139.10, 136.71, 130.39, 129.34, 129.12, 127.91, 125.47, 124.96, 124.32, 111.80 (Ar-C), 97.36, 42.57, 33.04, 31.83, 31.61, 29.21, 29.14, 27.83, 27.61, 22.67, 14.16. Anal. calcd for C48H66Cl6N2O2S2Ti2: C, 53.60; H, 6.19; N, 2.60%. Found: C, 53.39; H, 6.37; N, 2.81%. IR (KBr, cm−1): 2956, 2925, 2854, 1617, 1574, 1560, 1483, 1461, 1264, 1146, 891, 762.
C), 139.10, 136.71, 130.39, 129.34, 129.12, 127.91, 125.47, 124.96, 124.32, 111.80 (Ar-C), 97.36, 42.57, 33.04, 31.83, 31.61, 29.21, 29.14, 27.83, 27.61, 22.67, 14.16. Anal. calcd for C48H66Cl6N2O2S2Ti2: C, 53.60; H, 6.19; N, 2.60%. Found: C, 53.39; H, 6.37; N, 2.81%. IR (KBr, cm−1): 2956, 2925, 2854, 1617, 1574, 1560, 1483, 1461, 1264, 1146, 891, 762.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O), 173.45 (Ar-N
C–O), 173.45 (Ar-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 156.12, 147.72, 144.86, 139.70, 134.83, 134.21, 132.26, 130.47, 129.63, 129.50, 129.43, 121.37, 109.07, 107.56 (Ar-C), 86.99, 31.58, 22.65. Anal. calcd for C44H42Cl6N2O2S2Ti2: C, 52.67; H, 4.22; N, 2.79%. Found: C, 52.18; H, 4.86; N, 3.31%. IR (KBr, cm−1): 3331, 2963, 1707, 1557, 1496, 1475, 1439, 1347, 1290, 1210, 1122, 1087, 1059, 1023, 793, 750, 691.
C), 156.12, 147.72, 144.86, 139.70, 134.83, 134.21, 132.26, 130.47, 129.63, 129.50, 129.43, 121.37, 109.07, 107.56 (Ar-C), 86.99, 31.58, 22.65. Anal. calcd for C44H42Cl6N2O2S2Ti2: C, 52.67; H, 4.22; N, 2.79%. Found: C, 52.18; H, 4.86; N, 3.31%. IR (KBr, cm−1): 3331, 2963, 1707, 1557, 1496, 1475, 1439, 1347, 1290, 1210, 1122, 1087, 1059, 1023, 793, 750, 691.4.4 Mononuclear titanium complex (TiL6)
TiL6 was prepared using the same procedure as for Ti2L1, except that the molar ratio of ligand L6 and TiCl4 was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2. Yield: 80%. 1H NMR (400 MHz, CDCl3) δ 7.53 (d, J = 7.7 Hz, 1H), 7.39 (d, J = 7.4 Hz, 1H), 7.34 (t, J = 7.3 Hz, 2H), 7.21 (d, J = 6.0 Hz, 2H), 7.06 (t, J = 7.1 Hz, 1H), 6.92 (t, J = 7.6 Hz, 1H), 6.45 (d, J = 8.2 Hz, 1H), 6.26 (s, 1H), 3.50 (s, 2H), 2.06 (dd, J = 14.9, 7.5 Hz, 2H), 1.36 (s, 9H), 1.21 (t, J = 7.4 Hz, 3H). 13C NMR (101 MHz, CDCl3): δ 190.20 (
1.2. Yield: 80%. 1H NMR (400 MHz, CDCl3) δ 7.53 (d, J = 7.7 Hz, 1H), 7.39 (d, J = 7.4 Hz, 1H), 7.34 (t, J = 7.3 Hz, 2H), 7.21 (d, J = 6.0 Hz, 2H), 7.06 (t, J = 7.1 Hz, 1H), 6.92 (t, J = 7.6 Hz, 1H), 6.45 (d, J = 8.2 Hz, 1H), 6.26 (s, 1H), 3.50 (s, 2H), 2.06 (dd, J = 14.9, 7.5 Hz, 2H), 1.36 (s, 9H), 1.21 (t, J = 7.4 Hz, 3H). 13C NMR (101 MHz, CDCl3): δ 190.20 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O), 173.64(Ar-N
C–O), 173.64(Ar-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 152.70, 138.76, 133.40, 133.36, 130.56, 129.59, 129.15, 128.67, 128.51, 127.33, 125.72, 112.00 (Ar-C), 100.13, 47.03, 39.50, 27.81, 21.81, 13.36. Anal. calcd for C22H26Cl3NOSTi: C, 52.15; H, 5.17; N, 2.76%. Found: C, 52.58; H, 5.49; N, 2.36%. IR (KBr, cm−1): 2964, 1617, 1559, 1506, 1087, 769.
