Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Deep eutectic solvent-catalyzed arylation of benzoxazoles with aromatic aldehydes†
Phuong Hoang Tran * and
Anh-Hung Thi Hang
* and
Anh-Hung Thi Hang
Department of Organic Chemistry, Faculty of Chemistry, University of Science, Viet Nam National University, Ho Chi Minh City 721337, Viet Nam. E-mail: thphuong@hcmus.edu.vn
First published on 20th March 2018
Abstract
A novel and efficient methodology for the arylation of benzoxazoles with aromatic aldehydes catalyzed by deep eutectic solvent has been developed. The reaction smoothly proceeded with a wide range of substrates to give the desired products in high yields within short reaction time. Deep eutectic solvents are easily recovered and reused without significant loss of catalytic activity.
Introduction
2-Arylbenzoxazoles are important structural subunits in natural products, pharmaceuticals, agrochemicals, and dyes.1–4 Therefore, the development of efficient synthetic methods for C2-arylation of benzoxazoles has received much attention.5,6 Among them, palladium-catalyzed cross-coupling is one of the most powerful and reliable tools to achieve aryl-substituted benzoxazoles through direct C–H activation and subsequent C–C bond formation.7–9 However, this protocol still suffered from inherent drawbacks associated with the use of uncommon, toxic, and expensive agents such as aryltrimethylammonium triflates,7 aryl halides or triflates,10–17 aryl boronic acids,18 sodium arylsulfinates,19 arylsulfonyl hydrazides,20 and 3-phenylpropionic acid.21 Additionally, the requirement of high-cost palladium catalysts and other expensive additives also prevents the palladium-catalyzed cross-coupling procedure from being employed in large-scale synthesis.22 Recently, the replacement of palladium by other transition metals, such as bis(diisopropylphosphinomethyl)amine nickel(II) and nickel(0) complexes,23 metal–organic frameworks,24,25 Cu/Fe system,26 CeO2/Fe3O4,27 CuCN(PPh3)2,28 and Ni(COD)2,29 has been investigated. These direct arylation methods have received much attention due to their advantage of avoiding the use of stoichiometric amounts of expensive organometallic reagents or additives. However, these protocols still encounter several problems including low yields, long reaction times, poor substrate scope, and high toxicity. Therefore, the development of an effective alternative method for the arylation of benzoxazoles remains highly desirable. Aromatic aldehydes are considered as preferable reagents in the arylation of benzoxazoles owing to their widespread availability, non-toxicity, and low-cost production. The reaction between benzoxazoles and aromatic aldehydes can be conducted in the presence of a suitable catalyst, e.g., molecular I2 or FeSO4.30,31 Until now, there has been no further report on the arylation of benzoxazoles using aldehydes as reagents.In an attempt to develop a cost-effective and environmentally benign protocol, we focus on exploring a new and affordable catalyst for direct arylation of benzoxazoles under “greener” conditions. Deep eutectic solvents (DESs) which were discovered for the first time by Abbott in 2001 have been known as a new class of ionic liquids possessing many outstanding characteristics.32–34 DESs have found many applications as green solvents in diverse fields including nanotechnology,35 separation processes,36 transition metal catalyzed reactions,37 material chemistry,38 stabilization of DNA,39 and organic synthesis.40 Besides, DESs have been known as preferable alternative solvents/catalysts for organic synthesis due to their non-hazardous, non-toxic, stable, non-flammable, and inexpensive nature.41–49 Recently, we have reported DESs-catalyzed organic transformations such as Friedel–Crafts acylation and esterification of sterically hindered alcohols.50,51 As our ongoing efforts to develop environmentally benign syntheses, it is the aim of this communication to describe our preliminary results in the arylation of benzoxazoles with aromatic aldehyde using DES as a green catalyst. Notable features of our report include: (i) straightforward and affordable preparation of catalyst, (ii) simple work-up, (iii) removable additive agents, (iv) recyclable, biodegradable, and low-toxic catalyst.
