Open Access Article
Open Access ArticleRevealing the in situ NaF generation balance for user-friendly controlled synthesis of sub-10 nm monodisperse low-level Gd3+-doped β-NaYbF4:Er†
Ji-Wei Shen *,
Zhiqing Wang
*,
Zhiqing Wang ,
Xiaoxuan Wei,
Jiawei Liu
,
Xiaoxuan Wei,
Jiawei Liu and
Yinmao Wei
and
Yinmao Wei *
*
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of the Ministry of Education, College of Chemistry & Materials Science, Northwest University, Xi'an 710069, China. E-mail: jiweish@nwu.edu.cn; ymwei@nwu.edu.cn
First published on 6th March 2018
Abstract
Herein, user-friendly control of the synthesis of sub-10 nm hexagonal (β-) NaYbF4:Er nanocrystals (NCs) with extremely low-level Gd3+ doping (0%, 10 mol%) was achieved. We reveal for the first time that the effective sodium/fluoride levels during the formation of cubic (α-) nuclei are not only controlled by the sodium/fluoride to rare-earth precursor ratios used, but also sensitively restricted by the in situ NaF generation reaction in a sodium oleate-based solvothermal system. Excessive in situ NaF generation will lead to a respective sodium- and fluoride-deficient environment, delayed α-to-β transition and larger β-NCs. Based on these effects, sub-10 nm monodisperse low-level Gd3+-doped β-NaYbF4:Er was obtained with a user-friendly low fluoride dosage by finely balancing this NaF generation reaction and achieving an intrinsic optimized sodium-fluoride level for NC nucleation. Notably, our work represents the first example where the focus is on the competing in situ NaF generation reaction and its use for nucleation regulation, as well as for the user-friendly control of the solvothermal synthesis of sub-10 nm β-NaYbF4:Er.
Introduction
The controlled synthesis of multifunctional rare-earth upconversion nanocrystals (UCNCs) is useful for various applications.1–6 As a facile strategy, Gd3+ doping could provide synergistic controlled synthesis and multiple functionalities for promising UCNCs obtained via the solvothermal method, especially for β-NaYbF4-based materials.7–10 The sodium and fluoride sources in the solvothermal synthesis of Gd3+-doped β-NaYbF4 are generally supplied by a methanolic solution containing NaOH and NH4F according to a typical optimal sodium/fluoride/rare-earth (Na/F/RE) ratio of 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.11 When the molar doping ratio of Gd3+ reaches as high as 30% and 40%, the diameter of the Gd3+-doped β-NaYbF4 particles is ∼20 nm and ∼12 nm, respectively. However, the particle diameters are still as high as ∼80 nm and ∼44 nm when the molar doping ratios of Gd3+ are 10% and 20%, respectively.9,10 It is highly desirable to achieve sub-10 nm β-NaYbF4 by using low-level Gd3+ doping (e.g., 0% or 10%), which may be more favorable for high upconversion efficiency.
1.11 When the molar doping ratio of Gd3+ reaches as high as 30% and 40%, the diameter of the Gd3+-doped β-NaYbF4 particles is ∼20 nm and ∼12 nm, respectively. However, the particle diameters are still as high as ∼80 nm and ∼44 nm when the molar doping ratios of Gd3+ are 10% and 20%, respectively.9,10 It is highly desirable to achieve sub-10 nm β-NaYbF4 by using low-level Gd3+ doping (e.g., 0% or 10%), which may be more favorable for high upconversion efficiency.
Insights into the intrinsic UCNC nucleation process should provide new strategies that can overcome the above synthesis control issues. Surveying the literature, we noticed that the previous solvothermal nucleation investigations were mainly focused on regulating the nucleation time, solvent compositions and Na/F/RE ratio from a macroscopic viewpoint.12–14 For achieving small-sized β-UCNCs, sodium oleate (NaOA) used as a sodium source has recently shown its attractively superior properties compared to the most often used NaOH sodium source. Haase and co-workers pioneered the α-nucleus composition versus Na/RE ratio investigations and achieved an efficient NaOA-based solvothermal UCNC controlled synthesis strategy.14,15 However, the Na/RE ratio control in their work was also conducted from the macroscopic point of view. A high Na/F/RE ratio (e.g., 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) that was far in excess of the typical value of 2.5
1) that was far in excess of the typical value of 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was essential for achieving α-nuclei with a high sodium content in order to obtain a fast α-to-β transition and small-sized β-UCNCs according to their strategy. The formation of sodium-deficient α-nuclei under a low Na/F/RE ratio of 2
1 was essential for achieving α-nuclei with a high sodium content in order to obtain a fast α-to-β transition and small-sized β-UCNCs according to their strategy. The formation of sodium-deficient α-nuclei under a low Na/F/RE ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was unreasonable to some degree because such a ratio was close to the typical value of 2.5
1 was unreasonable to some degree because such a ratio was close to the typical value of 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The same high Na/F/RE ratio of 8
1. The same high Na/F/RE ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was also used for the preparation of sub-10 nm β-NaYbF4:Tm, and reagent-consuming synthesis was seemingly unavoidable for sub-10 nm β-NaYbF4.16 The intrinsic reasons for the variations in the number of β-nuclei and the UCNC controlled synthesis effects via variation of the Na/F/RE ratio are still unclear.14,16
1 was also used for the preparation of sub-10 nm β-NaYbF4:Tm, and reagent-consuming synthesis was seemingly unavoidable for sub-10 nm β-NaYbF4.16 The intrinsic reasons for the variations in the number of β-nuclei and the UCNC controlled synthesis effects via variation of the Na/F/RE ratio are still unclear.14,16
Fine-tuning of the intrinsic sodium/fluoride levels, as well as their functional morphologies, and the resultant impacts on α-nuclei and β-UCNC formation appear to have been overlooked somewhat in the past. Recently, we achieved small-sized β-NaYbF4:Er (∼11.0 nm) with a size close to 10 nm by using a NaOA-based solvothermal strategy, but with a low level of sodium and fluoride sources (NaOA/F/RE, 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).17 Such small-sized β-NaYbF4:Er was formed due to low in situ NaOA/NH4F-to-NaF conversion reactivity but enhanced NH4F-to-HF decomposition reactivity. We can imagine that the high Na/F/RE ratio (e.g., 8
1).17 Such small-sized β-NaYbF4:Er was formed due to low in situ NaOA/NH4F-to-NaF conversion reactivity but enhanced NH4F-to-HF decomposition reactivity. We can imagine that the high Na/F/RE ratio (e.g., 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-based solvothermal systems described in the literature will provide not only a large amount of in situ generated NaF, but also the user-unfriendly HF. The UCNC nucleation process actually consists of two separate but competing reactions of NaF generation and α-nuclei formation. To date, this in situ NaF generation reaction has received almost no attention in UCNC controlled synthesis. Our motivation was thus to achieve the user-friendly control of the synthesis of sub-10 nm low-level Gd3+-doped β-NaYbF4:Er by using a low Na/F/RE ratio.
