Open Access Article
Open Access ArticleShape and structure controlling of calcium oxalate crystals by a combination of additives in the process of biomineralization†
Nian Liuab,
Hao Xie *ab,
Hang Pingb,
Lin Wanga,
Zewen Liuab,
Fei Taoab,
Junhui Guoa and
Bao-Lian Su*bc
*ab,
Hang Pingb,
Lin Wanga,
Zewen Liuab,
Fei Taoab,
Junhui Guoa and
Bao-Lian Su*bc
aSchool of Chemistry, Chemical Engineering and Life Sciences, Wuhan University of Technology, Wuhan, 430070, China. E-mail: h.xie@whut.edu.cn
bLaboratory of Living Materials at the State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan, 430070, China. E-mail: bao-lian.su@unamur.be
cLaboratory of Inorganic Materials Chemistry, University of Namur, B-5000 Namur, Belgium
First published on 20th March 2018
Abstract
The origin of complex hierarchical superstructures of biomaterials and their unique self-assembly mechanisms of formation are important in biological systems and have attracted considerable attention. In the present study, we investigated the morphological changes of calcium oxalate (CaOx) crystals induced by additives including chiral aspartic acid, sodium citrate, Mg2+, casein and combinations of these molecules. The morphology and structure of CaOx were identified with the use of various techniques. The morphogenesis of CaOx crystals were significantly affected by chiral aspartic acid, sodium citrate or Mg2+. However, they only formed calcium oxalate monohydrate (COM). It was observed that the chiral aspartic acid, sodium citrate and casein adhered to the surface of the crystals. The adherence of Mg2+ to crystals was not evident. Casein significantly affected the formation of COM and calcium oxalate dihydrate (COD). The ratio of different CaOx crystal forms is associated with the casein concentration. In combination with Mg2+ or citrate ions, casein showed improved formation of COD. The present study mimics biomineralization with a simple chemical approach and provides insight into the complicated system of CaOx biomineralization as well as facilitates the understanding of urinary stone treatment.
Introduction
The increasing incidences and prevalence of stone disease is taking a toll on the social health system worldwide.1–4 The cause, the process of stone formation, and the different inhibitive mechanisms remain elusive.5–7CaOx is the most frequently encountered crystalline phase in urethral calculi and renal calculi.3,8–10 CaOx is present in four crystalline types, namely, anhydrous (COA),11 COM, COD and trihydrate (COT)12 and an amorphous type (ACO).13,14 The major component of human urinary stones is COM, COD, or a mixture of the two species.15,16 Thermodynamically stable COM crystals with higher adsorptive capability and larger (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 01) surface area than those of COD crystals can easily come in contact with renal tubular cells.17
01) surface area than those of COD crystals can easily come in contact with renal tubular cells.17
Understanding morphogenesis of COM facilitates the explanation of the mechanism of calculus formation and provides effective strategies for lithiasis diagnosis and treatment. The controlled crystallization of CaOx has been investigated in a variety of organic systems including Langmuir monolayers,18 vesicles,19 micelles,20 citrate,21–23 amino acids24 and natural macromolecules.15,18,25–28 Some urinary proteins2 and polypeptides29 were observed to inhibit stone formation. These discoveries are significant for setting up effective therapies for stone disease, which have affected human beings for thousands of years6,30 and shed light on the complex structure formation of inorganic materials in an easier way. Due to the complicated functions and changeable activities of biological materials during the formation of inorganic materials, more research needs to be carried out on the shape and structure control of CaOx crystals.
L-Aspartic acid is abundant in the human body and is frequently presents in the form of calculus. Some researchers believe that L-aspartic acid plays a significant role in preventing CaOx stone formation.5,31,32 For example, Golovanova et al. observed that aspartic acid and glutamic acid inhibited the growth of CaOx crystals.31 However, other researchers did not agree that L-aspartic acid has a significant role in affecting the CaOx stone growth. Guo et al. found that aspartate and glutamate drastically reduced the aspect ratio during pit filling, but only weakly inhibited the crystal growth.15 The influence of L-aspartic acid on CaOx formation is ambiguous. Chirality is an important issue in crystallization. Many researchers have reported that different chiral materials have significant difference in their performances in vitro and in vivo.33 Therefore, the roles of both L- and D-aspartic acid in CaOx crystallization were investigated in the present study.
Sodium citrate (Na3 Citrate) is a therapeutic agent of urinary stone disease. It has been used in clinical treatments for many years.34,35 Mg2+ is abundantly available in nature as well as in the human body. It can decrease oxalate absorption in the gut,36 which in turn decreases the formation rate of CaOx. Since ions doped calcium oxalate crystals can be obtained by co-precipitation,37 Mg2+ may also influence the property of crystallization of CaOx in this manner. In the present study, the effects of these two chemicals on CaOx crystallization were also investigated.
