Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
In vitro combinations of inert phenolato Ti(IV) complexes with clinically employed anticancer chemotherapy: synergy with oxaliplatin on colon cells†
N. Ganot and
E. Y. Tshuva *
*
The Institute of Chemistry, The Hebrew University of Jerusalem, Jerusalem 9190401, Israel. E-mail: edit.tshuva@mail.huji.ac.il
First published on 6th February 2018
Abstract
Advanced anticancer phenolato titanium(IV) complexes were combined with known chemotherapeutic anticancer drugs applied in the clinic and were analyzed in vitro on cell lines most sensitive to the Ti(IV) complex and relevant to the clinical application of the known drugs. Combination of the Ti(IV) complex with cisplatin on ovarian cells showed mostly an additive behavior, also on a line resistant to cisplatin. Combination of the Ti(IV) complex with fluorouracil on colon cells gave near additive behavior, and that with oxaliplatin gave a synergistic behavior at a wide range of Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt ratios, but only when the drugs were administered together. Increasing the time intervals between the administration of Ti and of Pt turned the behavior to antagonistic, suggesting some deactivation of Pt by the Ti agent. For combinations where the drugs were applied together, the behavior depended on the effect level, and higher effects gave greater synergism, implying that technical aspects such as solubility are influential. Nevertheless, more complex patterns recorded for combinations where the drugs had been applied separately suggested multiple mechanisms with different concentration dependence. Overall the results point to high medicinal potential for the tested compounds for anticancer combination treatments.
Pt ratios, but only when the drugs were administered together. Increasing the time intervals between the administration of Ti and of Pt turned the behavior to antagonistic, suggesting some deactivation of Pt by the Ti agent. For combinations where the drugs were applied together, the behavior depended on the effect level, and higher effects gave greater synergism, implying that technical aspects such as solubility are influential. Nevertheless, more complex patterns recorded for combinations where the drugs had been applied separately suggested multiple mechanisms with different concentration dependence. Overall the results point to high medicinal potential for the tested compounds for anticancer combination treatments.
Introduction
Cisplatin (Chart 1) and its derivatives are significant components in the chemotherapeutic treatment of many types of cancer.1–3 Cisplatin is often used for ovarian, testicular, and bladder cancers, applied mostly in combination with other drugs.4–12 Oxaliplatin (Chart 1), a second generation platinum-based drug, serves as a common treatment for colon cancer, especially in combination with the thymidylate synthase inhibitor fluorouracil (Chart 1).13–15 Nevertheless, the high toxicity of the platinum ion and the development of drug resistance in many cases encourage scientists to search for other metal based drugs.16–24 Among the metals studied, titanium based complexes exhibit reduced toxicity and wide activity range, without resistance development known to date.25–37 In particular, the advanced diaminobis(phenolato)-bis(alkoxo)Ti(IV) complexes that we have introduced show enhanced cytotoxic activity and exceptional water resistance with no decomposition for weeks in water solutions and no activity decrease following one week in biological medium.38 Importantly, the leading phenolato Ti(IV) complex L1Ti (Chart 2) not only showed efficacy in vivo in reducing mortality in treated mice inoculated with lymphoma, but also showed no clinical signs of toxicity to the treated animals.38 This complex also exhibited promising results when evaluated on the NCI-60 panel of the Developmental Therapeutics Program (DTP) of the National Cancer Institute (NCI), with a wide range of activity and significant cytotoxicity toward all cell lines tested, whereby especially high sensitivity was recorded for colon and ovarian cell lines.38Combination therapy is therefore a common method for treating cancer diseases. Combination of two or more drugs enables achieving the desired effect but with reduced dose of each drug, which may consequently reduce the side effects of the drugs and the chances for resistance development.39,40 In a previous research, early generation of phenolato Ti(IV) (“salan”) complexes displayed synergistic and additive behaviours when combined with randomly selected organic or inorganic anticancer drugs for in vitro testing on certain cell types.41 Herein, specific in vitro combinations of the advanced phenolato Ti(IV) complexes with known drugs are presented; the NCI-60 results provide an opportunity to select the most suitable cell lines for analysis. Thus, the lines were chosen as those particularly sensitive to L1Ti based on the NCI-60 screen, namely, ovarian and colon lines,38 and the combined drugs were chosen as those commonly employed in the clinic for a tested cell type, namely, cisplatin and oxaliplatin/fluorouracil, respectively. Additive and synergistic behaviours were often detected, both with a significant medical value.