Results and discussion
Preparation and characterization of [ZnCl2][ethylene glycol]4
DESs were synthesized by mixing zinc chloride and ethylene glycol (HBDs) at a defined molar ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) and heating at 120 °C for 1 h at atmospheric pressure under constant magnetic stirring until a homogeneous liquid was formed. The charge delocalization which occurs through hydrogen bonding between the halide anion and ethylene glycol is responsible for the decrease in the Tm of the mixture.52,53
4) and heating at 120 °C for 1 h at atmospheric pressure under constant magnetic stirring until a homogeneous liquid was formed. The charge delocalization which occurs through hydrogen bonding between the halide anion and ethylene glycol is responsible for the decrease in the Tm of the mixture.52,53
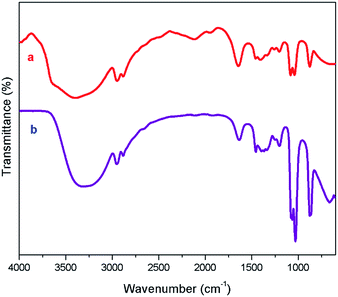
The NMR characterization are in good agreement with the structure of [ZnCl2][ethylene glycol]4 and the NMR spectra show that the catalyst is free of impurities. DESs with high viscosity and the inter- as well as intra-dipolar interactions can cause the broadening effect on the resonance signals of NMR spectrum.54 The high viscosity of DES used in the current work involves the formation of massive hydrogen bond network between each component.55 Fig. 1 displays the FT-IR spectra of ethylene glycol and [ZnCl2][ethylene glycol]4. The spectrum of the [ZnCl2][ethylene glycol]4 is an overlap of those of ethylene glycol. The result showed that the structure of ethylene glycol was not destroyed in the [ZnCl2][ethylene glycol]4. Particularly, the absorption bands of ethylene glycol at 3390 cm−1 could be ascribed to stretch vibration of O–H functional group. As observed in Fig. 1, the O–H stretching vibration of [ZnCl2][ethylene glycol]4 shifts to lower wavenumber, indicating that O–H of ethylene glycol takes part in the formation of the hydrogen bond with the anion of zinc chloride.47,53,55
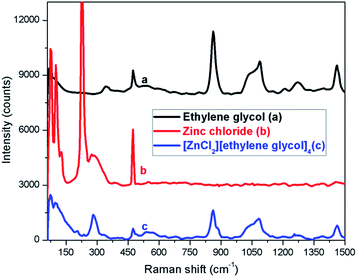
The Raman spectra of ethylene glycol, zinc chloride, and [ZnCl2][ethylene glycol]4 are presented in Fig. 2 for a comparative analysis in the region from 50 to 1500 cm−1. In pure ZnCl2, we have observed a strong signal at 225 cm−1 and another weak signal at 290 cm−1; however, at low ZnCl2 molar fraction (in deep eutectic solvent) the feature at 290 cm−1 becomes strong and the signal at 225 cm−1 disappears. Rubim et al. have also observed the same feature for ZnCl2 in eutectic mixture with 1-butyl-3-methylimidazolium chloride.56 Thus, the Raman spectrum of [ZnCl2][ethylene glycol]4 does not change as compared with the signal of ethylene glycol but additional peaks from ZnCl2 appear at 80 and 290 cm−1.
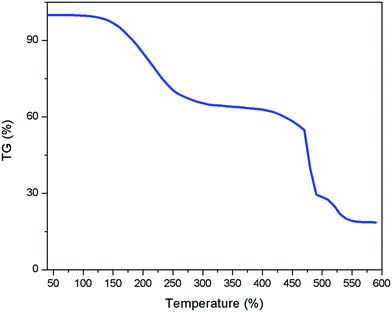
Thermal gravimetric analysis (TGA) of [ZnCl2][ethylene glycol]4 was performed in Fig. 3. The major weight loss occurs in the temperature range from 200 °C to 475 °C, which make the [ZnCl2][ethylene glycol]4 suitable for high-temperature reaction conditions.
