 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Multifunctional Nd3+ substituted Na0.5Bi0.5TiO3 as lead-free ceramics with enhanced luminescence, ferroelectric and energy harvesting properties†
Kumara Raja Kandula,
Saket Asthana * and
Sai Santosh Kumar Raavi
* and
Sai Santosh Kumar Raavi *
*
Advanced Functional Materials Laboratory, Department of Physics, Indian Institute of Technology Hyderabad, Kandi, Sangareddy 502285, Telangana, India. E-mail: sskraavi@iith.ac.in; asthanas@iith.ac.in
First published on 23rd April 2018
Abstract
Herein, we present comprehensive investigations of the optical and electrical properties of Nd3+ substitution in sodium bismuth titanate ceramics (NBNT) with varying Nd3+ concentration. The room temperature photoluminescence (PL) emission for both unpoled and poled samples is observed to be a maximum for an Nd3+ substitution of 1 mol%. Upon poling, the PL intensity is observed to be quenched, consistent with the obtained XRD data, indicating an electric-field induced structural ordering towards higher symmetry, confirmed with the help of structural refinement. The evaluated ferroelectric to relaxor and antiferroelectric relaxor T(F–R) was observed clearly from the poled dielectric–loss curve for the 1 mol% of Nd3+ substitution. Furthermore, the optimized NBNT exhibited a lower Ec and a higher off-resonance figure of merit (FOMoff) for energy harvesting by 12% and 30%, respectively, in comparison with un-doped NBT.
1. Introduction
Multifunctional ceramics, exhibiting two or more properties from among magnetic, ferroelectric, pyroelectric, piezoelectric and optoelectronic properties have received a remarkable amount of interest.1–3 Sodium bismuth titanate, Na0.5Bi0.5TiO3 (NBT), with a lead-free perovskite structure is a proven candidate showing excellent ferroelectric properties.4,5 Enhanced piezoelectric properties of NBT and its solid solutions by cation substitution at the A and B-sites have been reported.6–9 In particular, it was observed that site-specific substitution with optically active rare-earth (RE) ions resulted in enhanced electrical properties with the additional functionality of photoluminescence (PL), making them attractive for promising applications.10–15 Recent reports on RE substituted NBT ceramics indicated that both PL and electrical properties can be modified upon electrical poling.16,17 Among all the optically active RE ions, Nd3+ with emission in the near IR spectral region has found a plethora of optoelectronics applications. M. Zannen et al.13 reported the best emission together with better ferroelectric properties for 5 mol% substitution of Nd3+ for NBT ceramics. Another investigation18 revealed better piezoelectric coefficients with 2 mol% substitution of Nd3+. These contradictory reports, however, did not deliberate on the effect of electrical poling.In this report, we focus our attention on obtaining the optimal substitution of Nd3+ for enhanced properties and multifunctionality. Here, we relate the role of structural changes in the host lattice to the obtained PL intensity from Nd3+ substituted [Na0.5Bi0.5−xNdx]TiO3 (referred to hereafter as NBNT) ceramics with varying Nd3+ concentration in the range of x = 0.003–0.1 [≅(0.3–10) mol%] by photo excitation at 532 nm. We observed the maximum PL intensity for x = 0.01 (≅1 mol%), which is the critical concentration for the onset of PL quenching. Upon poling, the applied electric-field induces structural order that is manifested in further PL quenching. The obtained hysteresis (P–E) data for NBNT showed a reduced coercive electric field (Ec) with almost identical remnant-polarization (Pr), implying that optimized NBNT maintains a ferroelectric behaviour similar to that of NBT. Further, the estimated off-resonance figure of merit (FOMoff) for energy harvesting showed an increase of ≈30%, compared to NBT, making the optimised NBNT a promising candidate for multifunctional applications. The literature reports multifunctionality achieved in different solid-solutions which, no doubt provide better properties. Yet processing these materials from simple bulk ceramic compounds to thin-film devices is challenging. Thus, advances in simple perovskite compounds are useful in this respect and this communication aims to show that simple substitution of Nd3+ into the A-site of NBT enhances the ferroelectric, piezoelectric, and energy harvesting properties with the added functionality of it being photo-luminescent.