In the human body, macromolecules such as proteins may also affect the CaOx crystallization. Casein is the major phosphate protein in bovine milk. It has brilliant Ca2+ binding property38 and contains numerous L-aspartic residues.39 Higher concentration of both calcium ions and casein with stirring has been explored previously.39 However, lower concentration and non-stirring condition is beneficial to obtain the relationship between additives and the crystal forming process.33 Therefore, casein was employed as a macromolecular additive to study its influence on CaOx crystallization.
The aim of this study was to investigate the crystallization of CaOx under the influence of L-aspartic acid, D-aspartic acid, Mg2+, Na3 Citrate, and casein. Considering the complex environment in vivo, where different compositions co-exist, the effects of combinations of macromolecules and micro molecules on the CaOx crystallization were studied. The morphology of CaOx as well as polycrystalline superstructures had been identified by multiple techniques. The formation and complexity of hierarchical structures of CaOx were evaluated. A possible self-assembly process of CaOx was discussed. The present study suggests that it is possible to control the morphogenesis of CaOx crystalline superstructures by a simple chemical method mimicking biomineralization.
Experimental
Materials
L-Aspartic acid (Bioxtra, ≥ 99%, HPLC), D-aspartic acid (99%), and Casein sodium salt from bovine milk were purchased from Sigma Aldrich. All other chemicals were of analytical grade, obtained commercially, and used without further purification. Ultra-pure water (18.2 MΩ) was used for the preparation of aqueous solutions in all experiments.Synthetic procedures
Fresh 2.5 mmol L−1 Tris–HCl buffer was used to prepare 2 mmol L−1 CaCl2 and 2 mmol L−1 Na2C2O4. All solutions were adjusted to pH 6 at 37 °C by HCl or NaOH unless otherwise stated. CaOx crystals were prepared by mixing equal volume of CaCl2 and Na2C2O4 solutions and incubating overnight at 37 °C. Appropriate amounts of additives were added into the CaCl2 solution before adjusting the pH if required. Then, the crystal products were collected by centrifugation (10000 g, 10 minutes) or filtration through a 0.22 μm nylon membrane and rinsed three times with Ultra-pure water. The crystals were then dried at 37 °C.Characterization techniques
Results and discussion
Influence of small molecular additives on mineralization of COM
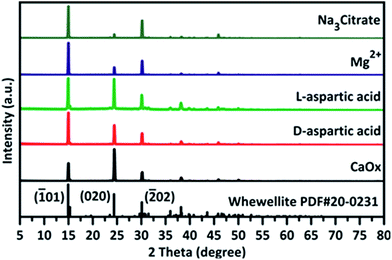
In the present study, the concentration of Ca2+ and C2O42− in crystallization assays was adjusted to 1 mmol L−1 to avoid fast precipitation of CaOx and acquire sufficient amount of crystals for analysis. In a preliminary study, to optimise the concentration of additives, a broad range of concentrations were explored from 0.1 mmol L−1 to 2 mmol L−1 (Fig. S1–S4, see ESI†). The results indicated that for additives Mg2+, D-aspartic acid, and L-aspartic acid, better crystal morphology can be viewed at 2 mmol L−1 of additive concentration. However, it was difficult to obtain crystals for Na3 Citrate at 2 mmol L−1 even on using centrifugation (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g, 10 minutes) to collect crystals. Since the concentration of Na3 Citrate in healthy urine sample is around 1.1–1.6 mmol L,40–42 the additive concentration of Na3 Citrate was adjusted to 1 mmol L−1 for the rest of the study. Although effects of concentrated additives on crystallization may be more evident, it is rare in vivo. The addition of small molecular additives did not change the crystal form of COM (Fig. 1). Under these conditions, only COM was acquired in the presence or absence of additives. However, the addition of Mg2+ or Na3 Citrate drastically influenced the orientation of the crystals.
000 g, 10 minutes) to collect crystals. Since the concentration of Na3 Citrate in healthy urine sample is around 1.1–1.6 mmol L,40–42 the additive concentration of Na3 Citrate was adjusted to 1 mmol L−1 for the rest of the study. Although effects of concentrated additives on crystallization may be more evident, it is rare in vivo. The addition of small molecular additives did not change the crystal form of COM (Fig. 1). Under these conditions, only COM was acquired in the presence or absence of additives. However, the addition of Mg2+ or Na3 Citrate drastically influenced the orientation of the crystals.