Experimental
The ligands L1H4 and L2H4 and their complexes L1Ti and L2Ti were synthesized as previously described.38 cis-Dichlorodiammine platinum(II) 99% was purchased from Acros, 5-fluorouracil 99% was purchased from Apollo Scientific Ltd, and oxaliplatin was purchased from Glentham Life Sciences Ltd.Cytotoxicity was measured on HT-29 colon cancer cells obtained from ATCC Inc, and A2780 ovarian and A2780-cp cisplatin-resistant ovarian cancer cells obtained from ECACC Inc., using the MTT assay as previously described.42 Approximately 0.6 × 106 cells in medium (contains: 1% penicillin/streptomycin antibiotics; 1% L-glutamine; 10% fetal bovine serum (FBS) and 88% medium RPMI-1640, all purchased from Biological Industries Inc.) were seeded into a 96-well plate and allowed to attach for a day. The cells were consequently treated with the reagent or combination of reagents tested at fixed ratios at 10 different concentrations. After a standard of 3 days incubation at 37 °C in 5% CO2 atmosphere, MTT (0.1 mg in 20 μL) was added and the cells were incubated for additional 3 hours. For experiments where the Pt complex was inserted with certain time intervals after the Ti complex, the incubation time was measured starting from the first administration. After the incubation period, the MTT solution was removed, and the cells were dissolved in 200 μL isopropanol. The absorbance at 550 nm was measured by a Bio-Tek EL-800 microplate reader spectrophotometer or by a Spark 10M Multimode Microplate Reader spectrophotometer. Relative IC50 values were determined by a nonlinear regression of a variable slope (four parameters) model by Graph Pad Prism 5.04 program, with error values based on the STD of the at least 3 × 3 repetitions (three separate measurements conducted on three different days to give nine repeats altogether). Some dose response curves can be found in the ESI.†
The interactions between the combined reagents were evaluated with the isobolographic method and the multiple effect analysis (based on the median effect principle),43,44 using Graph Pad Prism 5.04 program.
Pt cellular accumulation studies were conducted using ICP-MS Agilent 7500cx (Agilent Technologies Inc., USA). HT-29 cells were cultured in 6-well plates at density of ∼800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well and allowed to attach overnight. Cisplatin, oxaliplatin, or the combination of each with L1Ti complex were separately added for incubation of 24 h (37 °C, 5% CO2 atmosphere). The medium was then removed and the cells were washed three times with DPBS (purchased from Biological Industries Inc., Israel). The cells underwent three freeze/thaw cycles, and total protein was determined using the Lowry protein assay.45,46 A known amount of each sample was lyophilized using a VirTis Benchtop K Lyophilized (SP industries, USA). The dried cell samples were dissolved in >68% HNO3 (Primar Plus – Trace analysis grade, purchased from Fisher Chemical) and were left overnight for complete cell digestion and degradation under heat. The samples were then dissolved in 1% HNO3 in TDW (obtained from PURELAB Classic, ELGA). The Pt concentration in the cell samples are presented as a mean ± SD of three independent experiments relative to the control (Pt complex alone) normalized to 100%.
000 cells per well and allowed to attach overnight. Cisplatin, oxaliplatin, or the combination of each with L1Ti complex were separately added for incubation of 24 h (37 °C, 5% CO2 atmosphere). The medium was then removed and the cells were washed three times with DPBS (purchased from Biological Industries Inc., Israel). The cells underwent three freeze/thaw cycles, and total protein was determined using the Lowry protein assay.45,46 A known amount of each sample was lyophilized using a VirTis Benchtop K Lyophilized (SP industries, USA). The dried cell samples were dissolved in >68% HNO3 (Primar Plus – Trace analysis grade, purchased from Fisher Chemical) and were left overnight for complete cell digestion and degradation under heat. The samples were then dissolved in 1% HNO3 in TDW (obtained from PURELAB Classic, ELGA). The Pt concentration in the cell samples are presented as a mean ± SD of three independent experiments relative to the control (Pt complex alone) normalized to 100%.