Optimization of reaction conditions
We initiated our studies by investigating various reaction conditions for the C2-arylation of benzoxazole by benzaldehyde (Tables 1 and 2). The optimized condition revealed that the air-stable and environmentally benign [ZnCl2][ethylene glycol]4 proved to be an effective catalyst for the highly selective C2-arylation of benzoxazole with benzaldehyde in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio. The method provided the desired product in high yield in the absence of other additives such as organic solvents or bases. It is also noteworthy that this arylation of benzoxazole proceeded smoothly without the need of inert atmosphere.
1 molar ratio. The method provided the desired product in high yield in the absence of other additives such as organic solvents or bases. It is also noteworthy that this arylation of benzoxazole proceeded smoothly without the need of inert atmosphere.
| Entry | Catalytic amount (mol%) | Time (h) | Temperature (°C) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: benzoxazole (1 mmol), benzaldehyde (0.5 mmol), solvent-free.b Isolated yield. | ||||
| 1 | 1 | 6.0 | 120 | 60 |
| 2 | 2 | 6.0 | 120 | 75 |
| 3 | 3 | 6.0 | 120 | 80 |
| 4 | 5 | 6.0 | 120 | 95 |
| 5 | 10 | 6.0 | 120 | 95 |
| 6 | 5 | 1.0 | 120 | 0 |
| 7 | 5 | 1.5 | 120 | 0 |
| 8 | 5 | 2.5 | 120 | 0 |
| 9 | 5 | 4.0 | 120 | 30 |
| 10 | 5 | 4.5 | 120 | 75 |
| 11 | 5 | 6.0 | 120 | 95 |
| 12 | 5 | 6.5 | 120 | 97 |
| 13 | 5 | 6.0 | 90 | 40 |
| 14 | 5 | 6.0 | 100 | 45 |
| 15 | 5 | 6.0 | 110 | 80 |
| 16 | 5 | 6.0 | 120 | 95 |
| 17 | 5 | 6.0 | 130 | 95 |
| 18 | 5 | 6.0 | 140 | 95 |
With the optimized catalyst in hand, the scope of the benzoxazoles and aromatic aldehydes in the arylation reaction was studied (Table 3). The results demonstrated that the developed pathway provided the C2-arylation products in high yields. Generally, the robust DES between ethylene glycol and zinc chloride allowed a variety of substituted benzoxazoles and aldehydes to transform into the desired products in good to excellent yields with 100% selectivity in C2-arylation. First, a large number of aromatic aldehydes, regardless of containing electron-donating substituents (methyl, t-butyl, hydroxy, methoxy) or electron-withdrawing substituents (nitro, halide), can react with benzoxazoles to produce the expected products under the given reaction conditions (Table 3). However, low yields of the desired products were observed for benzaldehydes bearing severe electron-withdrawing groups such as nitro or fluoro substituents (Table 3, entries 5, 6, 8, 10, 18, 26, 35). Next, the scope of various benzoxazoles was also evaluated. As our expectation both 5-methylbenzoxazole and 5-chlorobenzoxazole generally underwent the arylation to give the corresponding products in very good yields ranging from 82 to 95% except the case of 4-fluorobenzaldehyde and 4-hydroxybenzaldehyde whose resulting arylated products were only obtained in significantly diminished yields of 70–75% (Table 3, entries 14–30). For the benzoxazole bearing a nitro substituent, the lower yields of arylation products with various aldehydes were recorded even though harsher conditions (higher temperature for prolonged reaction time) were employed (Table 3, entries 31–37). It is noteworthy that the condensation between benzoxazole and benzaldehyde was successfully performed on a 10 mmol or 20 mmol scale, and the yield is virtually the same as on 1 mmol scale (Table 3, entry 1).