The orientation changes of crystals were also observed along with the pH changes from 4 to 7 (Fig. S5†). Upon the addition of Mg2+ or Na3 Citrate, larger (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 01) face of COM was observed as compared to those of other additives. In addition, relatively smaller (020) face and (121) face were observed in the presence of Na3 Citrate, resulting in the hexagon thin plate morphology of COM. The addition of chiral aspartic acid increased the size of COM and smoothed the edge. To prove the effects of aspartic acid on crystals, higher concentration of D-aspartic acid and L-aspartic acid at 20 mmol L−1 were tested at both pH 6 and pH 7 (Fig. 2). It was found that the size of the as-formed crystals were almost double in comparison with that formed in the absence of additives. However, the amounts of additives attached to the crystals were insufficient to be detected by TGA (Fig. S6, Table S1†). In addition, there is evidence of interaction between CaOx and these additives, particularly for Na3 Citrate, which is a strong bonding additive to CaOx crystals (Fig. 1).
01) face of COM was observed as compared to those of other additives. In addition, relatively smaller (020) face and (121) face were observed in the presence of Na3 Citrate, resulting in the hexagon thin plate morphology of COM. The addition of chiral aspartic acid increased the size of COM and smoothed the edge. To prove the effects of aspartic acid on crystals, higher concentration of D-aspartic acid and L-aspartic acid at 20 mmol L−1 were tested at both pH 6 and pH 7 (Fig. 2). It was found that the size of the as-formed crystals were almost double in comparison with that formed in the absence of additives. However, the amounts of additives attached to the crystals were insufficient to be detected by TGA (Fig. S6, Table S1†). In addition, there is evidence of interaction between CaOx and these additives, particularly for Na3 Citrate, which is a strong bonding additive to CaOx crystals (Fig. 1).
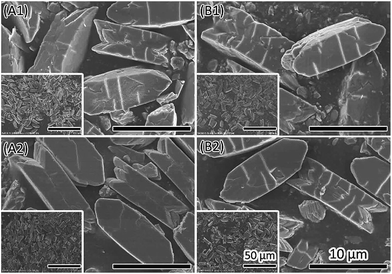 | ||
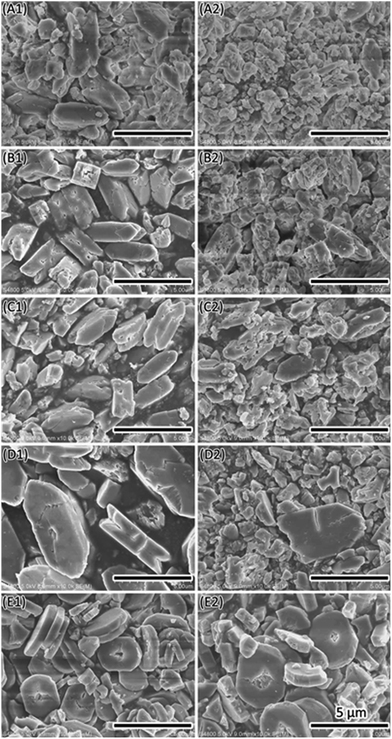
| Fig. 2 SEM images of COM formed in the presence of 20 mmol L−1 of D-aspartic acid (A1, A2) or L-aspartic acid (B1, B2). The pH solution was pH6 (A1, B1) and pH7 (A2, B2). | ||
In most cases, the pH of human urine is around 6. Therefore, the pH was set to 6 in the present study to investigate the effect of additives as a function of crystallization time. CaOx crystal formation is a rapid process. It only takes several minutes for the precipitation of CaOx minerals after mixing calcium chloride solution with sodium oxalate solution at 37 °C. The resultant crystals were imperfect with a rough surface, rough edges and small fragments (Fig. S7†). However, there were differences between the effects of different additives. Chiral aspartic acid did not have significant influence on the crystals at this concentration. Both Mg2+ and Na3 Citrate can make the (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 01) face larger even in a short crystallization time. Additives might affect the crystal morphogenesis in two ways. One is that additives attached to the crystal face increased the stability by inactivating the dissolution and recrystallization process on this face. The other is that additives reduced the dissolution process by increasing the compete stress in solution.
01) face larger even in a short crystallization time. Additives might affect the crystal morphogenesis in two ways. One is that additives attached to the crystal face increased the stability by inactivating the dissolution and recrystallization process on this face. The other is that additives reduced the dissolution process by increasing the compete stress in solution.
Since there may be dissolution and recrystallization cycles, roles of additives were further studied in the ageing process of crystallization (Fig. 3). In a relatively long crystalline process without any additives, CaOx crystals formed irregular shapes in 3 days. This is owing to the constant dissolution and recrystallization process with limited ion resources. In the presence of small molecular additives, most crystals retain their relatively complete (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 01) face. After 3 days, there were holes on the (020) face of crystals formed in chiral aspartic acid added solution. Some even changed to a sponge-like morphology in 10 days (Fig. S8†).