Results and discussion
General
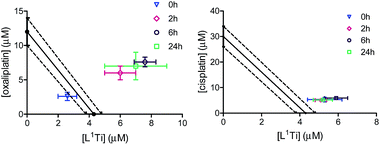
Two phenolato Ti(IV) complexes, L1Ti and L2Ti (Chart 2), were synthesized as previously published, as confirmed by 1H NMR.38 The cytotoxicity of these complexes alone and in combination with known drugs – cisplatin, oxaliplatin, and fluorouracil, was measured by the MTT assay according to a published protocol (See ESI† for dose–response curves).42 The behaviours of the combinations were first analysed by the well-established isobolographic method.43,44 An isobologram graph represents the concentrations of the drugs required to achieve a defined effect, normally applied for inhibition of 50% of cell viability. Each axe presents the concentration of one of the combined drugs; the IC50 values of each drug when administrated alone are plotted on the axes, and the line connecting these dots is the additive line, which represents eqn (1) when the combination index (CI) is 1 ((ICx)A,B: the concentration of drug A and B that inhibits x% of cell viability when administrated alone; CA, CB: the concentration of drugs A, B that inhibits x% of cell viability when administrated in combination).
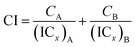 | (1) |
The additive line is accompanied by the error range presented by dashed lines, as derived from the error values of the individual IC50 values. In the combination experiment the drugs are combined at a fixed ratio, the 50% cell growth inhibition point is identified, and the concentration of each drug at that point is plotted on the isobologram graph. Dots located above the additive region indicate antagonistic behaviour, and those located bellow it indicate synergism.
Combinations on ovarian cancer cells
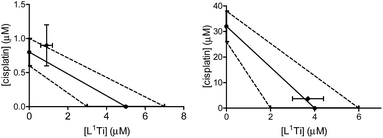
L1Ti was combined with cisplatin, commonly employed in the clinic for ovarian cancer,8,9 and the combination was tested on human ovarian A2780 cell line and cisplatin-resistant human ovarian A2780-cp cell line. The drugs were combined at a fixed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. The results are presented in Fig. 1.
1 ratio. The results are presented in Fig. 1.
The combination on both cell lines showed additive or near-additive behaviours. This implies that the two anti cancer agents act as if they were administered alone, and there are no significant beneficial or destructive interactions between them. The even more profound additive effect on the line resistant to cisplatin implies that the presence of the Ti complex did not affect the cell resistance,38,47–49 to the better or to the worse. Nevertheless, the additive behaviour has a marked medicinal value, as it should enable lowering the dose of the toxic cisplatin.
Combinations on colon cancer cells
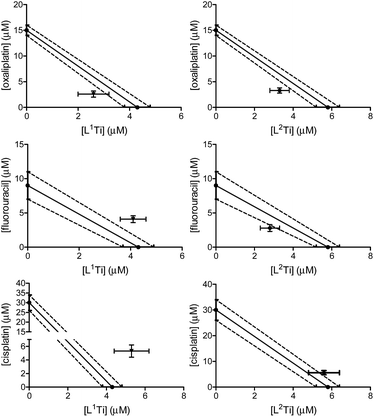
The cytotoxicity of L1,2Ti, separately combined with the colon cancer drugs oxaliplatin and fluorouracil,13–15 was tested on human colon HT-29 cells. Cisplatin was also evaluated as a lead drug for comparison. The drugs were first combined at a fixed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. The results are presented in Fig. 2.
1 ratio. The results are presented in Fig. 2.