| Entry | Product | Condition | Yielda (%) |
|---|---|---|---|
| a Isolated yield.b On 10 mmol or 20 mmol scale. | |||
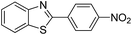
| 1 | 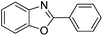 |
120 °C, 6 h | 95 (94)b |
| 2 | 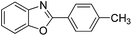 |
120 °C, 5 h | 93 |
| 3 | 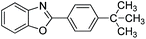 |
120 °C, 5 h | 94 |
| 4 | 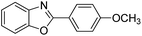 |
120 °C, 4 h | 95 |
| 5 | 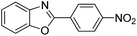 |
140 °C, 6 h | 75 |
| 6 | 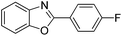 |
120 °C, 4 h | 80 |
| 7 | 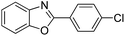 |
120 °C, 4 h | 92 |
| 8 | 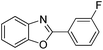 |
120 °C, 5 h | 70 |
| 9 | 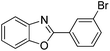 |
120 °C, 5 h | 90 |
| 10 | 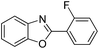 |
120 °C, 5 h | 72 |
| 11 | 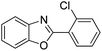 |
120 °C, 4 h | 90 |
| 12 | 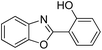 |
120 °C, 6 h | 93 |
| 13 | 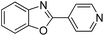 |
120 °C, 6 h | 80 |
| 14 | 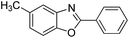 |
120 °C, 5 h | 94 |
| 15 | 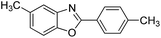 |
120 °C, 4 h | 90 |
| 16 | 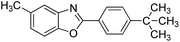 |
120 °C, 4 h | 90 |
| 17 | 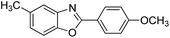 |
120 °C, 4 h | 95 |
| 18 | 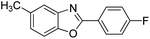 |
120 °C, 4.5 h | 75 |
| 19 | 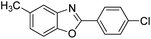 |
120 °C, 4.5 h | 85 |
| 20 | 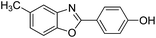 |
120 °C, 6.5 h | 70 |
| 21 | 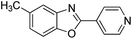 |
120 °C, 6 h | 82 |
| 22 | 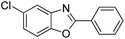 |
120 °C, 5 h | 95 |
| 23 | 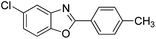 |
120 °C, 4 h | 95 |
| 24 | 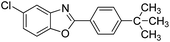 |
120 °C, 4 h | 90 |
| 25 | 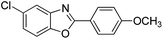 |
120 °C, 4 h | 95 |
| 26 | 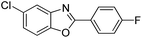 |
120 °C, 4 h | 75 |
| 27 | 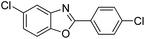 |
120 °C, 4 h | 95 |
| 28 | 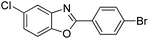 |
120 °C, 6 h | 85 |
| 29 | 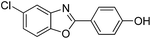 |
120 °C, 6.5 h | 75 |
| 30 | 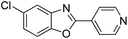 |
120 °C, 6 h | 85 |
| 31 | 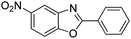 |
140 °C, 6 h | 70 |
| 32 | 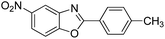 |
140 °C, 5 h | 75 |
| 33 | 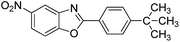 |
140 °C, 5 h | 75 |
| 34 | 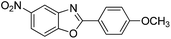 |
140 °C, 5 h | 80 |
| 35 | 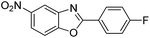 |
140 °C, 6 h | 72 |
| 36 | 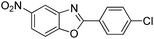 |
140 °C, 6 h | 80 |
| 37 | 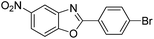 |
140 °C, 6 h | 85 |
The versatility of benzothiazole and benzimidazole as substrates in the replacement of benzoxazole was also reported. The adducts resulted from the condensation of benzothiazole with various aromatic aldehydes were isolated in comparative yields with those derived from benzoxazole (Table 4, entries 1–6). Meanwhile, a failure in the formation of the desired product was noted for the case of benzimidazole (Table 4, entry 7), probably due to the existence of intermolecular hydrogen bonds between the NH of benzimidazole and DES.