01) face. After 3 days, there were holes on the (020) face of crystals formed in chiral aspartic acid added solution. Some even changed to a sponge-like morphology in 10 days (Fig. S8†).
In combining this result with TGA (Fig. S6 and Table S1†) and energy dispersive spectrometry (EDS) (Fig. S9†), it is supposed that the binding of chiral aspartic acid to crystals is specific, which is coincident with the results reported by Grohe et al.43 and He et al.24 Na3 Citrate could function in the same manner, while Mg2+ functions in another. EDS analysis showed that Mg2+ was not present in COM, although addition of Mg2+ did change the crystal shape. If MgC2O4·H2O or MgC2O4·2H2O were present, the TGA spectrum would confirm that the total weight loss was higher than that of pure COM. However, this did not occur as shown in Table S1.† Therefore, we concluded that there was no detectable Mg2+ in the formed crystals on addition of 2 mmol L−1 Mg2+. This situation is quite different from Fe2+ doping in calcium oxalate.37
Influence of casein on introducing dihydrate form of CaOx
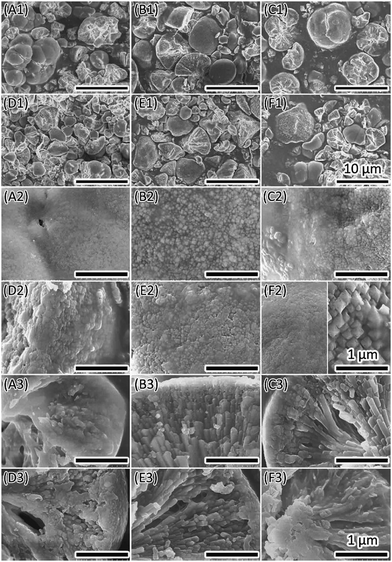
Macromolecules such as proteins can function as templates or catalysts in the biomineralization processes. Liu et al. reported the production of dumbbell-shaped CaOx in the presence of amphiphilic phosphoproteins.25,39 They investigated effects of casein on the morphogenesis of CaOx at pH 6.9 at room temperature. However, this condition is different to that in vivo. In order to obtain insight of natural biomineralization, we explored the influence of casein on mineralization of CaOx at pH 6 at 37 °C, which mimics the in vivo condition. Completely different morphology and crystal types were obtained in comparing with the previous study.25,39Casein (ranging from 0.1 g L−1 to 4.0 g L−1) induced changes in the crystal morphology as well as crystal form (Fig. 4 and 5). Most of the crystals are fragments with a rough surface and branch inside (Fig. 4, A1 to F1). The crystal surface changed along with the addition of different amounts of casein (Fig. 4, A2 to F2), but not in a streak manner. The core region of the cross section was a bunch of branch-like crystals, elongating near the surface, which converted into particles (Fig. 4, A3 to F3).
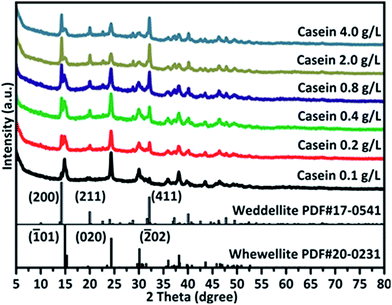 | ||
| Fig. 5 XRD results illustrate changing from COM to COD formed with different concentration of casein. | ||
XRD spectra (Fig. 5) showed that the intensity of the peaks belonging to COM decreased upon increasing casein concentration, while intensity of COD peaks increased. This implied that more COD was formed along with involvement of more casein. Upon casein treatment, XRD peaks of CaOx minerals were lower with wider full width at half maximum (FWHM) and uneven baselines. This implied the change of crystallinity or crystallographic texture. The change in XRD pattern might be due to the deposition of casein on the crystals and changes in the crystals structure. The impure crystals were fragile and readily broke during the treatment process, resulting in most crystals broken into small fragments.
Theoretically, the total weight loss of pure COM is 61.62%, while this value is 65.83% for that of pure COD. Addition of casein induced formation of COD (as proven by XRD), which also affected the weight loss. When adding higher amount of casein in solution (Table S2†), total weight loss was higher than 65.83%, indicating that COD is not the only composition in the sample (can be proven by XRD). This implies that casein may contribute to the increase in weight loss.