2. Experimental details
Polycrystalline NBT compounds with different Nd3+ substitutions were synthesized by a conventional solid-state route using high-quality powders of Na2CO3, Bi2O3, TiO2, and Nd2O3 (99.99% Sigma-Aldrich Chemicals, USA). Considering the loss on ignition, the precursors are weighed carefully according to stoichiometric ratios given as [Na0.5Bi0.5−xNdx]TiO3 where x = 0, 0.003, 0.005, 0.007, 0.01, 0.02, 0.03, 0.04, 0.05, 0.07, and 0.1) and subsequently milled and calcined at 800 °C for 3 h. The calcined powders are further milled, sieved, and compacted into a circular pellet (ϕ = 10 mm) and finally sintered at 1150 °C for 3 h in air with a heating rate of 5°C min−1. Phase analysis of the obtained ceramic pellets was performed by using a powder X-ray diffractometer (PANalyticalX'pertpro; CuKα radiation λ = 1.5406 Å) over the angles 20° < 2θ < 80°. The relative density (RD) of the NBNTs was measured using the Archimedes method and was estimated to be in the range of 94–95% relative to the theoretical density of 5.94 g cm3 (ref. 19 and 20) for pure NBT ceramics. Diffused-reflectance measurements were performed using a Shimadzu UV-3600 spectrometer. The room temperature PL spectrum was measured by photo-excitation at 532 nm from a cw-DPSS laser (LSR532NL-300, Lasever) and the subsequent PL was collected by a fiber coupled spectrometer (AvaSpec-ULS2048L-RS, Avantes) with a spectral resolution of 1.4 nm.21 The PL spectrum, averaged over 3 spots on the sample, is presented as obtained. All the ceramics are thinned down to a thickness of 0.5 mm to get enough dense silver electrodes on both sides of the pellets. The samples were electrically poled at room temperature in a silicon oil (DOW CORNING 704) bath by applying a DC electric field of (0–10–50) kV cm−1 for 30 min and all the measurements were repeated thereafter. The hysteresis (P–E) data were obtained with a TF-Analyzer 2000 (aixACCT systems, GmbH) from samples of 0.5 mm thickness, using silver-coated electrodes on both sides. The temperature- and frequency-dependent dielectric measurements on the silver-coated pellets were performed with an impedance analyzer (Wayne Kerr 6500B). The piezoelectric harvesting parameters were obtained using a Piezometer System PM300 (Piezotest Ltd.) operated at a frequency of 100 Hz.3. Results and discussion
The prepared NBNT ceramics with Nd3+ substitution [x = 0.003–0.1] allowed a sufficient number of samples to get the optimized NBNT with the best multifunctionality. The absorption spectra of the NBNTs, shown in Fig. 1, had the specific signature of Nd3+ in an ordered host medium, even though the peak intensities differ in different hosts.22 With an increase in x these bands are observed to be getting stronger, with no absorption feature for pure NBT in the visible spectral region (inset).Fig. 2 shows the measured PL spectra of NBNT ceramics exhibiting multiple peaks within the possible detection range. Evidently, the best PL intensity is observed for x = 0.01 substitution and the PL intensity decreases with an increase in the Nd3+ substitution. Importantly, the PL peak position is not influenced by x. PL is not observed from the pure NBT sample, as expected. The spectroscopic terms related to the radiative relaxation of Nd3+ are well known.22 The observed PL spectrum consists of overlapping lines arising from transitions between the crystal field split 4F3/2 level and various sublevels of 4I9/2 and 4I11/2. The inset of Fig. 2 shows the PL spectrum from NBNT with x = 0.01 substitution, at a log-scale to highlight the various peaks, the maxima being at 880 nm and 910 nm. The choice of rejection color filters at detection enabled observation of the peaks at 808 nm and 832 nm, which were not presented in earlier reports.14,15 The measured spectrum in (1000–1100) nm with a maximum at 1064 nm is weak since the detector sensitivity21 drops sharply in this spectral region. The shoulder at 1083 nm can be conjectured to be the effect of structural disorder, which could be due to the individual excitation of Nd3+ ions in the Bi3+ site.
Considering NBNT is a ferroelectric ceramic, it will be subjected to electric fields during routine device operation, making it vital to understand the effect of an applied electric field on the PL. Upon electrical poling, PL is observed to have been quenched in intensity compared to the respective unpoled samples. Nonetheless, the variation in emission with respect to substitution is similar to the trend seen for unpoled ceramics, with the best emission for x = 0.01 substitution, similar to emission from unpoled samples. To make a quantitative comparison, we calculated the area under the PL spectra of poled and unpoled ceramics in the spectral range of (800–1000) nm. Fig. 3 demonstrates the effect of electrical poling, with a reduction in PL spectral intensity of ≈30% upon poling to 50 kV cm−1 being observed. It is interesting to note that the shapes of the PL spectral profiles remain unchanged upon poling, unlike a similar report12 on Eu3+ doped NBT ceramics for which the formation of a new spectral peak was observed. The PL intensity is found to be a maximum for x = 0.01 substitution.