1)-based solvothermal systems described in the literature will provide not only a large amount of in situ generated NaF, but also the user-unfriendly HF. The UCNC nucleation process actually consists of two separate but competing reactions of NaF generation and α-nuclei formation. To date, this in situ NaF generation reaction has received almost no attention in UCNC controlled synthesis. Our motivation was thus to achieve the user-friendly control of the synthesis of sub-10 nm low-level Gd3+-doped β-NaYbF4:Er by using a low Na/F/RE ratio.
In this work, the definite competing reactions of in situ NaF generation and α-nuclei formation during NaOA-based solvothermal UCNC nucleation were proposed. In contrast with the typical highly skewed NH4F/RE ratio optimization process (e.g., from 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 8
1 to 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), we revealed for the first time that fine-tuning of the NH4F/RE ratio in the vicinity of the stoichiometric value could sensitively balance the competing in situ NaF generation reaction during NaOA-based solvothermal α-nuclei formation and thus achieve user-friendly and reagent-saving control of the synthesis of sub-10 nm low-level Gd3+-doped β-NaYbF4:Er by using a very low Na/F/RE ratio.
1), we revealed for the first time that fine-tuning of the NH4F/RE ratio in the vicinity of the stoichiometric value could sensitively balance the competing in situ NaF generation reaction during NaOA-based solvothermal α-nuclei formation and thus achieve user-friendly and reagent-saving control of the synthesis of sub-10 nm low-level Gd3+-doped β-NaYbF4:Er by using a very low Na/F/RE ratio.
Experimental
Materials
All chemicals used were of at least analytical grade. Oleic acid (HOA, technical grade, 90%), 1-octadecene (ODE, technical grade, 90%), sodium oleate and rare-earth acetates were obtained from Sigma-Aldrich (St. Louis, MO, USA). Ammonium fluoride (NH4F, 98%) was purchased from Aladdin Industrial Corporation (Shanghai, China).Fine tuning of the sodium/fluoride level for NaOA-based solvothermal controlled synthesis of NaYbF4:Er (2 mol%)
The synthesis procedures were performed according to the NaOA-based solvothermal strategy for the preparation of UCNCs reported in the literature with some modifications.18 A mixed solution of HOA/ODE (3.6 mL/9 mL) was added to a three-necked flask containing the corresponding 0.6 mmol of rare-earth acetates. A heating procedure (160 °C for 30 min) was then performed to obtain rare-earth oleates. On cooling down to 35 °C, NaOA (1.5 mmol) was introduced into this system, followed by stirring at this temperature for 10 min. The designed amount of NH4F dissolved in methanol was then added, followed by stirring at 35 °C for 30 min and an increase in the system temperature to 100 °C. The system was cooled down naturally after final UCNC growth at 300 °C.The as-obtained NaYbF4:Er was purified and re-dispersed in cyclohexane, and the insoluble component was isolated via centrifugation prior to product characterization. The X-ray powder diffraction (XRD) results indicated that NaF comprised the majority of the insoluble component. The mass of the insoluble component was thus used to reflect the NaF content of the system.
To achieve a reagent-saving controlled synthesis strategy for sub-10 nm β-NaYbF4:Er, unless otherwise specified, the Na/RE ratio was kept constant at 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in this work.
1 in this work.
Monitoring of the NaF level during solvothermal synthesis
The solvothermal reactions were stopped by rapid cooling with the help of cool air flow. NaF was collected via washing the centrifugal precipitates with cyclohexane.Yb(OA)3 preparation
A mixed solution of HOA/ODE (3.6 mL/9 mL) was added to a 50 mL flask containing 0.6 mmol of Yb(CH3COO)3. Then, the reaction system was heated at 160 °C for 30 min to obtain Yb(OA)3. The as-formed Yb(OA)3 was collected via centrifugation after addition of ethanol (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 5 min).
000 rpm, 5 min).