Influence of combinations of additives on CaOx mineralization
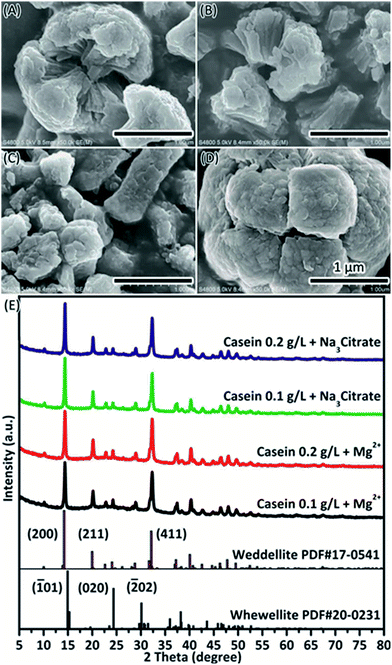
A living organism is a quite complex system, in which various molecules work together to maintain the essential biochemical and physiological processes. In a biomineralization process, the formation of biomineral requires functions from both macromolecules (mineral proteins) and small molecules (ions). In the present study, the effects of casein on CaOx mineralization was further explored in the presence of Mg2+ or Na3 Citrate (Fig. 6). Small crystals were obtained with rough surface and different shapes.The involvement of casein in CaOx mineralization in the presence of Mg2+ or Na3 Citrate was analysed by TGA. The amount of casein contained in the crystals increased on addition of Mg2+ (Table 1, Fig. S10†). In this study, COM was acquired in the presence of Mg2+ (Fig. 1) and it did not significantly affect the TGA results (Fig. S6, Table S1†). When casein was included in the crystallization assay, it resulted in weight loss, which was much more significant if Mg2+ was also included (Table 1). The weight loss may be either due to the increase in amount of COD or due to the increase in amount of casein in the crystals. This is supported by the fact that weight loss of crystals formed in the presence of 0.2 g L−1 casein and Mg2+ is higher than that of pure COD and close to that formed in the presence of 0.8 g L−1 casein (Table S2†). However, the weight loss of the crystals formed in the presence of 0.1 g L−1 casein and Mg2+ is close to that of pure COD since COM may be included. We previously reported that addition of Mg2+ can increase the size of casein micelles.44 The unique structure of casein micelles facilitates absorption of Ca2+ and subsequent interaction with the oxalate ions. Then, casein micelles form single small particles, which can function as the core of crystallization and assembly template. It allows fusion of small COD particles into branch-like structures of the resultant morphology. Since citrate can co-deposit with crystals (Table S1†), weight change (Table 1) of crystals can be due to COD (Fig. 6) and co-deposited citrate (Table S1†). Citrate can attract more Ca2+ and increase the ratio of Ca2+/C2O42− around crystal, which functions with casein to induce COD formation. Significant depletion of water was observed at low temperature in the TGA for crystals in the presence of Mg2+ or Na3 Citrate. This may be due to the water in COD, which is weakly bonded in crystals than in COM45 or can be attributed to the interactions between deposited casein and water molecules.
| Sample | 40–250 °C | 250–550 °C | 550–800 °C | Total |
|---|---|---|---|---|
| CaOx | 14.5 | 18.4 | 27.3 | 60.2 |
| Casein from Sigma | 14.7 | 60.8 | 17.3 | 92.8 |
| Casein 4 g L−1 | 18.2 | 36.9 | 17.6 | 72.7 |
| Casein 0.2 g L−1 + Mg2+ | 25.8 | 24.1 | 19.5 | 69.4 |
| Casein 0.2 g L−1 + Na3 Citrate | 22.5 | 24.4 | 18.7 | 65.6 |
| Casein 0.2 g L−1 | 16.7 | 27.5 | 20.3 | 64.5 |
| Casein 0.1 g L−1 + Mg2+ | 22.2 | 24.5 | 18.6 | 65.3 |
| Casein 0.1 g L−1 + Na3 Citrate | 21.6 | 23.6 | 18.0 | 63.2 |
| Casein 0.1 g L−1 | 15.6 | 26.7 | 20.7 | 63.0 |
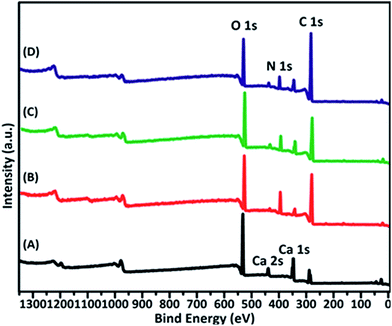
The deposition of casein on CaOx crystals were also analysed by XPS spectrometry (Fig. 7 & Table 2). XPS results show the percentage of atoms in the crystal surface. It confirmed the presence of casein by increasing the amount of N atoms, which were present only in casein in this situation. The increasing amount of C was attributed to casein or Na3 Citrate. The peak of magnesium is not evident. This implies that the role of Mg2+ in solution is to upregulate the stress of bivalent cations and then decrease the dissolution process of CaOx.