Considering the paucity of reports on the computational study of crystal-field effects on rare-earth emission in doped perovskite material systems, we try to elucidate our observation qualitatively. It is well known that the Nd3+ emission is extremely sensitive to the local host symmetry. The Judd–Ofelt theory, which is widely used to estimate the intensities of transitions for the RE ions, defines a set of three intensity parameters Ωt (t = 2, 4 and 6) that are sensitive to the local environment of the luminescent centers.23,24 Here, the important factors affecting the local environments of Nd3+ in NBT pertain to fluctuations in the Na![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bi ratio from 1
Bi ratio from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as a result of substitution, different chemical ordering at the local scale and disorder associated with atomic displacements. With a solid matrix with fixed lattice positions for Na and Bi, the external electric field is expected to induce long-range order in the polar displacements of the atoms. This in turn affects the transition intensities. Therefore, in line with an earlier report16 for Pr-doped BNT-BT, where there was an enhancement in PL intensity upon poling, the observed reduction in PL can be conjectured to be a result of electric-field induced structural order towards higher symmetry.
1 as a result of substitution, different chemical ordering at the local scale and disorder associated with atomic displacements. With a solid matrix with fixed lattice positions for Na and Bi, the external electric field is expected to induce long-range order in the polar displacements of the atoms. This in turn affects the transition intensities. Therefore, in line with an earlier report16 for Pr-doped BNT-BT, where there was an enhancement in PL intensity upon poling, the observed reduction in PL can be conjectured to be a result of electric-field induced structural order towards higher symmetry.
To confirm the role of structural ordering, XRD measurements were performed in different poling fields (0–50) kV cm−1 with the interval of the poling field 10 kV cm−1 to identify the structural symmetry variation in the optimal specimen, i.e. NBT-0.01Nd. Unpoled NBNTs exhibited a single phase structure stabilizing into a monoclinic structure with Cc space group symmetry in the substitution range 0 ≤ x ≤ 0.1, with no co-existing secondary phases within the sensitivity of XRD.22 Fig. 4 shows the variation in the XRD pattern for various poling fields. The poling field induces a distinct broadness in the main prominent peak (110)PC of the perovskite structure, as shown in the left panel of Fig. 4. Notably, the structurally induced changes we observe upon electrical poling are in line with the reduction in PL intensity presented in Fig. 3.
 | ||
| Fig. 4 X-Ray diffraction pattern of NBT-0.01Nd under different poling fields (0–10–50) kV cm−1, showing the enlarged portion of (110)PC. | ||
Further analysis of this prominent peak presented the structural evolution of other phases from the peak broadening as well the change in peak profile. In order to extract the possible structural evolution under the poling field in (110)PC, the obtained peak is deconvoluted by using Lorentzian multi-peak fits, as shown in Fig. 5. The new structural phase rhombohedral (R3c) gradually evolves over the poling fields (represented by the blue shaded portion of the area under the curve). From the 40 kV cm−1 spectrum we can observe a prominent doublet instead of a singlet, representing the effect of electrical poling on the crystal structure. The XRD data showing the effect of poling throughout the series is provided in the ESI.†
 | ||
| Fig. 5 Deconvolution of the (110)PC peak for extracting the rhombohedral R3c phase along with monoclinic Cc under different electrical poling fields (0–50) kV cm−1. | ||
To strengthen the above arguments, further structural refinement of the XRD measurements was carried out by Rietveld refinement for the poled and unpoled [Na0.5Bi0.5−xNdx]TiO3 (x = 0.01) using Fullproof software, as presented in Fig. 6. Notably, the shape of the Bragg profile was modified upon poling, suggesting a gradual change throughout the series [Na0.5Bi0.5−xNdx]TiO3 (x = 0 and 0.1). The structure throughout the series stabilizes to monoclinic with the Cc space group. Upon poling the induced structural modification from Cc to R3c changes, observed as clear splitting at the (110)PC reflection. The unpoled XRD pattern of [Na0.5Bi0.5−xNdx]TiO3 (x = 0.01) has fitted reasonably well with the recently proposed monoclinic Cc structure. The poled pattern of the same composition could be fitted well with the pure rhombohedral (R3c) structure. The goodness of fit is depicted in the inset of Fig. 6 for the Bragg reflection at 40°. The right panel of Fig. 6 illustrates the proposed crystal structure corresponding to poled and unpoled ceramics, using the refined parameters as input to the Diamond-Crystal and Molecular Structure Visualization software. Evidently distinct structural modulation can be observed upon poling. Further, the XRD patterns of the poled compositions x = 0.04 onwards could be fitted well with the Cc model, showing a reduced contribution of the fraction of the R3c phase upon poling the compounds with increased Nd3+ substitution. Table 1 shows the refined crystal parameters and corresponding positions.