The combinations of both complexes with oxaliplatin showed a synergistic behaviour. This behaviour may result from beneficial interactions between the drugs, related mechanisms of action, or simply improved technical aspects such as solubility or cellular penetration. The combinations of fluorouracil with L1,2Ti exhibited near additive behaviours, where that of L1Ti is more antagonistic and that of L2Ti is on the borderline with synergistic behaviour. Because these complexes are suspected to operate by a similar (although still undetermined) mechanism, it is yet to be concluded whether this difference is indeed meaningful, as different ligand substitution was shown previously to impact the behaviours of the combination.41 Nevertheless, it is not unlikely that these differences simply derive from different solubility/accessibility of the differently substituted complexes.
Interestingly, combinations of both complexes with cisplatin on the colon cancer cells showed an antagonistic or near-antagonistic behaviour. Because cisplatin is not a common drug to treat colon cancer and provides relatively high IC50 values,50,51 it is reasonable that the big difference in IC50 values between the two compounds would result in decreased accuracy of the isobolographic analysis. Nevertheless, some destructive interaction between the drugs cannot be ruled out.
One most promising combinations showing a synergistic behaviour, L1Ti with oxaliplatin, were selected for further studies. Because previous studies showed that the ratio of the combined agents may affect the behaviour of the combination,41 L1Ti was combined with oxaliplatin at different ratios up to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Ti
1, Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt (Fig. 3). All the ratios tested consistently showed synergistic behaviour, with little impact of the particular ratio on the behaviour of the combination. This observation provides additional flexibility to an envisioned potential treatment, especially as the Ti agent shows markedly reduced side effects.38,47,52–54 Interestingly, combinations of L1Ti with cisplatin at different rations analysed for comparison showed a clear impact of the ratio employed (Fig. 3). Although the 1
Pt (Fig. 3). All the ratios tested consistently showed synergistic behaviour, with little impact of the particular ratio on the behaviour of the combination. This observation provides additional flexibility to an envisioned potential treatment, especially as the Ti agent shows markedly reduced side effects.38,47,52–54 Interestingly, combinations of L1Ti with cisplatin at different rations analysed for comparison showed a clear impact of the ratio employed (Fig. 3). Although the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio gave antagonism, increasing the ratio in favour of Ti turned the behaviour to additive, whereby increasing further the ratio to 10
1 ratio gave antagonism, increasing the ratio in favour of Ti turned the behaviour to additive, whereby increasing further the ratio to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Ti
1 Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt turned the behaviour back to antagonistic. This may again derive from the potentially decreased accuracy of the analysis due to the low activity of cisplatin relative to that of L1Ti.
Pt turned the behaviour back to antagonistic. This may again derive from the potentially decreased accuracy of the analysis due to the low activity of cisplatin relative to that of L1Ti.
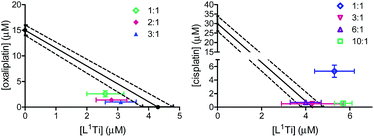 | ||
| Fig. 3 Isobolographic analysis of the anti-proliferative activity of the combinations of L1Ti with oxaliplatin (left) and cisplatin (right) at different ratios against human colon HT-29 cancer cells. | ||
To shed additional light on possible interactions between the combined drugs and their time dependence,41 the combinations of L1Ti with oxaliplatin or cisplatin in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Ti
1 Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt ratio were analysed with varying time intervals between the administration of the two drugs, applying the Ti(IV) complex first (Fig. 4). Interestingly, similar IC50 values were obtained in all experiments involving combination with cisplatin, regardless of the period between administrations. This observation supports the notion that the antagonistic behaviour observed for the 1
Pt ratio were analysed with varying time intervals between the administration of the two drugs, applying the Ti(IV) complex first (Fig. 4). Interestingly, similar IC50 values were obtained in all experiments involving combination with cisplatin, regardless of the period between administrations. This observation supports the notion that the antagonistic behaviour observed for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of the drugs applied together is not a result of specific destructive interactions, which should have been reduced with increasing the time between administrations. For oxaliplatin, however, the behaviour became antagonistic when the drugs are not applied simultaneously, as observed previously for related combinations.41 This behaviour may reflect either a beneficial interactions of the drugs upon mixing, or cellular interactions of L1Ti that hamper the reactivity of oxaliplatin or affect its cell entry.