| Sample | C 1s | Ca 2p | N 1s | O 1s | Mg 1s |
|---|---|---|---|---|---|
| CaOx | 15.11 | 15.58 | 1.25 | 67.85 | 0.22 |
| Casein | 62.18 | 1.55 | 13.78 | 22.34 | 0.15 |
| Casein–Mg2+ | 61.81 | 3.33 | 10.21 | 24.52 | 0.13 |
| Casein–Na3 Citrate | 71.20 | 2.67 | 7.15 | 18.88 | 0.11 |
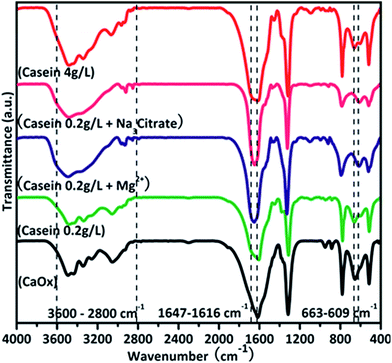
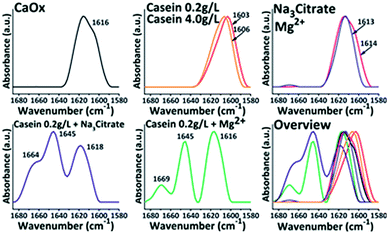
The interaction between casein and minerals was analysed by FTIR (Fig. 8). The symmetrical stretching vibration and asymmetrical stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds from oxalate were assigned to 1317 cm−1 and 1616 cm−1. The absorption bands in amide I region between 1600–1700 cm−1 corresponds to the secondary structure of the polypeptide backbone.38 This area was explored to obtain structural information of casein during the crystallization (Fig. 9). In the absence of casein, the acquired crystals only exhibit a characteristic peak of CaOx around 1616 cm−1. Upon addition of casein without other additives, there was a red shift of the absorbance peak to 1616 cm−1. However, no characteristic peaks of proteins were observed in the spectrum. Upon addition of casein together with Mg2+ or Na3 Citrate, there were characteristic peaks at 1645 cm−1 and 1664 (1669) cm−1, which are related to the uncoiling and turning of the protein structure. Therefore, Mg2+ or Na3 Citrate might promote the interactions between casein and crystals.
O bonds from oxalate were assigned to 1317 cm−1 and 1616 cm−1. The absorption bands in amide I region between 1600–1700 cm−1 corresponds to the secondary structure of the polypeptide backbone.38 This area was explored to obtain structural information of casein during the crystallization (Fig. 9). In the absence of casein, the acquired crystals only exhibit a characteristic peak of CaOx around 1616 cm−1. Upon addition of casein without other additives, there was a red shift of the absorbance peak to 1616 cm−1. However, no characteristic peaks of proteins were observed in the spectrum. Upon addition of casein together with Mg2+ or Na3 Citrate, there were characteristic peaks at 1645 cm−1 and 1664 (1669) cm−1, which are related to the uncoiling and turning of the protein structure. Therefore, Mg2+ or Na3 Citrate might promote the interactions between casein and crystals.
Conclusions
In the present study, we explored the morphological and structural changes of CaOx crystals induced by additives including chiral aspartic acid, sodium citrate, Mg2+, casein and a variety of combination of these molecules. The difference was not significant between L-aspartic acid and D-aspartic acid on affecting the COM formation. The adhesion of aspartic acid on CaOx (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 01) face was proved by monitoring the ageing process of CaOx mineralization. Casein could affect the morphogenesis of CaOx minerals. However, its function was limited at low concentrations. Adherence of Na3 Citrate to the crystals was observed. It facilitated the Ca2+ ions to adhere around the crystals, which affected the morphogenesis and improved the COD formation in the presence of casein. Mg2+ regulated interactions between casein and CaOx minerals through increasing casein micelle size, which subsequently improved the function of casein at low concentration. This observation is helpful for understanding molecular interactions and behaviours in vivo, for systems which are full of ions, proteins and other small or macro molecules. These molecules may function together in various physiological and biochemical processes as that of casein and other small molecules in biomineralization of CaOx. The present study helps us explore principles and mechanisms for the formation of crystalline superstructures as well as understand complex biomineralization in systems.