| Unpoled (Cc) | x | y | z |
|---|---|---|---|
| Na/Bi/Nd | 0.0000 | 0.2517 | 0.0000 |
| Ti | 0.2519 | 0.2504 | 0.7386 |
| O1 | −0.0016 | 0.2521 | 0.5107 |
| O2 | 0.1982 | 0.4906 | −0.0686 |
| O3 | 0.3013 | 0.0296 | 0.0614 |
| Lattice parameters (Å) | a = 9.518, b = 5.4876, c = 5.4982, α = γ = 90°, β = 125.331°, χ2 = 1.85 | ||
| Poled (R3c) | x | y | z |
|---|---|---|---|
| Na/Bi/Nd | 0.0000 | 0.0000 | 0.2713 |
| Ti | 0.0000 | 0.0000 | 0.0068 |
| O | 0.1335 | 0.3379 | 0.0838 |
| Lattice parameters (Å) | a = 5.4789, b = 5.4789, c = 13.5465, α = β = 90°, γ = 120°, χ2 = 2.45 | ||
In order to understand the difference in the state of dipolar ordering in unpoled and poled specimens of NBT-0.01Nd, we performed temperature-dependent studies of the dielectric constant and loss tangent (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) at different frequencies within the range of 5–100 kHz, as shown in Fig. 7. There are two prominent dielectric anomalies, corresponding to the temperatures Td (the depolarization temperature) and Tm (the maximum temperature), which are responsible for the phase transitions from ferroelectric to antiferroelectric and antiferroelectric to paraelectric over the temperature range. The Td and Tm were found to be 140 °C and 360 °C, respectively, from the poled and unpoled samples. In the case of the poled specimen, the dielectric constant improved compared to the unpoled specimen and a strong frequency dispersion with frequency dependence was observed at broad maxima of Td and Tm. After poling, a new dielectric anomaly, which is associated with the transition from ferroelectric to relaxor and antiferroelectric relaxor at T(F–R), can be observed clearly from the dielectric and loss curve at 110 °C in the poled specimen.
δ) at different frequencies within the range of 5–100 kHz, as shown in Fig. 7. There are two prominent dielectric anomalies, corresponding to the temperatures Td (the depolarization temperature) and Tm (the maximum temperature), which are responsible for the phase transitions from ferroelectric to antiferroelectric and antiferroelectric to paraelectric over the temperature range. The Td and Tm were found to be 140 °C and 360 °C, respectively, from the poled and unpoled samples. In the case of the poled specimen, the dielectric constant improved compared to the unpoled specimen and a strong frequency dispersion with frequency dependence was observed at broad maxima of Td and Tm. After poling, a new dielectric anomaly, which is associated with the transition from ferroelectric to relaxor and antiferroelectric relaxor at T(F–R), can be observed clearly from the dielectric and loss curve at 110 °C in the poled specimen.