1 ratio of the drugs applied together is not a result of specific destructive interactions, which should have been reduced with increasing the time between administrations. For oxaliplatin, however, the behaviour became antagonistic when the drugs are not applied simultaneously, as observed previously for related combinations.41 This behaviour may reflect either a beneficial interactions of the drugs upon mixing, or cellular interactions of L1Ti that hamper the reactivity of oxaliplatin or affect its cell entry.
To further examine whether L1Ti affects cell entry of cisplatin or oxaliplatin as a source of the antagonistic or synergistic behaviors, respectively, the amount of Pt in the cells after treatment with cisplatin/oxaliplatin alone, or combined with L1Ti, was determined. Colon HT-29 cells were exposure to the platinum complex at 3× IC50 concentrations (90 μM for cisplatin; 36 μM for oxaliplatin) or the platinum complex at the same concentration combined with L1Ti (3× IC50; 13 μM) for 24 hours. The total protein as a measure of the number of cells was determined by the Lowry protein assay, and the Pt concentration was measured by ICP-MS (Fig. 5). L1Ti did not impact Pt entry to cells, neither for cisplatin nor for oxaliplatin, with similar Pt concentrations obtained for both experiments. Similar results were obtained when the Ti was administered prior to Pt administration (see ESI†). These results rule out altered cell entry as a source for the antagonistic/synergistic behaviors.
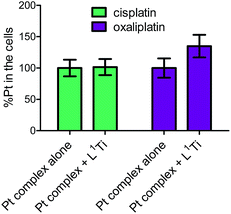 | ||
| Fig. 5 Normalized Pt levels in cells treated with cisplatin (90 μM) or oxaliplatin (36 μM) with or without L1Ti (13 μM). | ||
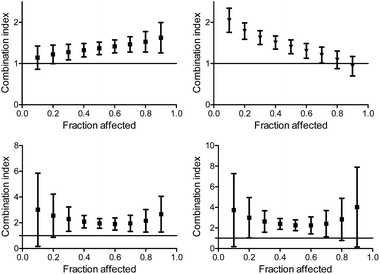
The multiple drug effect analysis
The isobologram analysis, although can conveniently present several experiments on a single plot, is limited to the presentation of only a single effect for each experiment (herein 50% cell growth inhibition). The combination index analysis of multiple effects (based on the median effect principle)43,44,55 presents the behaviour of a combination in a given experiment at various cell growth inhibition levels. The x axe represents the different effect levels, and the y axe is the calculated combination index (CI) according to eqn (1). CI = 1 represents an additive behaviour, CI < 1 represents synergism, and CI > 1 represents antagonism. This more complex analysis was therefore applied for selected experiments to get a deeper understanding of the parameters of influence.The CI curves of the combination of L1Ti with oxaliplatin or cisplatin at different ratios (Fig. 6, ESI†) interestingly demonstrate that the behavior of the combination is effect-dependent, and a behavior observed for the mid-point (50% cell growth inhibition) is not necessarily the same for all effects. The combinations studied herein for different ratios of the compounds administered together all exhibit a similar trend, where the larger the affect, the stronger the synergism (or the weaker the antagonism). Thus, aiming at maximal effects, this relatively wide synergism obtained for the combination with oxaliplatin is highly beneficial, as the antagonism at low effects is medically insignificant. Additionally, for the combination with cisplatin, some high effects in the 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 6
1 and 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Ti
1 Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt ratios also fall in the near synergistic zone (Fig. 6, ESI†), although the edge ratio points tested of 1
Pt ratios also fall in the near synergistic zone (Fig. 6, ESI†), although the edge ratio points tested of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 10
1 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 combinations are completely antagonistic for all effects.
1 combinations are completely antagonistic for all effects.