Monitoring of the nucleation reactions in NaOA-based solvothermal controlled synthesis of NaYbF4:Er UCNCs
The NaOA-based solvothermal UCNC synthesis reactions were stopped immediately after the system reached the predetermined temperature, followed by rapid cooling down to room temperature with the help of cool air flow. The precipitate was collected by direct centrifugation or centrifugation after the addition of ethanol. Then, washing procedures were performed by re-dispersing the corresponding precipitate in cyclohexane, followed by the addition of ethanol, and centrifugation.Seed-mediated epitaxial growth of NaYF4 onto β-NaYbF4:Gd/Er
A shell of NaYF4 was grown onto the β-NaYbF4:Gd/Er UCNCs by using procedures based on the conventional seed-mediated epitaxial growth strategy.17Preparation of β-NaYbF4:Gd/Er by using a nano-sized NaF-based solvothermal strategy
The β-NaYbF4:Gd/Er was prepared according to the procedure reported in the literature.10 Nano-sized NaF was formed by dissolving NaOH and NH4F into methanol.17 Following the literature, nano-sized NaF, instead of NaOA, was used as the source of the sodium supply.Characterization
High-resolution transmission electron microscopy (HRTEM), high-angle annular dark-field (HAADF), element mapping and energy-dispersive X-ray analysis (EDXA) were performed using a Tecnai G2 F20 microscope (FEI, USA). A D8 ADVANCE X-ray diffractometer (Bruker, Germany) with Cu Kα radiation (λ = 0.15418 nm) was used to collect XRD patterns. Upconversion luminescence spectra of the different samples were collected using a RF-5301 spectrofluorometer (Shimadzu, Japan). The samples were excited by a 980 nm laser source.Results and discussion
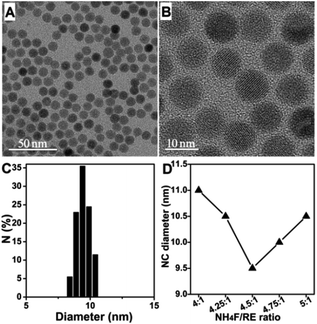
Tuning the NH4F/RE ratio in the vicinity of the stoichiometric value had a significant effect on the NC size in the NaOA-based solvothermal NaYbF4:Er synthesis. As reported previously, a conventional stoichiometric ratio of NH4F/RE (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) provided β-NaYbF4:Er particles with an average diameter of 11.0 nm ± 6.5% by reacting at 300 °C for 20 min.17 In this work, we found that a slight increase in the NH4F/RE ratio from 4
1) provided β-NaYbF4:Er particles with an average diameter of 11.0 nm ± 6.5% by reacting at 300 °C for 20 min.17 In this work, we found that a slight increase in the NH4F/RE ratio from 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4.25
1 to 4.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 provided monodisperse and smaller β-NaYbF4:Er particles (10.5 nm, Fig. S1A†), showing an attractive NC diameter decrease effect.
1 provided monodisperse and smaller β-NaYbF4:Er particles (10.5 nm, Fig. S1A†), showing an attractive NC diameter decrease effect.
Therefore, fine-tuning of the NH4F/RE ratio (4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4.75
1, 4.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 5
1 and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was carried out to explore the possibility of achieving sub-10 nm β-NaYbF4:Er. The NC diameter showed a decreasing and then an increasing tendency with an increased NH4F/RE ratio, and sub-10 nm narrowly size-distributed β-NaYbF4:Er (9.5 nm ± 5.6%) was obtained at the NH4F/RE ratio of 4.5
1) was carried out to explore the possibility of achieving sub-10 nm β-NaYbF4:Er. The NC diameter showed a decreasing and then an increasing tendency with an increased NH4F/RE ratio, and sub-10 nm narrowly size-distributed β-NaYbF4:Er (9.5 nm ± 5.6%) was obtained at the NH4F/RE ratio of 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
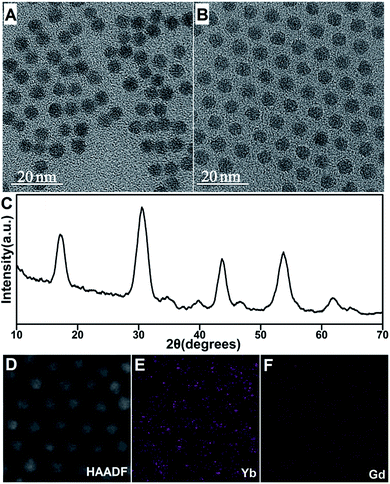
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 1A–D, S1†). The peak width evolution of the as-prepared β-NaYbF4:Er in the XRD pattern results was in agreement with the NC diameter variation tendency (Fig. S2†). Encouragingly, the use of precise modification of the NH4F/RE ratio overcame the issue of controlled synthesis of sub-10 nm monodisperse β-NaYbF4:Er. Notably, the optimal NH4F/RE ratio differed from the conventional optimal ratio of 4
1 (Fig. 1A–D, S1†). The peak width evolution of the as-prepared β-NaYbF4:Er in the XRD pattern results was in agreement with the NC diameter variation tendency (Fig. S2†). Encouragingly, the use of precise modification of the NH4F/RE ratio overcame the issue of controlled synthesis of sub-10 nm monodisperse β-NaYbF4:Er. Notably, the optimal NH4F/RE ratio differed from the conventional optimal ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
To reveal the origin of the above sensitive dependence of the NaYbF4:Er growth characteristics on the fluoride level, further investigations of the UCNC growth characteristics under different NaOA/NH4F/RE ratios were performed (Table 1). An even higher NH4F/RE ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 provided α-, (α + β)- and β-NaYbF4:Er by reacting at 300 °C for 20 min, 30 min and 45 min, respectively, showing a definite delayed phase transition (Fig. S3†). The diameter of the β-NaYbF4:Er particles obtained by using a higher NH4F/RE ratio (6
1 provided α-, (α + β)- and β-NaYbF4:Er by reacting at 300 °C for 20 min, 30 min and 45 min, respectively, showing a definite delayed phase transition (Fig. S3†). The diameter of the β-NaYbF4:Er particles obtained by using a higher NH4F/RE ratio (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was much larger than that of the particles obtained by using a lower NH4F/RE ratio (49.6 nm ± 4.5%, Fig. 2A vs. Fig. 1A).