 | ||
| Fig. 7 The dielectric constant and tangent loss with temperature of NBT-0.01Nd for (top) unpoled and (bottom) poled samples. | ||
The PL intensity variation and subsequent PL quenching with higher Nd3+ substitution can be understood by invoking concentration quenching.25,26,27 Theoretically, the rate of relaxation due to concentration quenching varies as r−6 (where r denotes the inter-ion distance). As the concentration increases, the distance between neighboring Nd3+ ions reduces, leading to an energy exchange among Nd3+ ions. The inter-ionic radius for different x is estimated by using the relation.28  Here, NNd3+ is the Nd3+ concentration in ions per cm3. As expected, an increase in x leads to a reduction in RNd. For the unpoled NBNT with x = 0.01, the estimated RNd ≈ (23.2 ± 1.3) Å. Similar calculations can be made for the poled NBNTs. From Fig. 2, we deduce the critical concentration XCritical = 0.01, after which concentration quenching by energy transfer begins. The theoretical transfer distance, RC (defined as the distance for which the energy transfer probability between neighboring Nd3+ ions is equal to the emission probability of the Nd3+ ions) is estimated using the relation29
Here, NNd3+ is the Nd3+ concentration in ions per cm3. As expected, an increase in x leads to a reduction in RNd. For the unpoled NBNT with x = 0.01, the estimated RNd ≈ (23.2 ± 1.3) Å. Similar calculations can be made for the poled NBNTs. From Fig. 2, we deduce the critical concentration XCritical = 0.01, after which concentration quenching by energy transfer begins. The theoretical transfer distance, RC (defined as the distance for which the energy transfer probability between neighboring Nd3+ ions is equal to the emission probability of the Nd3+ ions) is estimated using the relation29  , where V is the volume of one unit cell, XCritical is the optimal concentration of the activator ion and Z is the number of cations present in the unit cell. Using the values, V = 234.1 Å3, Z = 4 for a monoclinic structure (Cc, unpoled), we estimated RC ≈ (22.3639 ± 1.5) Å which is in the vicinity of the value of RNd for x = 0.01. Similarly, for the poled compound, using V = 351.5 Å3, Z = 6 for the rhombohedral (R3c) structure, and we estimated RC ≈ (22.3612 ± 1.5) Å, which is similar to the value for the unpoled structure and in the vicinity of the value of RNd for x = 0.01. From our calculations we deduce that the critical energy transfer radius (RC) and the inter-ionic radius (RNd) are equal within the error limits for x = 0.01 substitution and beyond this concentration quenching begins.
, where V is the volume of one unit cell, XCritical is the optimal concentration of the activator ion and Z is the number of cations present in the unit cell. Using the values, V = 234.1 Å3, Z = 4 for a monoclinic structure (Cc, unpoled), we estimated RC ≈ (22.3639 ± 1.5) Å which is in the vicinity of the value of RNd for x = 0.01. Similarly, for the poled compound, using V = 351.5 Å3, Z = 6 for the rhombohedral (R3c) structure, and we estimated RC ≈ (22.3612 ± 1.5) Å, which is similar to the value for the unpoled structure and in the vicinity of the value of RNd for x = 0.01. From our calculations we deduce that the critical energy transfer radius (RC) and the inter-ionic radius (RNd) are equal within the error limits for x = 0.01 substitution and beyond this concentration quenching begins.
To achieve multifunctionality, it is vital to have this PL functionality without compromising on the existing ferroelectric nature of the parent NBT compound. Fig. 8(a) presents a comparison of the hysteresis (P–E) loops obtained for the parent NBT and the optimized NBNT (x = 0.01). These hysteresis loops are well saturated, which is characteristic of ferroelectric behavior. We performed the measurement on two devices of each composition and estimated the average coercive electric fields (Ec) to be ≈65 kV cm−1 and 59 kV cm−1 and the remnant polarization values (Pr) to be 32.7 μC cm−2 and 33.8 μC cm−2 for NBT and NBT-0.01Nd, respectively. NBT-0.01Nd exhibits a slightly higher Pr and much lower Ec values by 3% and 12%, respectively, relative to pure NBT, confirming the better suitability of NBNT for possible applications in piezochromic devices. The requirement for such NBT based devices is lower coercivity (for easy switching) without compromising Pr. Thus, it can be inferred that the NBT-0.01Nd compound can be considered as a possible candidate for optimizing future piezochromic devices.