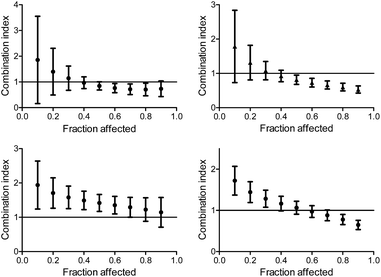 | ||
Fig. 6 Combination index for different effects on human colon HT-29 cancer cells by the combination of L1Ti with oxaliplatin (top) and cisplatin (bottom) at ratios 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (left) and 1 1 (left) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (right). 3 (right). | ||
When applying the multiple effect analysis on the measurements with time intervals between administrations, the behavior remained antagonistic for all effects but a different trend is observed (Fig. 7); whereas for the combination with oxaliplatin, the mid effects are least antagonistic, for the combination with cisplatin the trend depends on the period between administrations. This complicated behavior may reflect more than a single mechanism ongoing for each drug, with different concentration dependence.
Conclusions
This paper presents the in vitro combinations of two advanced phenolato Ti(IV) complexes with known, clinically employed anticancer chemotherapeutic drugs, on lines that were identified as particularly sensitive to the Ti(IV) agent based on the NCI-60 screen. When the combined drugs were analysed on the lines relevant to their reactivity in the clinic, mostly additive and synergistic behaviours were observed; specifically, combination with cisplatin on ovarian cells gave an additive behaviour, and combination with oxaliplatin on colon cells gave synergism at various ratios. Both behaviours are medically valuable to potentially enable lowering the doses of each drug to reduce side effects, especially that of the more toxic platinum drug.The Ti(IV) complex did not affect the penetration of the Pt complexes to the cancer cells; nevertheless, it is important to administer the combined drugs simultaneously to achieve the optimal effect.41 It thus cannot be ruled out that activation of some cellular pathways by the Ti(IV) complex when it is administered prior to the Pt agent may hamper the reactivity of the Pt compound, developing partial Pt resistance. This is especially plausible as the more profound CI analysis of multiple effect levels clearly indicated antagonism for all effect points when the compounds were administered with certain time intervals in between, although different patterns of concentration dependence were detected; this implies that multiple mechanisms with different concentration dependence may be ongoing for one or both of the drugs. Interestingly, this analysis run on simultaneously applied combinations often showed behaviour change upon concentration increase, implying that the reasons for non-additive behaviours in these cases more likely relate to technical aspects (solubility, accessibility), rather than complex cellular bio-interactions. It is thus a plausible conclusion that different mechanisms are ongoing for the two drugs, especially concerning the COMPARE analysis previously reported, showing no distinct correlations of the results obtained on the NCI-60 panel with those of known drugs.38 It is yet to be determined whether the target of the Ti(IV) complexes is also DNA.36
To conclude, we found that specific combinations of promising anticancer phenolato Ti(IV) complexes with known drugs applied simultaneously at a range of possible ratios on the relevant cancer cells were mostly additive or synergistic. The behaviour may change depending on the effect level, and mostly, the higher the effect level the better is the behaviour, as would be preferred for medicinal applications. The results presented herein call for additional carefully designed and clinically relevant in vivo combination studies, which are currently ongoing in our laboratory.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Funding was received from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement 681243).References
- D. Wang and S. J. Lippard, Nat. Rev. Drug Discovery, 2005, 4, 307 CrossRef CAS PubMed.
- L. Kelland, Nat. Rev. Cancer, 2007, 7, 573 CrossRef CAS PubMed.
- S. Dasari and P. Bernard Tchounwou, Eur. J. Pharmacol., 2014, 740, 364 CrossRef CAS PubMed.
- H. B. Grossman, R. B. Natale, C. M. Tangen, V. O. Speights, N. J. Vogelzang, D. L. Trump, R. W. deVere White, M. F. Sarosdy, D. P. Wood, D. Raghavan and E. D. Crawford, N. Engl. J. Med., 2003, 349, 859 CrossRef CAS PubMed.
- H. von der Maase, S. W. Hansen, J. T. Roberts, L. Dogliotti, T. Oliver, M. J. Moore, I. Bodrogi, P. Albers, A. Knuth, C. M. Lippert, P. Kerbrat, P. Sanchez Rovira, P. Wersall, S. P. Cleall, D. F. Roychowdhury, I. Tomlin, C. M. Visseren-Grul and P. F. Conte, J. Clin. Oncol., 2000, 18, 3068 CrossRef CAS PubMed.