1) was much larger than that of the particles obtained by using a lower NH4F/RE ratio (49.6 nm ± 4.5%, Fig. 2A vs. Fig. 1A).
| NaOA/NH4F/RE ratio | 20 min | 30 min | 45 min |
|---|---|---|---|
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
α | α + β | β |
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
α | α | α + β |
1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
α + β | β | |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
α | α + β | β |
The formation of larger β-UCNCs under a much higher fluoride level may be attributed to the high surface coverage of F− ions on the NCs according to the theory proposed by Gao et al.19 It is possible that a universal and clearer NC nucleation theory could be established to render this F− ion coverage theory compatible with the abovementioned α-nuclei sodium abundance theory15 and thus supply useful guidelines for solvothermal UCNC controlled synthesis.
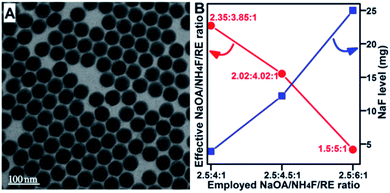
The important role of the sodium and fluoride levels in the solvothermal UCNC growth suggested that the in situ NaF generation reaction may have significant effects on the NC nucleation and growth. First, the system NaF levels were monitored after reacting at 300 °C for 0 min in the NaOA-based NaYbF4:Er synthesis system. Since the α-to-β transition of NaYbF4:Er was difficult, α-nuclei and NaF were collected at this stage. The mass of the collected NaF was 3.9 mg, 12.2 mg and 25.0 mg when the NaOA/NH4F/RE ratio was 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2.5
1, 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5
4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2.5
1 and 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively, showing an obvious tendency of increasing with increased NH4F/RE ratio (Fig. 2B). Therefore, the actual sodium and fluoride source, which could participate in the α-nuclei formation rather than in the inert NaF generation, supplied effective NaOA/NH4F/RE ratios of 2.35
1, respectively, showing an obvious tendency of increasing with increased NH4F/RE ratio (Fig. 2B). Therefore, the actual sodium and fluoride source, which could participate in the α-nuclei formation rather than in the inert NaF generation, supplied effective NaOA/NH4F/RE ratios of 2.35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.85
3.85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2.02
1, 2.02![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.02
4.02![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1.5
1 and 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for the used NaOA/NH4F/RE ratios of 2.5
1 for the used NaOA/NH4F/RE ratios of 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2.5
1, 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5
4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2.5
1 and 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively (Fig. 2B). A significant amount of sodium/fluoride participated in NaF generation rather than in UCNC nucleation, and we therefore can suggest that the larger β-NaYbF4:Er particles obtained at low and high NH4F/RE ratios were due to the formation of fluoride-deficient and sodium-deficient α-nuclei formation environments, respectively. The sodium-deficient environment under a high NH4F/RE ratio (e.g., 6
1, respectively (Fig. 2B). A significant amount of sodium/fluoride participated in NaF generation rather than in UCNC nucleation, and we therefore can suggest that the larger β-NaYbF4:Er particles obtained at low and high NH4F/RE ratios were due to the formation of fluoride-deficient and sodium-deficient α-nuclei formation environments, respectively. The sodium-deficient environment under a high NH4F/RE ratio (e.g., 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) should supply sodium-deficient α-nuclei, and thus, the formation of larger β-UCNCs could be supported by the sodium content-dependent α-to-β transition characteristics of α-UCNCs reported in the literature.15
1) should supply sodium-deficient α-nuclei, and thus, the formation of larger β-UCNCs could be supported by the sodium content-dependent α-to-β transition characteristics of α-UCNCs reported in the literature.15
Interestingly, sub-10 nm β-NaYbF4:Er was achieved at the effective and nearly stoichiometric NH4F/RE ratio of 4.02![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which was realized by compensating the system fluoride consumption in the NaF generation reaction via a slight NH4F dosage increase (Fig. 1A and 2B). In fact, the classic NaOA/NH4F/RE ratio of 2.5
1, which was realized by compensating the system fluoride consumption in the NaF generation reaction via a slight NH4F dosage increase (Fig. 1A and 2B). In fact, the classic NaOA/NH4F/RE ratio of 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 supplied a fluoride-deficient nucleation environment for the preparation of β-NaYbF4:Er. The reactivity between sodium and fluoride was strengthened under elevated temperatures, leading to the competing and unfavorable NaF generation reaction during nucleation. The in situ NaF generation reaction competes with the α-nuclei formation reaction in consuming both sodium and fluoride sources during nucleation. Further monitoring of the α-nuclei formation process supported the effects of the competing NaF generation reaction (Fig. 3).
1 supplied a fluoride-deficient nucleation environment for the preparation of β-NaYbF4:Er. The reactivity between sodium and fluoride was strengthened under elevated temperatures, leading to the competing and unfavorable NaF generation reaction during nucleation. The in situ NaF generation reaction competes with the α-nuclei formation reaction in consuming both sodium and fluoride sources during nucleation. Further monitoring of the α-nuclei formation process supported the effects of the competing NaF generation reaction (Fig. 3).