A comparative study between the current method and previous ones was presented in Table 5. Deep eutectic solvent-catalyzed arylation of benzoxazole afforded the arylated benzoxazole products in excellent yields under a mild and simple condition without the demand for any additives as in preceding reports (Table 5, entry 6). Remarkably, no loss of catalytic activity in the recycling test of DES is the most prominent artifact of this protocol.
| Entry | Catalyst | Reagent | Condition | Yield (%) |
|---|---|---|---|---|
| 1 | FeSO4 (0.2 equiv.), H2O/diglyme/O2 | 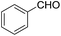 |
150 °C, 20 h | 70 (ref. 31) |
| 2 | I2 (2 equiv.), PhCl, DMF | 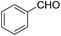 |
130 °C, 30 h | 75 (ref. 30) |
| 3 | [Pd(π-allyl)Cl]2 (0.1 equiv.), PCy3, NaOtBu (2 equiv.), DMF | 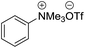 |
120 °C, 12 h | 43 (ref. 7) |
| 4 | CuCN(PPh3)2 (10 mol%), PPh3, Cs2CO3, pivalonitrile | 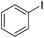 |
Reflux, 24 h | 85 (ref. 28) |
| 5 | Ni(COD)2 (0.1 equiv.), dcype (0.2 equiv.), Cs2CO3 (1.5 equiv.), p-xylene | 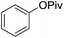 |
140 °C, 22 h | 91 (ref. 29) |
| 6 | Current work: [ZnCl2][ethylene glycol]4 (5 mol%), solvent-free |  |
120 °C, 6 h | 95 |
As asserted in previous literature through isotope-labeling mechanistic studies, the arylation of benzoxazoles with aromatic aldehydes underwent the ring-opening step assisted by Lewis acids such as I2 or FeSO4 to afford the key intermediate 2-aminophenol. Its nucleophilic addition to aldehydes followed by oxidative ring closure provided arylated benzoxazoles as final products.30,31 As an extra part in our research to check the conformability of the proposed mechanism for the same reaction catalyzed by [ZnCl2][ethylene glycol]4, we also carried out the acylation–cyclization of 2-aminophenol with benzaldehyde under the same optimized conditions which were previously applied for the arylation of benzoxazole by benzaldehyde. As the result, the same arylated benzoxazole product was obtained in a comparable yield of 85%. Additionally, in another control experiment whereby benzoxazole reacted with [ZnCl2][ethylene glycol]4 in the absence of benzaldehyde, 2-aminophenol was obtained in 62% yield. Thus, it is not doubtful that the arylation of benzoxazole studied herein must also undergo the ring-opening step prior to the condensation step with aldehydes. Although the mechanism is not clear now, the method possesses attractive merits including cheap and recyclable catalyst, non-toxicity, and wide scope of substrates.
The recyclability is an important feature for applying a catalyst in industrial processes. The recyclability of deep eutectic solvent of zinc chloride and ethylene glycol under study in this work was investigated in the model reaction. After completion of the reaction, the product was extracted with diethyl ether (10 × 5 mL), the catalyst was separated from the ethereal solution and dried under vacuum. The recovered catalyst was reused in the model reaction to the next run. As illustrated in Fig. 4, the efficiency of deep eutectic solvent was found to be constantly excellent even after five consecutive recycles. IR spectroscopy of fresh and recovered deep eutectic solvent indicated that no detectable structural degradation can be seen (Fig. 5). After each recycling test, a very small amount of DES leaching to diethyl ether phase during the work-up step was indirectly estimated by means of ICP-MS technique in which Zn content of about 0.08 ppm in the ethereal phase was determined. A slight decrease of catalytic activity was observed due to a little loss of deep eutectic solvent during the work-up process.