C), 152.70, 138.76, 133.40, 133.36, 130.56, 129.59, 129.15, 128.67, 128.51, 127.33, 125.72, 112.00 (Ar-C), 100.13, 47.03, 39.50, 27.81, 21.81, 13.36. Anal. calcd for C22H26Cl3NOSTi: C, 52.15; H, 5.17; N, 2.76%. Found: C, 52.58; H, 5.49; N, 2.36%. IR (KBr, cm−1): 2964, 1617, 1559, 1506, 1087, 769.
4.5. Crystallographic analysis
Crystal data were collected on a Bruker APEX-II CCD diffractometer with graphite-monochromated Mo Kα radiation (λ = 0.71073 Å) at 130 K for Ti2L2. Crystals were coated in oil and then directly mounted on the diffractometer under a stream of cold nitrogen gas. A total of N reflections were collected by using ω scan mode. Corrections were applied for Lorentz and polarization effects as well as absorption using multi-scans (SADABS). All the structures were solved by direct method (SHELXS-97). The remaining non-hydrogen atoms were obtained from the successive difference Fourier maps. All non-H atoms were refined with anisotropic displacement parameters, while the H atoms were constrained to the parent sites, using a riding mode (SHELXTL). Details of the X-ray structure determinations and refinements are provided in Table 1. Other details are shown in the ESI.† CCDC numbers for L2 and Ti2L2 are CCDC 1587147 and 1587146,† respectively.4.6. Ethylene polymerization and copolymerization
A flame-dried Schlenk flask purged with N2 was filled with ethylene gas. 30 ml of freshly distilled toluene was added and raised to the reaction temperature for 10 min. MMAO was then injected using a syringe and the mixture was stirred for 5 min. The polymerization was initiated by adding a solution of the titanium complex in toluene with a syringe. After a desired time, the polymerization was quenched with acidified ethanol (100 mL, 8 vol% HCl in ethanol). The precipitated polymer was filtered off, washed with ethanol, then dried under vacuum overnight at 60 °C till a constant weight. For copolymerization, α-olefins (1-hexene, 1-octene or 1-decene) and MMAO were injected in sequence via a syringe.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for the financial support from the National Natural Science Foundation of China (21172269).Notes and references
- (a) H. Sinn and W. Kaminsky, Adv. Organomet. Chem., 1980, 18, 99 CrossRef CAS; (b) H. Sinn, W. Kaminsky, H. J. Vollmer and R. Woldt, Angew. Chem., Int. Ed., 1980, 19, 390 CrossRef; (c) H. G. Alt and A. Köppl, Chem. Rev., 2000, 100, 1205 CrossRef CAS PubMed; (d) L. Resconi, L. Cavallo, A. Fait and F. Piemontesi, Chem. Rev., 2000, 100, 1253 CrossRef CAS PubMed; (e) G. Fink, B. Steinmetz, J. Zechlin, C. Przybyla and B. Tesche, Chem. Rev., 2000, 100, 1377 CrossRef CAS PubMed; (f) G. W. Coates, Chem. Rev., 2000, 100, 1223 CrossRef CAS PubMed.
- Selected review article, see: (a) G. J. P. Britovsek, V. C. Gibson and D. F. Wass, Angew. Chem., Int. Ed., 1999, 38, 428 CrossRef CAS; (b) S. D. Ittel, L. K. Johnson and M. Brookhart, Chem. Rev., 2000, 100, 1169 CrossRef CAS PubMed; (c) S. Mecking, Angew. Chem., Int. Ed., 2001, 40, 534 CrossRef CAS; (d) V. C. Gibson and S. K. Spitzmesser, Chem. Rev., 2003, 103, 283 CrossRef CAS PubMed; (e) L. Bourget-Merle, M. F. Lappert and J. R. Severn, Chem. Rev., 2002, 102, 3031 CrossRef CAS PubMed; (f) V. C. Gibson, C. Redshaw and G. A. Solan, Chem. Rev., 2007, 107, 1745 CrossRef CAS PubMed; (g) K. Nomura and S. Zhang, Chem. Rev., 2011, 111, 2342 CrossRef CAS PubMed; (h) S. Budagumpi, K. H. Kim and I. Kim, Coord. Chem. Rev., 2011, 255, 2785 CrossRef CAS; (i) D. Zhang and G. Zi, Chem. Soc. Rev., 2015, 44, 1898 RSC; (j) B. L. Small, Acc. Chem. Res., 2015, 48, 2599 CrossRef CAS PubMed; (k) H. Mu, L. Pan, D. Song and Y. Li, Chem. Rev., 2015, 115, 12091 CrossRef CAS PubMed.