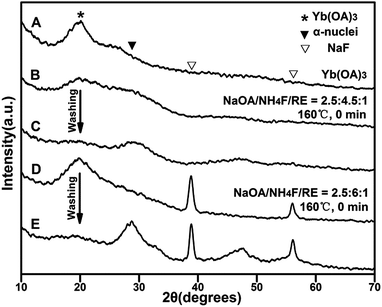
No NaF could be observed in the optimized NH4F/RE ratio (4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-based solvothermal NaYbF4:Er synthesis system (reaction at 160 °C, 0 min) via direct centrifugation, and only rare-earth oleates and α-nuclei were obtained via centrifugation after the addition of ethanol in such a system (Fig. 3A–C, S4†). In contrast, a large amount of precipitate was obtained in a high NH4F/RE ratio (6
1)-based solvothermal NaYbF4:Er synthesis system (reaction at 160 °C, 0 min) via direct centrifugation, and only rare-earth oleates and α-nuclei were obtained via centrifugation after the addition of ethanol in such a system (Fig. 3A–C, S4†). In contrast, a large amount of precipitate was obtained in a high NH4F/RE ratio (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-based solvothermal system (reacting at 160 °C, 0 min) via direct centrifugation (Fig. S4†). The obtained XRD patterns indicated that this precipitate was a mixture of rare-earth oleates, NaF and α-nuclei (Fig. 3A, D and E). The formation of rare-earth oleates with low dispersity should be due to the excessive surface binding of F− ions on the oleates under a high NH4F/RE ratio. The excessive surface binding of F− ions onto rare-earth oleates not only strengthened the NaF generation reaction, but also retarded the formation of monodisperse α-nuclei, as well as the α-to-β transition, by consuming the active sodium source of the system. These results confirmed the importance of the competing in situ NaF generation reaction for UCNC nucleation.
1)-based solvothermal system (reacting at 160 °C, 0 min) via direct centrifugation (Fig. S4†). The obtained XRD patterns indicated that this precipitate was a mixture of rare-earth oleates, NaF and α-nuclei (Fig. 3A, D and E). The formation of rare-earth oleates with low dispersity should be due to the excessive surface binding of F− ions on the oleates under a high NH4F/RE ratio. The excessive surface binding of F− ions onto rare-earth oleates not only strengthened the NaF generation reaction, but also retarded the formation of monodisperse α-nuclei, as well as the α-to-β transition, by consuming the active sodium source of the system. These results confirmed the importance of the competing in situ NaF generation reaction for UCNC nucleation.
The effects of the competing NaF generation reaction on UCNC nucleation were also verified by the NC evolution characteristics under fluoride- or sodium-deficient environments. The NaOA/NH4F/RE ratio of 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5
3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
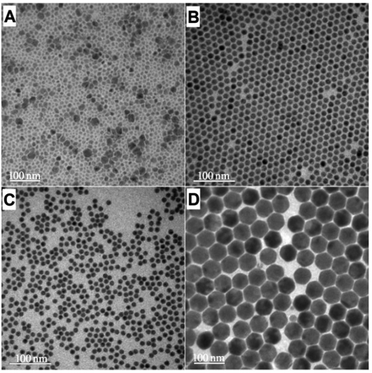
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 provided (α + β)-NaYbF4:Er even after reacting at 300 °C for 45 min, but large β-UCNCs had already appeared (Fig. 4A, S5,† Table 1), indicating that insufficient system fluoride actually retarded the UCNC α-to-β transition.
1 provided (α + β)-NaYbF4:Er even after reacting at 300 °C for 45 min, but large β-UCNCs had already appeared (Fig. 4A, S5,† Table 1), indicating that insufficient system fluoride actually retarded the UCNC α-to-β transition.
To further show the sensitive effect of fluoride level variation on the NC size evolution, we attempted to prepare NaYbF4:Er by partial fluoride consumption by slightly elevating the NaOA dosage (NaOA/NH4F/RE, 2.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5
4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and strengthening NaF generation. Approximately 20.6 mg of NaF was collected in the NaOA-based solvothermal system after reacting at 300 °C for 0 min. The intrinsic and effective NaOA/NH4F/RE ratio for α-nuclei formation was thus only 1.93
1) and strengthening NaF generation. Approximately 20.6 mg of NaF was collected in the NaOA-based solvothermal system after reacting at 300 °C for 0 min. The intrinsic and effective NaOA/NH4F/RE ratio for α-nuclei formation was thus only 1.93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.68
3.68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for the used NaOA/NH4F/RE ratio of 2.75
1 for the used NaOA/NH4F/RE ratio of 2.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5
4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which supplied an obvious fluoride-deficient nucleation environment. Compared to the β-NaYbF4:Er obtained by using the NaOA/NH4F/RE ratio of 2.5
1, which supplied an obvious fluoride-deficient nucleation environment. Compared to the β-NaYbF4:Er obtained by using the NaOA/NH4F/RE ratio of 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5
4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the expected much larger β-NaYbF4:Er particles (13.4 nm ± 5.9%) were obtained after reacting at 300 °C for 20 min (Fig. 4B).
1, the expected much larger β-NaYbF4:Er particles (13.4 nm ± 5.9%) were obtained after reacting at 300 °C for 20 min (Fig. 4B).
A slight active sodium deficiency of the system also led to a significantly delayed α-to-β transition process for NaYbF4:Er (Fig. S6,† Table 1). The α-to-β transition was finished after reacting at 300 °C for 30 min rather than 20 min by using the sodium-deficient NaOA/NH4F/RE ratio of 1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5
4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Such sodium deficiency led to larger-sized β-NaYbF4:Er particles (11.5 nm ± 6.3%, Fig. 4C). The α-to-β transition of NaYbF4:Er was still not finished after reacting at 300 °C for 30 min under an even more severe sodium-deficient environment (NaOA/NH4F/RE, 1.5
1. Such sodium deficiency led to larger-sized β-NaYbF4:Er particles (11.5 nm ± 6.3%, Fig. 4C). The α-to-β transition of NaYbF4:Er was still not finished after reacting at 300 °C for 30 min under an even more severe sodium-deficient environment (NaOA/NH4F/RE, 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5
4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), whereas larger β-NaYbF4:Er particles with an average diameter of ∼15.0 nm had emerged (Fig. S7†). These results were in agreement with the hypothesis of sodium-deficient α-nuclei for the formation of larger UCNCs and delayed α-to-β transition under high NH4F/RE ratios.15 The system sodium and fluoride levels played cooperative roles in regulating the effective sodium-fluoride levels during the nucleation.