01) face was proved by monitoring the ageing process of CaOx mineralization. Casein could affect the morphogenesis of CaOx minerals. However, its function was limited at low concentrations. Adherence of Na3 Citrate to the crystals was observed. It facilitated the Ca2+ ions to adhere around the crystals, which affected the morphogenesis and improved the COD formation in the presence of casein. Mg2+ regulated interactions between casein and CaOx minerals through increasing casein micelle size, which subsequently improved the function of casein at low concentration. This observation is helpful for understanding molecular interactions and behaviours in vivo, for systems which are full of ions, proteins and other small or macro molecules. These molecules may function together in various physiological and biochemical processes as that of casein and other small molecules in biomineralization of CaOx. The present study helps us explore principles and mechanisms for the formation of crystalline superstructures as well as understand complex biomineralization in systems.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has been performed as part of the program for Changjiang Scholars and Innovative Research Team (IRT_15R52) of Chinese Ministry of Education. This work was also supported by the National Natural Science Foundation of China (31771032) and the Fundamental Research Funds for the Central Universities (WUT 2016IB006).Notes and references
- B. W. Turney, J. M. Reynard, J. G. Noble and S. R. Keoghane, BJU Int., 2012, 109, 1082–1087 CrossRef PubMed.
- S. Narula, S. Tandon, S. K. Singh and C. Tandon, Life Sci., 2016, 164, 23–30 CrossRef CAS PubMed.
- Z. Zhao, Y. Xia, J. Xue and Q. Wu, Cryst. Growth Des., 2014, 14, 450–458 CAS.
- S. A. Strope, J. S. Wolf and B. K. Hollenbeck, Urology, 2010, 75, 543–546 CrossRef PubMed.
- H. Li, Q.-Z. Yao, Y.-Y. Wang, Y.-L. Li and G.-T. Zhou, Sci. Rep., 2015, 5, 7718 CrossRef PubMed.
- A. P. Evan, E. M. Worcester, F. L. Coe, J. Williams and J. E. Lingeman, Urolithiasis, 2015, 43, 19–32 CrossRef PubMed.
- D. E. Fleming, A. Van Riessen, M. C. Chauvet, P. K. Grover, B. Hunter, W. Van Bronswijk and R. L. Ryall, J. Bone Miner. Res., 2003, 18, 1282–1291 CrossRef CAS PubMed.
- Y. Shen, W. Yue, A. Xie, Z. Lin and F. Huang, Colloids Surf., A, 2004, 234, 35–41 CrossRef CAS.
- L. Maurice-Estepa, P. Levillain, B. Lacour and M. Daudon, Clin. Chim. Acta, 2000, 298, 1–11 CrossRef CAS.
- S. Wang, P. Du, N. Zhang, J. Liu, X. Tang, Q. Zhao and Y. Yang, Urolithiasis, 2016, 44, 203–210 CrossRef CAS PubMed.
- W. Zhao, N. Sharma, F. Jones, P. Raiteri, J. D. Gale and R. Demichelis, Cryst. Growth Des., 2016, 16, 5954–5965 CAS.
- T. Lee and Y. C. Lin, Cryst. Growth Des., 2011, 11, 2973–2992 CAS.
- S. Sun, D. Gebauer and H. Cölfen, Chem. Sci., 2017, 8, 1400–1405 RSC.
- J. Ihli, Y.-W. Wang, B. Cantaert, Y.-Y. Kim, D. C. Green, P. H. H. Bomans, N. A. J. M. Sommerdijk and F. C. Meldrum, Chem. Mater., 2015, 27, 3999–4007 CrossRef CAS.
- S. Guo, M. D. Ward and J. A. Wesson, Langmuir, 2002, 18, 4284–4291 CrossRef CAS.
- A. Langdon and B. Grohe, Colloids Surf., B, 2016, 146, 296–306 CrossRef CAS PubMed.
- J. Liu, H. Jiang and X.-Y. Liu, J. Phys. Chem. B, 2006, 110, 9085–9089 CrossRef CAS PubMed.
- S. R. Khan, P. A. Glenton and D. R. Talham, Kidney Int., 2002, 62, 2062–2072 CrossRef CAS PubMed.
- S. R. Khan, P. O. Whalen and P. A. Glenton, J. Cryst. Growth, 1993, 134, 211–218 CrossRef CAS.
- C. Zhong, Z. Deng, R. Wang and Y. Bai, Cryst. Growth Des., 2015, 15, 1602–1610 CAS.
- L. J. Wang, W. Zhang, S. R. Qiu, W. J. Zachowicz, X. Y. Guan, R. K. Tang, J. R. Hoyer, J. J. De Yoreo and G. H. Nancollas, J. Cryst. Growth, 2006, 291, 160–165 CrossRef CAS.
- S. Qiu, A. Wierzbicki, C. Orme, A. Cody, J. Hoyer, G. Nancollas, S. Zepeda and J. De Yoreo, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 1811–1815 CrossRef CAS PubMed.