- Selected recent references: (a) C. Rong, F. Wang, W. Li and M. Chen, Organometallics, 2017, 36, 4458 CrossRef CAS; (b) Z. Chen, X. Zhao, X. Gong, D. Xu and Y. Ma, Macromolecules, 2017, 50, 6561 CrossRef CAS; (c) P. Ji, J. B. Solomon, Z. Lin, A. Johnson, R. F. Jordan and W. Lin, J. Am. Chem. Soc., 2017, 139, 11325 CrossRef CAS PubMed; (d) Z. Chen, E. Yao, J. Wang, X. Gong and Y. Ma, Macromolecules, 2016, 49, 8848 CrossRef CAS; (e) M. Zhao and C. Chen, ACS Catal., 2017, 7, 7490 CrossRef CAS; (f) D. Zhang, Y. Zhang, W. Hou, Z. Guan and Z. Huang, Organometallics, 2017, 36, 3758 CrossRef CAS; (g) P. Kenyon and S. Mecking, J. Am. Chem. Soc., 2017, 139, 13786 CrossRef CAS PubMed; (h) K. Lian, Y. Zhu, W. Li, S. Dai and C. Chen, Macromolecules, 2017, 50, 6074 CrossRef CAS; (i) D. Bézier, O. Daugulis and M. Brookhart, Organometallics, 2017, 36, 2947 CrossRef; (j) S. Zhong, Y. Tan, L. Zhong, J. Gao, H. Liao, L. Jiang, H. Gao and Q. Wu, Macromolecules, 2017, 50, 5661 CrossRef CAS; (k) L. Zhong, G. Li, G. Liang, H. Gao and Q. Wu, Macromolecules, 2017, 50, 2675 CrossRef CAS; (l) M. Chen and C. Chen, ACS Catal., 2017, 7, 1308 CrossRef CAS; (m) F. Ölscher, I. Göttker-Schnetmann, V. Monteil and S. Mecking, J. Am. Chem. Soc., 2015, 137, 14819 CrossRef PubMed; (n) J. L. Rhinehart, L. A. Brown and B. K. Long, J. Am. Chem. Soc., 2013, 135, 16316 CrossRef CAS PubMed; (o) D. Shoken, M. Sharma, M. Botoshansky, M. Tamm and M. S. Eisen, J. Am. Chem. Soc., 2013, 135, 12592 CrossRef CAS PubMed; (p) D. Zhang and C. Chen, Angew. Chem., Int. Ed., 2017, 56, 14672 CrossRef CAS PubMed; (q) M. Li, X. Wang, Y. Luo and C. Chen, Angew. Chem., Int. Ed., 2017, 56, 11604 CrossRef CAS PubMed; (r) B. K. Long, J. M. Eagan, M. Mulzer and G. W. Coates, Angew. Chem., Int. Ed., 2016, 55, 7106 CrossRef CAS PubMed; (s) L. Falivene, G. Boffa, S. O. Sánchez, L. Caporaso, A. Grassi and S. Mecking, Angew. Chem., Int. Ed., 2016, 55, 14378 CrossRef PubMed; (t) Nakano, S. Ito and K. Nozaki, Angew. Chem., Int. Ed., 2016, 55, 2835 CrossRef PubMed; (u) Z. Jian and S. Mecking, Angew. Chem., Int. Ed., 2015, 54, 15845 CrossRef CAS PubMed; (v) J. Sun, F. Wang, W. Lia and M. Chen, RSC Adv., 2017, 7, 55051 RSC; (w) Y. Zhang, C. Huang, X. Hao, X. Hu and W.-H. Sun, RSC Adv., 2016, 6, 91401 RSC; (x) X. Xiao, X. Hao, J. Bai, J. Chao, W. Cao and X. Chen, RSC Adv., 2016, 6, 60723 RSC; (y) W. Li, F. Su, S. Yuan, X. Duan, S. Bai and D. Liu, RSC Adv., 2016, 6, 40741 RSC; (z) S. Yuan, E. Yue, C. Wen and W.-H. Sun, RSC Adv., 2016, 6, 7431 RSC.