1), whereas larger β-NaYbF4:Er particles with an average diameter of ∼15.0 nm had emerged (Fig. S7†). These results were in agreement with the hypothesis of sodium-deficient α-nuclei for the formation of larger UCNCs and delayed α-to-β transition under high NH4F/RE ratios.15 The system sodium and fluoride levels played cooperative roles in regulating the effective sodium-fluoride levels during the nucleation.
The NaF consumption process of the system also supported the important role of the competing NaF generation reaction during the nucleation for the UCNC evolutions. Considerable NaF (∼15.0 mg) still existed even after reacting at 300 °C for 20 min when using the NH4F/RE ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, while there was no obvious NaF collected when using a lower NH4F/RE ratio (e.g., 4
1, while there was no obvious NaF collected when using a lower NH4F/RE ratio (e.g., 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4.5
1, 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). It should be mentioned that the UCNC growth reaction of NaF + ReF3 + mβ-UCNCs (small) → β-UCNCs proposed by Ju and co-worker supported the NaF-dependent β-UCNC growth characteristics described in our work.20 In other words, larger-sized β-UCNCs would be formed when abundant NaF existed and was consumed at the NC growth stage.
1). It should be mentioned that the UCNC growth reaction of NaF + ReF3 + mβ-UCNCs (small) → β-UCNCs proposed by Ju and co-worker supported the NaF-dependent β-UCNC growth characteristics described in our work.20 In other words, larger-sized β-UCNCs would be formed when abundant NaF existed and was consumed at the NC growth stage.
Another attempt to improve the amount of active sodium source in the solvothermal system by raising the NaOA dosage (NaOA/NH4F/RE, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) also failed to achieve a better α-to-β transition environment and smaller-sized β-NaYbF4:Er (57.3 ± 4.5%, Fig. 4D, S8,† Table 1). The mass of NaF collected after reacting at 300 °C for 20 min was ∼25.5 mg, indicating that the newly added NaOA could not supply as effective an active sodium source for NC nucleation because most of it was consumed in promoting the NaF generation reaction by reacting with the active fluoride in the system instead. The active sodium level of the system was constrained by the NaF generation reaction and thus could not be promoted by a simple increase of the dosage.
1) also failed to achieve a better α-to-β transition environment and smaller-sized β-NaYbF4:Er (57.3 ± 4.5%, Fig. 4D, S8,† Table 1). The mass of NaF collected after reacting at 300 °C for 20 min was ∼25.5 mg, indicating that the newly added NaOA could not supply as effective an active sodium source for NC nucleation because most of it was consumed in promoting the NaF generation reaction by reacting with the active fluoride in the system instead. The active sodium level of the system was constrained by the NaF generation reaction and thus could not be promoted by a simple increase of the dosage.
In summary, the origin and mechanism of the competing NaF generation and α-nuclei formation reactions in the solvothermal β-NaYbF4:Er synthesis could be described as follows. (i) An in situ NaF generation reaction occurs between the sodium and fluoride sources during the system temperature increase, leading to consumption of both sodium and fluoride in the system. (ii) Since α-nuclei are formed by consuming the rare-earth oleates in the system, the sodium and fluoride sources reached the predetermined UCNC growth temperature (e.g., 300 °C) first, and the in situ NaF generation reaction competes with α-nuclei formation in the consumption of sodium and fluoride sources during α-nucleation. (iii) The effective system sodium-fluoride sources, which participate in α-nucleation rather than in in situ NaF generation, should determine the α-nucleation efficiency. For instance, the effective NaOA/NH4F/RE ratio for α-nucleation was only 1.93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.68
3.68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for the used NaOA/NH4F/RE ratio of 2.75
1 for the used NaOA/NH4F/RE ratio of 2.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5
4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 because the sodium-fluoride sources of the system were consumed in part by the in situ NaF generation reaction, resulting in low α-nucleation efficiency because the effective system fluoride level is lower than the necessary stoichiometric value. Briefly, the excessive system sodium source consumes the fluoride source via the in situ NaF generation reaction and thus leads to fluoride-deficient α-nucleation, and vice versa. (iv) The α-nucleation efficiency is positively related to the amount of β-nuclei formed at the UCNC growth temperature and therefore is inversely related to the size of the final β-NCs.
1 because the sodium-fluoride sources of the system were consumed in part by the in situ NaF generation reaction, resulting in low α-nucleation efficiency because the effective system fluoride level is lower than the necessary stoichiometric value. Briefly, the excessive system sodium source consumes the fluoride source via the in situ NaF generation reaction and thus leads to fluoride-deficient α-nucleation, and vice versa. (iv) The α-nucleation efficiency is positively related to the amount of β-nuclei formed at the UCNC growth temperature and therefore is inversely related to the size of the final β-NCs.