- D. F. Bajorin, M. F. Sarosdy, D. G. Pfister, M. Mazumdar, R. J. Motzer, H. I. Scher, N. L. Geller, W. R. Fair, H. Herr and P. Sogani, J. Clin. Oncol., 1993, 11, 598 CrossRef CAS PubMed.
- P. M. Wilkinson and G. Read, J. Clin. Oncol., 1997, 15, 594 CrossRef PubMed.
- R. Agarwal and S. B. Kaye, Nat. Rev. Cancer, 2003, 3, 502 CrossRef CAS PubMed.
- D. K. Armstrong, B. Bundy, L. Wenzel, H. Q. Huang, R. Baergen, S. Lele, L. J. Copeland, J. L. Walker and R. A. Burger, N. Engl. J. Med., 2006, 354, 34 CrossRef CAS PubMed.
- A. P. Jekunen, R. D. Christen, D. R. Shalinsky and S. B. Howell, Br. J. Cancer, 1994, 69, 299 CrossRef CAS PubMed.
- A. Jekunen, J. Vick, R. Sanga, T. C. Chan and S. B. Howell, Cancer Res., 1992, 52, 3566 CAS.
- T. Boulikas and M. Vougiouka, Oncol. Rep., 2004, 11, 559 CAS.
- D. Cunningham and D. Cunningham, Lancet, 2010, 375, 1030 CrossRef.
- T. André, C. Boni, L. Mounedji-Boudiaf, M. Navarro, J. Tabernero, T. Hickish, C. Topham, M. Zaninelli, P. Clingan, J. Bridgewater, I. Tabah-Fisch, A. de Gramont, F. Hôpital Tenon and D. Gramont, N. Engl. J. Med., 2004, 350, 2343 CrossRef PubMed.
- R. M. Goldberg, D. J. Sargent, R. F. Morton, C. S. Fuchs, R. K. Ramanathan, S. K. Williamson, B. P. Findlay, H. C. Pitot and S. R. Alberts, J. Clin. Oncol., 2004, 22, 23 CrossRef CAS PubMed.
- I. Ott and R. Gust, Arch. Pharm., 2007, 340, 117 CrossRef CAS PubMed.
- S. H. van Rijt and P. J. Sadler, Drug Discovery Today, 2009, 14, 1089 CrossRef CAS PubMed.
- M. A. Jakupec, M. Galanski, V. B. Arion, C. G. Hartinger and B. K. Keppler, Dalton Trans., 2008, 183 RSC.
- P. C. A. Bruijnincx and P. J. Sadler, Curr. Opin. Chem. Biol., 2008, 12, 197 CrossRef CAS PubMed.
- B. Desoize, Anticancer Res., 2004, 24, 1529 CAS.
- M. Galanski, V. B. Arion, M. A. Jakupec and B. K. Keppler, Curr. Pharm. Des., 2003, 9, 2078 CrossRef CAS PubMed.
- F. Trudu, F. Amato, P. Vaňhara, T. Pivetta, E. M. Peña-Méndez and J. Havel, J. Appl. Biomed., 2015, 13, 79 CrossRef.
- S. P. Fricker, Dalton Trans., 2007, 4903 RSC.
- M. Marloye, G. Berger, M. Gelbcke and F. Dufrasne, Future Med. Chem., 2016, 8, 2263 CrossRef CAS PubMed.
- P. M. Abeysinghe and M. M. Harding, Dalton Trans., 2007, 3474 RSC.
- E. Y. Tshuva and J. A. Ashenhurst, Eur. J. Inorg. Chem., 2009, 2203 CrossRef CAS.
- E. Y. Tshuva and D. Peri, Coord. Chem. Rev., 2009, 253, 2098 CrossRef CAS.
- E. Meléndez, Crit. Rev. Oncol. Hematol., 2002, 42, 309 CrossRef.