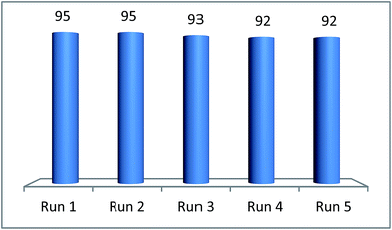 | ||
| Fig. 4 Reuse of [ZnCl2][ethylene glycol]4 catalyst in the arylation of benzoxazole with benzaldehyde. | ||
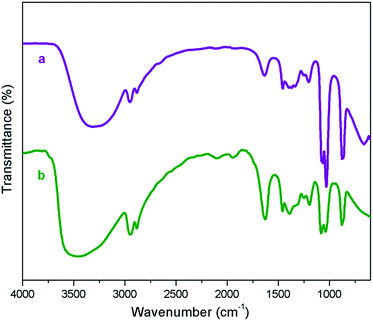 | ||
| Fig. 5 FT-IR of fresh [ZnCl2][ethylene glycol]4 (a) and [ZnCl2][ethylene glycol]4 after the fifth recovery (b). | ||
Experimental
General procedure for catalytic arylation of benzoxazole
Benzoxazole (119 mg, 1.0 mmol) was treated with benzaldehyde (53 mg, 0.5 mmol) in the presence of [ZnCl2][ethylene glycol]4 (5 mg, 0.01 mmol) at 120 °C for 6 h under solvent-free magnetic stirring. The completion of the reaction was checked by TLC and GC. The mixture was then diluted with diethyl ether (10 × 5 mL). The solvent was removed on a rotary evaporator. The crude product was purified by silica gel chromatography using acetone/petroleum ether (1/19) to afford the desired product (95% yield). The purity and identity of the product were confirmed by FT-IR, 1H NMR, 13C NMR, and MS. The recovered catalyst was activated by heating under reduced vacuum at 80 °C for 30 min and reused for next cycles.Conclusions
In conclusion, we have developed the first DES-mediated arylation of benzoxazoles with aromatic aldehydes at the C2 position under solvent-free condition. The most important highlight of the current method is the use of inexpensive, air- and water-stable, and recyclable deep eutectic solvent in the replacement of high-cost palladium catalysts. Interestingly, only a catalytic amount of deep eutectic solvent is requisite for the quantitative production of various 2-arylbenzoxazoles through a simplistic, environmentally benign, and low-cost synthetic procedure.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research is funded by Viet Nam National University, Ho Chi Minh City (VNU-HCM) under grant number 562-2018-18-03.Notes and references
- O. Daugulis, H.-Q. Do and D. Shabashov, Acc. Chem. Res., 2009, 42, 1074–1086 CrossRef CAS PubMed.
- K. Kuroda, S. Tsuyumine and T. Kodama, Org. Process Res. Dev., 2016, 20, 1053–1058 CrossRef CAS.
- C. S. Demmer and L. Bunch, Eur. J. Med. Chem., 2015, 97, 778–785 CrossRef CAS PubMed.
- K. Seth, S. K. Garg, R. Kumar, P. Purohit, V. S. Meena, R. Goyal, U. C. Banerjee and A. K. Chakraborti, ACS Med. Chem. Lett., 2014, 5, 512–516 CrossRef CAS PubMed.
- C. L. Sun and Z. J. Shi, Chem. Rev., 2014, 114, 9219–9280 CrossRef CAS PubMed.
- L. Yang and H. Huang, Chem. Rev., 2015, 115, 3468–3517 CrossRef CAS PubMed.
- F. Zhu, J.-L. Tao and Z.-X. Wang, Org. Lett., 2015, 17, 4926–4929 CrossRef CAS PubMed.
- F. Bellina and R. Rossi, Tetrahedron, 2009, 65, 10269–10310 CrossRef CAS.
- G. Wu, J. Zhou, M. Zhang, P. Hu and W. Su, Chem. Commun., 2012, 48, 8964–8966 RSC.