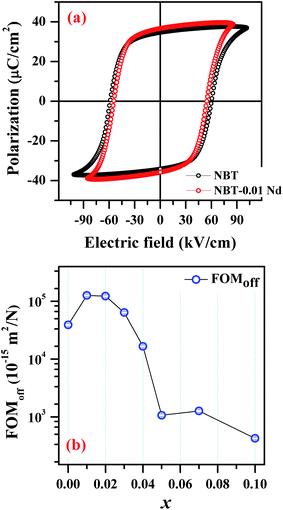 | ||
| Fig. 8 (a) Room temperature polarization (P)–electric field (E) measurement of NBT and NBT-0.01Nd. (b) Plot showing the variation of FOMoff as a function of x (Nd3+ substitution). | ||
The best enhancement in terms of device functionality upon substitution was observed for piezoelectric energy harvesting. The measured d33 were 84 pC N−1 and 95 pC N−1 for NBT and NBT-0.01Nd, suggesting an increase of 13%, implying that substitution of Nd3+ in NBT favors the mobility of domain walls (corroborated by the softer ferroelectric nature). From the same measurements we estimated the piezoelectric voltage coefficient (g33) to be 26.7 mV N−1, 48.4 mV N−1 and the dielectric loss (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) to be 0.0572, 0.0362 for NBT and NBT-0.01Nd, respectively. Together with the three quantities estimated so far, the off-resonance figure of merit (FOMoff) for energy harvesting can be estimated using the relation
δ) to be 0.0572, 0.0362 for NBT and NBT-0.01Nd, respectively. Together with the three quantities estimated so far, the off-resonance figure of merit (FOMoff) for energy harvesting can be estimated using the relation .30,31 The estimated values of FOMoff were 39
.30,31 The estimated values of FOMoff were 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 209.8 × 10−15 m2 N−1 and 127
209.8 × 10−15 m2 N−1 and 127![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 016.6 × 10−15 m2 N−1 for NBT and NBNT, implying an enhancement of ≈30%. Fig. 8(b) shows the variation in FOMoff for all the various composition used in the study. Interestingly, the variation in FOMoff follows the trend in PL emission for the varying amounts of Nd3+ substituted in NBT.
016.6 × 10−15 m2 N−1 for NBT and NBNT, implying an enhancement of ≈30%. Fig. 8(b) shows the variation in FOMoff for all the various composition used in the study. Interestingly, the variation in FOMoff follows the trend in PL emission for the varying amounts of Nd3+ substituted in NBT.
4. Conclusions
To conclude, we presented a comprehensive investigation of the effect of Nd3+ in Na0.5Bi0.5−xNdxTiO3 (NBNT) in order to obtain various functionalities. The optimal concentration of Nd3+ for the best PL emission was found to be x = 0.01 (≅1 mol%). Upon electrical poling, there was a significant quenching of PL intensity. We elucidated this to be a result of electric-field induced structural ordering to higher symmetry. Structural studies from XRD data corroborated this hypothesis, as the electrical poling resulted in the transformation of the stabilized NBNT phase from a monoclinic to a rhombohedral structure which has higher symmetry. Estimation of the critical radius (RC) for energy transfer and inter-ionic distance (RNd) suggested the onset of a concentration quenching effect after the substitution of x = 0.01. For the optimized NBNT the electrical characterization revealed a reduction in the coercive electric field (Ec) by 12% compared to undoped NBT. The piezoelectric characterization of the samples studied showed an enhanced d33coefficient for the optimized NBNT and therefore the estimated off-resonance figure of merit (FOMoff) for energy harvesting revealed ≈30% enhancement for NBT-0.01Nd. From a computational knowledge point of view, the effect of perovskite structure symmetry on Nd3+ emission has not been studied to date. Thus, we hope our results will encourage such investigations as the very possibility of having multifunctionality in a simple material system is elegant. The results presented here convey a simple material system, but with elegant multifunctional properties. Therefore, the optimally substituted NBNT displayed convincing reasons for it to be a potential multifunctional material exhibiting simultaneous ferroelectric, luminescent and energy harvesting properties.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support from various funding sources, namely, DST Project No. EMR/2014/000761 & YSS/2015/000008, CSIR project no. 03(1348)/16/EMR-II and DAE-CSR project no. CRS-M-250. Authors acknowledge helpful discussions with Dr Premkiran and Dr Venugopal Rao, ACRHEM, University of Hyderabad, Hyderabad, Telangana, India.References
- X. Wang, C. N. Xu, H. Yamada and K. Nishikubo, Adv. Mater., 2005, 17(10), 1254–1258 CrossRef CAS
.
- D. Peng, X. Wang, C. Xu, X. Yao, J. Lin and T. Sun, J. Am. Ceram. Soc., 2013, 96(1), 184–190 CrossRef CAS
.
- P. Du, L. Luo, Q. Yue and H. Chen, Appl. Phys. Lett., 2014, 104(15), 152902 CrossRef
.
- M. K. Niranjan, T. Karthik, S. Asthana, J. Pan and U. V. Waghmare, J. Appl. Phys., 2013, 113, 194106 CrossRef
.