- F. Caruso and M. Rossi, Mini-Rev. Med. Chem., 2004, 4, 49 CrossRef CAS PubMed.
- F. Caruso, M. Rossi and C. Pettinari, Expert Opin. Ther. Pat., 2001, 11, 969 CrossRef CAS.
- B. K. Keppler, C. Friesen, H. G. Moritz, H. Vongerichten and E. Vogel, Struct. Bonding, 1991, 78, U1 CrossRef.
- P. Koepf-Maier and H. Koepf, Chem. Rev., 1987, 87, 1137 CrossRef CAS.
- K. Strohfeldt and M. Tacke, Chem. Soc. Rev., 2008, 37, 1174 RSC.
- E. Y. Tshuva and M. Miller, in Metallo-Drugs: Development and Action of Anticancer Agents, Walter de Gruyter, Berlin, 2018, pp. 219–249 Search PubMed.
- S. A. Loza-Rosas, M. Saxena, Y. Delgado, K. Gaur, M. Pandrala and A. D. Tinoco, Metallomics, 2017, 9, 346 RSC.
- M. Cini, T. D. Bradshaw and S. Woodward, Chem. Soc. Rev., 2017, 46, 1040 RSC.
- I. Kostova, Adv. Anticancer Agents Med. Chem., 2009, 9, 827 CrossRef CAS.
- S. Meker, O. Braitbard, M. D. Hall, J. Hochman and E. Y. Tshuva, Chem.–Eur. J., 2016, 22, 9986 CrossRef CAS PubMed.
- S. M. Patel and L. D. Saravolatz, Med. Clin. North Am., 2006, 90, 1183 CrossRef CAS PubMed.
- M. L. Toews and D. B. Bylund, Proc. Am. Thorac. Soc., 2005, 2, 282 CrossRef CAS PubMed.
- N. Ganot, B. Redko, G. Gellerman and E. Y. Tshuva, RSC Adv., 2015, 5, 7874 RSC.
- N. Ganot, S. Meker, L. Reytman, A. Tzubery and E. Y. Tshuva, J. Visualized Exp., 2013, e50767 Search PubMed.
- R. J. Tallarida, F. Porreca and A. Cowan, Life Sci., 1989, 45, 947 CrossRef CAS PubMed.
- T.-C. Chou and P. Talalay, Adv. Enzyme Regul., 1984, 22, 27 CrossRef CAS PubMed.
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, J. Biol. Chem., 1951, 193, 265 CAS.
- G. L. Peterson, Anal. Biochem., 1979, 100, 201 CrossRef CAS PubMed.
- C. M. Manna, O. Braitbard, E. Weiss, J. Hochman and E. Y. Tshuva, ChemMedChem, 2012, 7, 703 CrossRef CAS PubMed.
- S. Meker, O. Braitbard, K. Margulis-Goshen, S. Magdassi, J. Hochman and E. Tshuva, Molecules, 2015, 20, 18526 CrossRef CAS PubMed.
- T. A. Immel, U. Groth and T. Huhn, Chem.–Eur. J., 2010, 16, 2775 CrossRef CAS PubMed.
- W. C. Reinhold, M. Sunshine, H. Liu, S. Varma, K. W. Kohn, J. Morris, J. Doroshow and Y. Pommier, Cancer Res., 2012, 72, 3499 CrossRef CAS PubMed.
- CellMiner – Analysis Tools, https://discover.nci.nih.gov/cellminer/analysis.do, accessed 3 January 2018.
- J. Schur, C. M. Manna, A. Deally, R. W. Koster, M. Tacke, E. Y. Tshuva and I. Ott, Chem. Commun., 2013, 49, 4785 RSC.
- K. M. Buettner and A. M. Valentine, Chem. Rev., 2012, 112, 1863 CrossRef CAS PubMed.
- M. Miller, O. Braitbard, J. Hochman and E. Y. Tshuva, J. Inorg. Biochem., 2016, 163, 250 CrossRef CAS PubMed.
- T. C. Chou, Cancer Res., 2010, 70, 440–446 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra00229k |
| This journal is © The Royal Society of Chemistry 2018 |