- B. Sezen and D. Sames, Org. Lett., 2003, 5, 3607–3610 CrossRef CAS PubMed.
- R. S. Sánchez and F. A. Zhuravlev, J. Am. Chem. Soc., 2007, 129, 5824–5825 CrossRef PubMed.
- F. Derridj, S. Djebbar, O. Benali-Baitich and H. Doucet, J. Organomet. Chem., 2008, 693, 135–144 CrossRef CAS.
- F. Yang, Y. Wu, Z. Zhu, J. Zhang and Y. Li, Tetrahedron, 2008, 64, 6782–6787 CrossRef CAS.
- J. Canivet, J. Yamaguchi, I. Ban and K. Itami, Org. Lett., 2009, 11, 1733–1736 CrossRef CAS PubMed.
- J. Roger, S. Mom, M. Beaupérin, S. Royer, P. Meunier, V. V. Ivanov, H. Doucet and J.-C. Hierso, ChemCatChem, 2010, 2, 296–305 CrossRef CAS.
- J. J. Dong, J. Roger, C. Verrier, T. Martin, R. Le Goff, C. Hoarau and H. Doucet, Green Chem., 2010, 12, 2053–2063 RSC.
- X.-B. Shen, Y. Zhang, W.-X. Chen, Z.-K. Xiao, T.-T. Hu and L.-X. Shao, Org. Lett., 2014, 16, 1984–1987 CrossRef CAS PubMed.
- S. Ranjit and X. Liu, Chem.–Eur. J., 2011, 17, 1105–1108 CrossRef CAS PubMed.
- M. Wang, D. Li, W. Zhou and L. Wang, Tetrahedron, 2012, 68, 1926–1930 CrossRef CAS.
- F. Kwong, O. Yuen, C. So and W. Wong, Synlett, 2012, 23, 2714–2718 CrossRef.
- D. Liu, B. Liu and J. Cheng, RSC Adv., 2013, 3, 9193–9196 RSC.
- K. E. Balsane, S. H. Gund and J. M. Nagarkar, Catal. Commun., 2017, 89, 29–33 CrossRef CAS.
- A. Kruckenberg, H. Wadepohl and L. H. Gade, Organometallics, 2013, 32, 5153–5170 CrossRef CAS.
- N. T. S. Phan, C. K. Nguyen, T. T. Nguyen and T. Truong, Catal. Sci. Technol., 2014, 4, 369–377 CAS.
- H. T. N. Le, T. T. Nguyen, P. H. L. Vu, T. Truong and N. T. S. Phan, J. Mol. Catal. A: Chem., 2014, 391, 74–82 CrossRef CAS.
- S.-L. Zhang, W.-Y. Hu and P.-P. Wang, Synthesis, 2014, 47, 42–48 CrossRef.
- R. S. Shelkar, K. E. Balsane and J. M. Nagarkar, Tetrahedron Lett., 2015, 56, 693–699 CrossRef CAS.
- D. Kim, K. Yoo, S. E. Kim, H. J. Cho, J. Lee, Y. Kim and M. Kim, J. Org. Chem., 2015, 80, 3670–3676 CrossRef CAS PubMed.
- D. F. Steinberg, M. C. Turk and D. Kalyani, Tetrahedron, 2017, 73, 2196–2209 CrossRef CAS PubMed.
- Y. C. Teo, S. N. Riduan and Y. Zhang, Green Chem., 2013, 15, 2365–2368 RSC.
- S. Liu, R. Chen, X. Guo, H. Yang, G. Deng and C.-J. Li, Green Chem., 2012, 14, 1577–1580 RSC.
- Q. Zhang, K. De Oliveira Vigier, S. Royer and F. Jerome, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC.
- B.-Y. Zhao, P. Xu, F.-X. Yang, H. Wu, M.-H. Zong and W.-Y. Lou, ACS Sustainable Chem. Eng., 2015, 3, 2746–2755 CrossRef CAS.