- M. L. Weaver, S. R. Qiu, J. R. Hoyer, W. H. Casey, G. H. Nancollas and J. J. De Yoreo, J. Cryst. Growth, 2007, 306, 135–145 CrossRef CAS.
- J. He, R. Lin, H. Long, Y. Liang and Y. Chen, J. Colloid Interface Sci., 2015, 454, 144–151 CrossRef CAS PubMed.
- Y. Liu, H. Mao, X. Liu, L. Qiao and R. Guo, CrystEngComm, 2014, 16, 8841–8851 RSC.
- B. Grohe, A. Taller, P. L. Vincent, L. D. Tieu, K. A. Rogers, A. Heiss, E. S. Sorensen, S. Mittler, H. A. Goldberg and G. K. Hunter, Langmuir, 2009, 25, 11635–11646 CrossRef CAS PubMed.
- S. E. R. Hernandez and N. H. de Leeuw, Cryst. Growth Des., 2015, 15, 4438–4447 CAS.
- T. Mandal, A. G. Shtukenberg, A. C. Yu, X. Zhong and M. D. Ward, Cryst. Growth Des., 2016, 16, 423–431 CAS.
- K. R. Cho, E. A. Salter, J. J. De Yoreo, A. Wierzbicki, S. Elhadj, Y. Huang and S. R. Qiu, CrystEngComm, 2013, 15, 54–64 RSC.
- M. López and B. Hoppe, Pediatr. Nephrol., 2008, 25, 49–59 CrossRef PubMed.
- O. A. Golovanova, E. Y. Achkasova, Y. O. Punin and E. V. Zhelyaev, Crystallogr. Rep., 2006, 51, 348–354 CrossRef CAS.
- D. Škrtić and H. Füredi-Milhofer, J. Cryst. Growth, 1993, 129, 449–455 CrossRef.
- W. G. Jiang, M. S. Pacella, D. Athanasiadou, V. Nelea, H. Vali, R. M. Hazen, J. J. Gray and M. D. McKee, Nat. Commun., 2017, 8, 1–13 CrossRef PubMed.
- H. A. Fuselier, K. Moore, J. Lindberg, F. E. Husserl, F. E. Cole, D. J. Kok, D. Whitehead, D. J. Galliano and D. T. Erwin, Urology, 1998, 52, 988–994 CrossRef CAS PubMed.
- J. Dey, A. Creighton, J. S. Lindberg, H. A. Fuselier, D. J. Kok, F. E. Cole and L. L. Hamm, J. Urol., 2002, 167, 169–171 CrossRef CAS PubMed.
- R. P. Holmes, J. Knight and D. G. Assimos, Urolithiasis, 2016, 44, 27–32 CrossRef CAS PubMed.
- K. K. Gangu, S. Maddila, S. N. Maddila and S. B. Jonnalagadda, RSC Adv., 2017, 7, 423–432 RSC.
- R. Gebhardt, N. Takeda, U. Kulozik and W. Doster, J. Phys. Chem. B, 2011, 115, 2349–2359 CrossRef CAS PubMed.
- Y. Liu, X. Liu, H. Mao and R. Guo, RSC Adv., 2015, 5, 83486–83493 RSC.
- R. Siener, A. Jahnen and A. Hesse, Eur. J. Clin. Nutr., 2004, 58, 270–276 CrossRef CAS PubMed.
- J. Dey, A. Creighton, J. S. Lindberg, H. A. Fuselier, D. J. Kok, F. E. Cole and L. L. Hamm, J. Urol., 2002, 167, 169–171 CrossRef CAS PubMed.
- H. A. Fuselier, K. Moore, J. Lindberg, F. E. Husserl, F. E. Cole, D. J. Kok, D. Whitehead, D. J. Galliano and D. T. Erwin, Urology, 1998, 52, 988–994 CrossRef CAS PubMed.
- B. Grohe, J. O'Young, D. A. Ionescu, G. Lajoie, K. A. Rogers, M. Karttunen, H. A. Goldberg and G. K. Hunter, J. Am. Chem. Soc., 2007, 129, 14946–14951 CrossRef CAS PubMed.
- A. Zhang, H. Xie, N. Liu, B.-L. Chen, H. Ping, Z.-Y. Fu and B.-L. Su, RSC Adv., 2016, 6, 110362–110366 RSC.
- C. Conti, M. Casati, C. Colombo, M. Realini, L. Brambilla and G. Zerbi, Spectrochim. Acta, Part A, 2014, 128, 413–419 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra00661j |
| This journal is © The Royal Society of Chemistry 2018 |