- E. B. Tjaden, D. C. Swenson, R. F. Jordan and J. L. Petersen, Organometallics, 1995, 14, 371 CrossRef CAS.
- P. G. Cozzi, E. Gallo, C. Floriani, A. Chiesivilla and C. Rizzoli, Organometallics, 1995, 14, 4994 CrossRef CAS.
- (a) H. Makio and T. Fujita, Acc. Chem. Res., 2009, 42, 1532 CrossRef CAS PubMed and references therein; (b) T. Matsugi and T. Fujita, Chem. Soc. Rev., 2008, 37, 1264 RSC and references therein; (c) H. Makio, H. Terao, A. Iwashita and T. Fujita, Chem. Rev., 2011, 111, 2363 CrossRef CAS PubMed and references therein; (d) T. Fujita and K. Kawai, Top. Catal., 2014, 57, 852 CrossRef CAS and references therein..
- (a) J. Tian and G. W. Coates, Angew. Chem., Int. Ed., 2000, 39, 3626 CrossRef CAS; (b) J. Tian, P. D. Hustad and G. W. Coates, J. Am. Chem. Soc., 2001, 123, 5134 CrossRef CAS PubMed; (c) P. D. Hustad and G. W. Coates, J. Am. Chem. Soc., 2002, 124, 11578 CrossRef CAS PubMed; (d) A. F. Mason and G. W. Coates, J. Am. Chem. Soc., 2004, 126, 10798 CrossRef CAS PubMed; (e) G. W. Coates, J. Am. Chem. Soc., 2008, 130, 4968 CrossRef PubMed.
- (a) X.-F. Li, K. Dai, W.-P. Ye, L. Pan and Y.-S. Li, Organometallics, 2004, 23, 1223 CrossRef CAS; (b) L. Pan, W.-P. Ye, J.-Y. Liu, M. Hong and Y.-S. Li, Macromolecules, 2008, 41, 2981 CrossRef CAS; (c) L. Pan, M. Hong, J.-Y. Liu, W.-P. Ye and Y.-S. Li, Macromolecules, 2009, 42, 4391 CrossRef CAS; (d) W.-P. Ye, J. Zhan, L. Pan, N.-H. Hu and Y.-S. Li, Organometallics, 2008, 27, 3642 CrossRef CAS; (e) M. Hong, J.-Y. Liu, B.-X. Li and Y.-S. Li, Macromolecules, 2011, 44, 5659 CrossRef CAS; (f) M. Hong, Y.-X. Wang, H.-L. Mu and Y.-S. Li, Organometallics, 2011, 30, 4678 CrossRef CAS.
- (a) C. Redshaw and Y. Tang, Chem. Soc. Rev., 2012, 41, 4484 RSC and references therein; (b) M. L. Gao, Y. F. Gu, C. Wang, X. L. Yao, X. L. Sun, C. F. Li, C. T. Qian, B. Liu, Z. Ma, Y. Tang, Z. W. Xie, S. Z. Bu and Y. Gao, J. Mol. Catal. A: Chem., 2008, 292, 62 CrossRef CAS; (c) X.-H. Yang, Z. Wang, X.-L. Sun and Y. Tang, Dalton Trans., 2009, 8945 RSC; (d) Z. Chen, J.-F. Li, W.-J. Tao, X.-L. Sun, X.-H. Yang and Y. Tang, Macromolecules, 2013, 46, 2870 CrossRef CAS; (e) C. Xu, Z. Chen, Q. Shen, X.-L. Sun and Y. Tang, J. Organomet. Chem., 2014, 761, 142 CrossRef CAS.
- (a) M. Delferro and T. J. Marks, Chem. Rev., 2011, 111, 2450 CrossRef CAS PubMed and references therein; (b) J. P. McInnis, M. Delferro and T. J. Marks, Acc. Chem. Res., 2014, 47, 2545 CrossRef CAS PubMed and references therein; (c) M. R. Salata and T. J. Marks, J. Am. Chem. Soc., 2008, 130, 12 CrossRef CAS PubMed; (d) M. R. Salata and T. J. Marks, Macromolecules, 2009, 42, 1920 CrossRef CAS.