The findings of the competing in situ NaF generation reaction and α-nucleation are also in agreement with the thermodynamically controlled UCNC α-to-β phase transition mechanism. As is well known, a higher UCNC growth temperature will facilitate the transformation from metastable α-nuclei to the thermodynamically stable β-nuclei. The intrinsic reason for the accelerated α-to-β phase transition under higher temperature should be the promoted reactivity of the involved ions (Re3+, Na+, and F−) and the resultant easier lattice modulation and rearrangement. In other words, the low reactivity of the involved ions would retard the UCNC α-to-β phase transition. NaF generation balance exists in the solvothermal β-NaYbF4:Er synthesis system, including the in situ NaF generation at low temperature α-nucleation and NaF consumption at high temperature β-nucleation/growth. Excessive in situ generated NaF will not only lead to a low α-nucleation efficiency, but also to a low reactivity of the Na+ and F− ions because of the inertness of NaF. Most of the in situ generated inert NaF would contribute to the later β-nuclei growth period rather than the initial β-nuclei formation period, leading to fewer β-nuclei, delayed α-to-β transition and larger β-NCs.
After overcoming the issue of facile control of the synthesis of sub-10 nm monodisperse β-NaYbF4:Er and revealing the competing reactions during the nucleation process, low-level Gd3+-doped (10%) β-NaYbF4:Er was prepared according to this strategy. The diameter of the β-NaYbF4:Er particles decreased from 9.5 nm ± 5.6% to 5.7 nm ± 7.8% after doping of 10% Gd3+, showing an impressive size decrease effect (Fig. 5A). The diameter of 5.7 nm is in sharp contrast to the much larger diameter of NaYbF4:Gd/Tm particles (10/0.5%) (∼80 nm) prepared by using a much longer reaction time (1.5 h) and a nano-sized NaF-based solvothermal strategy.9
Monodisperse β-NaYbF4:Gd/Er (10/2%) was also achieved by using an even shorter reaction time of 15 min under the optimized NC synthesis conditions for 9.5 nm β-NaYbF4:Er (Fig. 5B and C, 5.5 nm ± 6.9%). In fact, the NC size distribution was more narrow than that of the NCs prepared using the reaction time of 20 min as seen from the comparison of Fig. 5A and B, indicating the presence of NC Ostwald-ripening if the solvothermal reaction was not stopped in time. Gd3+ doping resulted in an earlier appearance of the Ostwald-ripening phenomenon for β-NaYbF4. Elemental analysis showed that the actual Gd3+-to-Yb3+ ratio in the as-prepared β-NaYbF4:Gd/Er (10/2%) was ∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.2, very close to the theoretical value (1
9.2, very close to the theoretical value (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.8), indicating the successful Gd3+ doping into β-NaYbF4 (Fig. 5D–F, S9†). These results demonstrated that balancing the competing NaF generation and α-nuclei formation reactions successfully overcame the challenge of the controlled solvothermal synthesis of sub-10 nm low-level Gd3+-doped β-NaYbF4:Er.
8.8), indicating the successful Gd3+ doping into β-NaYbF4 (Fig. 5D–F, S9†). These results demonstrated that balancing the competing NaF generation and α-nuclei formation reactions successfully overcame the challenge of the controlled solvothermal synthesis of sub-10 nm low-level Gd3+-doped β-NaYbF4:Er.
For control experiments, NaYbF4:Gd/Er (10/2%) was prepared according to the nano-sized NaF-based solvothermal strategy reported in the literature.10 The α-to-β transition was still not completed after 40 min of reaction at 300 °C (Fig. 6A). Pure β-UCNCs as large as ∼70 nm were achieved after reacting at 300 °C for 60 min (Fig. 6B). Moreover, the as-prepared β-UCNCs are very non-uniform in morphology. This phenomenon is not caused by the Ostwald-ripening mechanism because the α-to-β transition has just finished. The β-nuclei for β-UCNCs with sharp size differences should form in different nucleation periods, which could be explained by the slow release of the Na+ and F− ions from the inert nano-sized NaF. These comparisons confirmed that low-level Gd3+ doping could not obtain sub-10 nm β-UCNCs using the nano-sized NaF-based solvothermal strategy due to ex situ NaOH/NH4F-to-inert NaF conversion. The UCNCs prepared via the nano-sized NaF-based strategy were still too large for bio-applications (Fig. 6B).
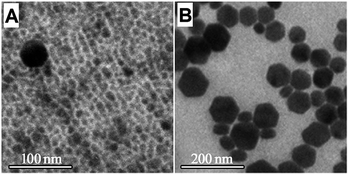 | ||
| Fig. 6 TEM images: NaYbF4:Gd/Er (10/2%) prepared by using the nano-sized NaF-based solvothermal strategy and reacting at 300 °C for 40 min (A) and 60 min (B). | ||
Finally, the upconversion intensity of the as-prepared 5.5 nm β-NaYbF4:Gd/Er (10/2%) was evaluated by comparison to high-level (40%) Gd3+-doped β-NaYbF4:Er, as well as the large-sized β-NaYbF4:Gd/Er (30/2%) with promising upconversion efficiency for practical applications. Similar-sized β-NaYbF4:Gd/Er (40/2%) (∼5.6 nm) was achieved by using the same synthesis procedures as those of the 5.5 nm β-NaYbF4:Gd/Er (10/2%) (Fig. S10A†). The low-level Gd3+-doped β-NaYbF4:/Er showed a stronger upconversion intensity than the high-level Gd3+-doped NaYbF4:/Er, suggesting that the NaYbF4 may be a more efficient upconversion host material than NaGdF4 (Fig. S10D†). Our previous work also supports the conclusion that NaYbF4 is an excellent upconversion host material.17 However, the upconversion intensity of the 5.5 nm β-NaYbF4:Gd/Er (10/2%) is much weaker than that of the large-sized β-NaYbF4:Gd/Er (30/2%) (∼20 nm, Fig. S10B†) obtained using the procedure based on the reports in the literature.10 In view of the much higher surface-to-volume ratio of the smaller-sized β-NaYbF4:Gd/Er and the resultant low upconversion intensity induced by surface quenchers, an optically inert NaYF4 shell was coated onto β-NaYbF4:Gd/Er (10/2%) for upconversion intensity strengthening. The upconversion intensity of the as-obtained small-sized core/shell structured β-NaYbF4:Gd/Er(10/2%)@NaYF4 (∼9 nm, Fig. S10C†) is comparable to that of the literature-based large-sized β-NaYbF4:Gd/Er (30/2%), indicating the potential of the as-obtained low-level Gd3+-doped β-NaYbF4:Er for practical application (Fig. S10E†).