- K. Reichmann, A. Feteira and M. Li, Materials, 2015, 8(12), 8467–8495 CrossRef CAS PubMed
.
- Y. Hiruma, H. Nagata and T. Takenaka, Appl. Phys. Lett., 2008, 92(26), 262904 CrossRef
.
- K. R. Kandula, S. S. K. Raavi and S. Asthana, Ferroelectrics, 2017, 518(1), 23 CrossRef CAS
.
- H. Sun, D. Peng, X. Wang, M. Tang, Q. Zhang and X. Yao, J. Appl. Phys., 2011, 110, 016102 CrossRef
.
- H. Zhou, X. Liu, N. Qin and D. Bao, J. Appl. Phys., 2011, 110(3), 034102 CrossRef
.
- X. Tian, Z. Wu, Y. Jia, R. K. Zheng, Y. Zhang and H. Luo, Appl. Phys. Lett., 2013, 102(4), 042907 CrossRef
.
- L. Luo, P. Du, W. Li, W. Tao and H. Chen, J. Appl. Phys., 2013, 114(12), 124104 CrossRef
.
- A. Kalaskar, B. N. Rao, T. Thomas and R. Ranjan, J. Appl. Phys., 2015, 117(24), 244106 CrossRef
.
- M. Zannen, A. Lahmar, M. Dietze, H. Khemakhem and M. Es-Souni, Mater. Chem. Phys., 2012, 134(2), 829–833 CrossRef CAS
.
- C. He, Y. Zhang, T. Wu and Y. Liu, J. Phys. D: Appl. Phys., 2013, 46(24), 245104 CrossRef
.
- Q. Yao, F. Wang, F. Xu and W. Shi, ACS Appl. Mater. Interfaces, 2015, 7(9), 5066–5075 CAS
.
- D. K. Khatua, A. Kalaskar and R. Ranjan, Phys. Rev. Lett., 2016, 116(11), 117601 CrossRef PubMed
.
- V. Pal, R. Dwivedi and O. P. Thakur, Mater. Res. Bull., 2014, 51, 189–196 CrossRef CAS
.
- W. Krupke, IEEE J. Quantum Electron., 1974, 10(4), 450–457 CrossRef CAS
.
- K. R. Kandula, S. S. K. Raavi and S. Asthana, Phys. Status Solidi A, 2018, 1700915 CrossRef
.
- Y. Huan, X. Wang, J. Fang and L. Li, J. Eur. Ceram. Soc., 2014, 34(5), 1445–1448 CrossRef
.
- https://www.avantes.com/support/theoretical-background/spectrometers.
- K. Kandula, S. S. K. Raavi and S. Asthana, J. Alloys Compd., 2018, 732, 233–239 CrossRef CAS
.
- J. Azkargorta, R. Balda and J. Fernández, Opt. Express, 2008, 16(16), 11894–11906 CrossRef CAS PubMed
.
- G. Singh, R. Selvamani, V. S. Tiwari and A. K. Karnal, J. Lumin., 2017, 1(192), 1084–1088 CrossRef
.
- Lanthanide luminescence: photophysical, analytical and biological aspects, ed. P. Hänninen and H. Härmä, Springer Science & Business Media, 2011, vol. 7 Search PubMed
.
- H. G. Danielmeyer and P. Balmer, Appl. Phys. A: Mater. Sci. Process., 1973, 1(5), 269–274 CAS
.
- A. Tarafder, K. Annapurna, R. S. Chaliha, V. S. Tiwari, P. K. Gupta and B. Karmakar, J. Alloys Compd., 2010, 489(1), 281–288 CrossRef CAS
.
- J. Hur, I. T. Seo, D. H. Kim, S. Nahm, J. Ryu, S. H. Han and S. J. Yoon, J. Am. Ceram. Soc., 2014, 97(10), 3157–3163 CrossRef CAS
.
- G. Blasse, J. Solid State Chem., 1986, 62(2), 207–211 CrossRef CAS
.
- M. Weber and T. E. Varitimos, J. Appl. Phys., 1971, 42(12), 4996–5005 CrossRef CAS
.
- I. Iparraguirre, J. Azkargorta, R. Balda and J. Fernández, Opt. Mater., 2015, 27(11), 1697–1703 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra01349g |
| This journal is © The Royal Society of Chemistry 2018 |