- A. P. Abbott, G. Capper, D. L. Davies, H. L. Munro, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2001, 2010–2011 RSC.
- A. Abo-Hamad, M. Hayyan, M. A. AlSaadi and M. A. Hashim, Chem. Eng. J., 2015, 273, 551–567 CrossRef CAS.
- B. Tang, H. Zhang and K. H. Row, J. Sep. Sci., 2015, 38, 1053–1064 CrossRef CAS PubMed.
- J. García-Álvarez, Eur. J. Inorg. Chem., 2015, 2015, 5147–5157 CrossRef.
- F. del Monte, D. Carriazo, M. C. Serrano, M. C. Gutierrez and M. L. Ferrer, ChemSusChem, 2014, 7, 999–1009 CrossRef CAS PubMed.
- H. Zhao, J. Chem. Technol. Biotechnol., 2015, 90, 19–25 CrossRef CAS.
- C. Vidal, J. García-Álvarez, A. Hernán-Gómez, A. R. Kennedy and E. Hevia, Angew. Chem., Int. Ed., 2016, 55, 16145–16148 CrossRef CAS PubMed.
- M. Starykevich, A. N. Salak, D. K. Ivanou, A. D. Lisenkov, M. L. Zheludkevich and M. G. S. Ferreira, Electrochim. Acta, 2015, 170, 284–291 CrossRef CAS.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed.
- J. I. García, H. García-Marín and E. Pires, Green Chem., 2014, 16, 1007–1033 RSC.
- J. Cao, B. Qi, J. Liu, Y. Shang, H. Liu, W. Wang, J. Lv, Z. Chen, H. Zhang and X. Zhou, RSC Adv., 2016, 6, 21612–21616 RSC.
- C. Mukesh, R. Gupta, D. N. Srivastava, S. K. Nataraj and K. Prasad, RSC Adv., 2016, 6, 28586–28592 RSC.
- G. García, S. Aparicio, R. Ullah and M. Atilhan, Energy Fuels, 2015, 29, 2616–2644 CrossRef.
- Q. Wang, X. Yao, Y. Geng, Q. Zhou, X. Lu and S. Zhang, Green Chem., 2015, 17, 2473–2479 RSC.
- O. S. Hammond, K. J. Edler, D. T. Bowron and L. Torrente-Murciano, Nat. Commun., 2017, 8, 14150–14157 CrossRef CAS PubMed.
- A. R. Hajipour, S. H. Nazemzadeh and F. Mohammadsaleh, Tetrahedron Lett., 2014, 55, 654–656 CrossRef CAS.
- P. H. Tran, H. T. Nguyen, P. E. Hansen and T. N. Le, RSC Adv., 2016, 6, 37031–37038 RSC.
- H. T. Nguyen and P. H. Tran, RSC Adv., 2016, 6, 98365–98368 RSC.
- Y. Dai, J. van Spronsen, G. J. Witkamp, R. Verpoorte and Y. H. Choi, J. Nat. Prod., 2013, 76, 2162–2173 CrossRef CAS PubMed.
- A. Pandey, Bhawna, D. Dhingra and S. Pandey, J. Phys. Chem. B, 2017, 121, 4202–4212 CrossRef CAS PubMed.
- C. D'Agostino, R. C. Harris, A. P. Abbott, L. F. Gladden and M. D. Mantle, Phys. Chem. Chem. Phys., 2011, 13, 21383–21391 RSC.
- J. Zhu, K. Yu, Y. Zhu, R. Zhu, F. Ye, N. Song and Y. Xu, J. Mol. Liq., 2017, 232, 182–187 CrossRef CAS.
- M. B. Alves, V. O. Santos Jr, V. C. D. Soares, P. A. Z. Suarez and J. C. Rubim, J. Raman Spectrosc., 2008, 39, 1388–1395 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra01094c |
| This journal is © The Royal Society of Chemistry 2018 |