Conclusions
We showed that the effective sodium-fluoride levels for α-nuclei formation were restricted sensitively by the competing in situ NaF generation reaction in NaOA-based solvothermal UCNC synthesis. The effects of the sodium and fluoride levels on the solvothermal UCNC evolution have been partially neglected in the respective F− ion coverage and the α-nuclei sodium abundance theories discussed in the literature.15,19 The competing NaF generation and α-nuclei formation reactions were the theoretical basis for both the F− ion coverage and the α-nuclei sodium abundance theories. Sub-10 nm monodisperse β-NaYbF4:Er (9.5 nm) and low-level Gd3+-doped (10 mol%) β-NaYbF4:Er (5.5 nm) were achieved via facile balancing of the competing in situ NaF generation and α-nuclei formation in the absence of high NaOA and NH4F dosages. Our work supplies new insights and a user-friendly tool for the control of solvothermal UCNC synthesis.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21505104, 21775121 and 21605122).References
- N. M. Idris, M. K. G. Jayakumar, A. Bansal and Y. Zhang, Chem. Soc. Rev., 2015, 44, 1449–1478 RSC.
- X. G. Liu, C. H. Yan and J. A. Capobianco, Chem. Soc. Rev., 2015, 44, 1299–1301 RSC.
- D. L. Zhou, D. L. Liu, W. Xu, X. Chen, Z. Yin, X. Bai, B. Dong, L. Xu and H. W. Song, Chem. Mater., 2017, 29, 6799–6809 CrossRef CAS.
- G. Y. Chen, H. L. Qju, P. N. Prasad and X. Y. Chen, Chem. Rev., 2014, 114, 5161–5214 CrossRef CAS PubMed.
- Q. S. Chen, X. J. Xie, B. L. Huang, L. L. Liang, S. Y. Han, Z. G. Yi, Y. Wang, Y. Li, D. Y. Fan, L. Huang and X. G. Liu, Angew. Chem., Int. Ed., 2017, 56, 7605–7609 CrossRef CAS PubMed.
- H. X. Mai, Y. W. Zhang, R. Si, Z. G. Yan, L. D. Sun, L. P. You and C. H. Yan, J. Am. Chem. Soc., 2006, 128, 6426–6436 CrossRef CAS PubMed.
- F. Shi and Y. Zhao, J. Mater. Chem. C, 2014, 2, 2198–2203 RSC.
- Q. Liu, Y. Sun, T. S. Yang, W. Feng, C. G. Li and F. Y. Li, J. Am. Chem. Soc., 2011, 133, 17122–17125 CrossRef CAS PubMed.
- J. A. Damasco, G. Y. Chen, W. Shao, H. Agren, H. Y. Huang, W. T. Song, J. F. Lovell and P. N. Prasad, ACS Appl. Mater. Interfaces, 2014, 6, 13884–13893 CAS.
- Y. L. Liu, K. L. Ai, J. H. Liu, Q. H. Yuan, Y. Y. He and L. H. Lu, Angew. Chem., Int. Ed., 2012, 51, 1437–1442 CrossRef CAS PubMed.
- Z. Q. Li and Y. Zhang, Nanotechnology, 2008, 19, 345606 CrossRef PubMed.
- C. S. Ma, X. X. Xu, F. Wang, Z. G. Zhou, S. H. Wen, D. M. Liu, J. H. Fang, C. I. Lang and D. Y. Jin, J. Phys. Chem. Lett., 2016, 7, 3252–3258 CrossRef CAS PubMed.
- D. D. Li, Q. Y. Shao, Y. Dong and J. Q. Jiang, Chem. Commun., 2014, 50, 15316–15318 RSC.
- T. Rinkel, A. N. Raj, S. Duhnen and M. Haase, Angew. Chem., Int. Ed., 2016, 55, 1164–1167 CrossRef CAS PubMed.
- T. Rinkel, J. Nordmann, A. N. Raj and M. Haase, Nanoscale, 2014, 6, 14523–14530 RSC.
- R. K. Shi, M. A. Ling, X. N. Li, L. Zhang, M. Lu, X. J. Xie, L. Huang and W. Huang, Nanoscale, 2017, 9, 13739–13746 RSC.
- J. W. Shen, Z. Q. Wang, J. W. Liu and H. Li, J. Mater. Chem. C, 2017, 5, 9579–9587 RSC.
- L. Q. Xiong, T. S. Yang, Y. Yang, C. J. Xu and F. Y. Li, Biomaterials, 2010, 31, 7078–7085 CrossRef CAS PubMed.
- C. Y. Liu, Z. Y. Gao, J. F. Zeng, Y. Hou, F. Fang, Y. L. Li, R. R. Qiao, L. Shen, H. Lei, W. S. Yang and M. Y. Gao, ACS Nano, 2013, 7, 7227–7240 CrossRef CAS PubMed.
- J. N. Shan and Y. G. Ju, Nanotechnology, 2009, 20, 275603 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra00655e |
| This journal is © The Royal Society of Chemistry 2018 |