- (a) P. Buchwalter, J. Rosé and P. Braunstein, Chem. Rev., 2015, 115, 28 CrossRef CAS PubMed; (b) I. Bratko and M. Gómez, Dalton Trans., 2013, 42, 10664 RSC.
- (a) M. R. Radlauer, A. K. Buckley, L. M. Henling and T. Agapie, J. Am. Chem. Soc., 2013, 135, 3784 CrossRef CAS PubMed; (b) M. R. Radlauer, M. W. Day and T. Agapie, J. Am. Chem. Soc., 2012, 134, 1478 CrossRef CAS PubMed.
- (a) S. L. Han, E. D. Yao, W. Qin, S. F. Zhang and Y. G. Ma, Macromolecules, 2012, 45, 4054 CrossRef CAS; (b) Q. Yan, K. Tsutsumi and K. Nomura, RSC Adv., 2017, 7, 41345 RSC; (c) M. Khoshsefat, S. Ahmadjo, S. M. M. Mortazavia and G. H. Zohuri, RSC Adv., 2016, 6, 88625 RSC; (d) D. Mandal, D. Chakraborty, V. Ramkumar and D. K. Chand, RSC Adv., 2016, 6, 21706 RSC; (e) M. Zhou, Q. Yang, H. Tong, L. Yan and X. Wang, RSC Adv., 2015, 5, 105292 RSC; (f) C. Redshaw, M. J. Walton, M. R. J. Elsegood, T. J. Priorand and K. Michiue, RSC Adv., 2015, 5, 89783 RSC; (g) M. Mandal, D. Chakrabortyand and V. Ramkumar, RSC Adv., 2015, 5, 28536 RSC; (h) Q. Xing, T. Zhao, Y. Qiao, L. Wang, C. Redshawand and W.-H. Sun, RSC Adv., 2013, 3, 26184 RSC.
- (a) G. Xie and C. Qian, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 211 CrossRef CAS; (b) G. Xie, Y. Li, J. Sun and C. Qian, Inorg. Chem. Commun., 2009, 12, 796 CrossRef CAS; (c) G. Xie, T. Li and A. Zhang, Inorg. Chem. Commun., 2010, 13, 1199 CrossRef CAS; (d) G. Xie, G. Liu, L. Li, T. Li, A. Zhang and J. Feng, Catal. Commun., 2014, 45, 7 CrossRef CAS; (e) G. Xie, X. Zhang, T. Li, L. Li, G. Liu and A. Zhang, J. Mol. Catal. A: Chem., 2014, 383–384, 121 CrossRef CAS; (f) G. Xie, W. Song, T. Li, X. Xu, Z. Lan, Y. Li and A. Zhang, J. Appl. Polym. Sci., 2014, 131, 41178 Search PubMed; (g) T. Li, W. Song, H. Ai, Q. You, A. Zhang and G. Xie, J. Polym. Res., 2015, 22, 631 CrossRef; (h) X. Xiao, Y. Wen, X. Wang, L. Lei, P. Xia, T. Li, A. Zhang and G. Xie, Russ. J. Coord. Chem., 2015, 41, 543 CrossRef CAS; (i) L. Wang, X. You, Q. You, T. Li, A. Zhang and G. Xie, Transition Met. Chem., 2016, 41, 857 CrossRef CAS.
- (a) T. Li, Z. Lan, G. Xie, D. Luo, L. Li, S. Xiong, L. Zhang, L. Ouyang and A. Zhang, Catal. Lett., 2017, 147, 996 CrossRef CAS; (b) L. Zhang, X. Chen, X. Xiao, D. Luo, Y. Zeng, T. Li, X. Li, A. Zhang and G. Xie, J. Organomet. Chem., 2018, 856, 50 CrossRef CAS.
- J. K. Clegg, D. J. Bray, K. Gloe, K. Gloe, K. A. Jolliffe, G. A. Lawrance, L. F. Lindoy, G. V. Meehane and M. Wenzel, Dalton Trans., 2008, 1331 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1587147 and 1587146 contain the supplementary crystallographic data for L2 and Ti2L2. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8ra00071a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |