Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Study on the flammability, thermal stability and diffusivity of polyethylene nanocomposites containing few layered tungsten disulfide (WS2) functionalized with metal oxides
K. Wenelska *,
K. Maślana and
E. Mijowska
*,
K. Maślana and
E. Mijowska
Nanomaterials Physicochemistry Department, Faculty of Chemical Technology and Engineering, West Pomeranian University of Technology, Szczecin, Piastow 45, 70-311 Szczecin, Poland. E-mail: kwenelska@zut.edu.pl
First published on 9th April 2018
Abstract
In this work, exfoliated tungsten disulfide (WS2) functionalized with metal oxides as a filler of polyethylene (PE) was used. An efficient exfoliation procedure resulted in the synthesis of 7–9 layered flakes of WS2. Flakes of exfoliated WS2 were functionalized by iron oxide and nickel oxide nanoparticles, respectively. The nanomaterials were mixed with polyethylene by extrusion. Methods such as Transmission Electron Microscopy (TEM), Atomic Force Microscopy (AFM), X-Ray Diffraction (XRD) or Thermogravimetric Analysis (TGA) were used to characterize the materials. Flame retardant properties were investigated by microcalorimetry. Comparing the obtained values of heat released during combustion, it can be observed that the addition of fillers reduces flammability significantly compared to neat polyethylene. It is revealed that this composite can provide a certain physical barrier and inhibit the diffusion of heat and gaseous products during combustion. Thermogravimetric analysis of composites showed increased thermal stability with addition of nanofillers and reduction of carbon monoxide generation in the whole range of the nanofiller addition (from 0.5 to 2 wt% in PE). Results suggested that the composite with Ni2O3 could endow the best flame retardance for PE. The peak heat release rate of this sample with 2 wt% nanofiller was reduced to 792 W g−1 (1216 W g−1 for PE), and the total heat release was decreased to 39 kJ g−1 (47 kJ g−1 for PE). A very significant increase in thermal conductivity for all composites was observed as well.
1. Introduction
The discovery of graphene and its superior properties have focused research interest on other two-dimensional (2D) groups of materials like hexagonal boron nitride (h-BN) and transition metal dichalcogenides (TMDs).1 A monolayer of TMD, the general chemical formula of which is MX2, consists of a metal atom (M = Mo, W) sandwiched between two chalcogenide atoms (X = S, Se)3 and it has a thickness between 6 and 7 Å.2 Depending on the coordination number and oxidation state of the metal atoms, TMDs can be metallic, semimetallic or semiconducting.4 These materials exhibit a layered structure5 with weak interlayer van der Waals forces which allow easy exfoliation.1,3 Unlike graphene, the band gap of these materials, which due to a reduced number of sheets, changes from an indirect band gap to a direct band gap.1,6–9 Therefore, these materials can have many new applications e.g. in electronics, optoelectronics,3,8 catalysis, energy storage and sensing.3,10Tungsten disulfide (WS2) is one of the most popular compound of semiconducting TMS's. The band gap of monolayer of WS2 is 2.1 eV, while in bulk is 1.3 eV and that results in enhancement of photoluminescence.11,12 This compound exhibit trigonal prismatic structure. Mechanically exfoliated atomically thin sheets of WS2 were shown to exhibit high in-plane carrier mobility and electrostatic modulation of conductance similar to MoS2.13 Many techniques have been reported to obtain atomically thin layers WS2 like mechanical exfoliation,13 chemical exfoliation4,14 and chemical vapor deposition.15 WS2 nanosheets have broad applications in optoelectronics.12,16–20
Recently this group of materials has attracted increased attention in the field of nanocomposites fillers, due their graphene-like properties such as high thermal and mechanical properties.21,22 Polymeric materials are widely used in the most important industries. However, they are known for high fire risk and most of them combust with emission toxic gases. It is important to modify these materials to reduce their flammability. There are three typical strategies to achieve that: use of inherently flame retardant polymers,21 flame retardants,22 and surface treatment/coating.23 Usually small amount of nanofiller improves thermal properties of composites. WS2, as a typical layered inorganic material, is expected to disperse and exfoliate in polymers and it results in the physical barrier formation which inhibits the diffusion of heat and the decomposition of polymer products. So it is reasonable that WS2 may improve the thermal stability, mechanical properties and fire resistance of polymer composites.24 To the best of our knowledge there is a gap in the scientific reports on the potential of few layered WS2 functionalized with metal oxides as a nanofiller of polymers used for flame retardancy.
Polyethylene is one of the most common polymer used in everyday object in our houses. However, its flammability and toxicity during the fire are the key motivations to perform the proposed study.
In this work we present a technique of liquid exfoliation of tungsten disulfide nanosheets and its functionalization with metal oxides (nickel and iron). We use these materials as fillers in polyethylene nanocomposites and investigate their flame retardants properties by pyrolysis combustion flow calorimeter (PCFC) and thermal conductivity by xenon flash method.
2. Experimental
2.1 Reagents
Bulk tungsten(IV) disulfide (WS2, powder 99.9%), Nickel(II) acetate (Ni(CH3COO)2·4H2O, powder <99%), Iron(II) acetate (Fe(CH3COO)2, powder 96%) polyethylene (d = 0.92 g mL−1, powder) were obtained from Sigma-Aldrich and cetyltrimethylammonium bromide (CTAB) was purchased from AppliChem.2.2 Characterization
The structures of materials before and after exfoliation/functionalization were studied by Transmission Electron Microscopy (Tecnai F20-based at 200 kV accelerating voltage). Atomic Force Microscopy (AFM NTEGRA Aura (NT-MTD) microscope) provide information about thickness and number of layers of WS2. X-ray diffraction (XRD) Philips X'Pert PRO X-ray diffractometer witch Cu Kα radiation was employed to identify phase identification of the samples. Thermogravimetric analysis (TGA) was carried out using a SDT Q 6000 thermoanalyzer instrument (TA Instruments Inc.) under air flow of 100 mL min−1. The samples with mass of about 5.0 mg were heated from room temperature to 900 °C at a linear heating rate of 10 °C min−1. During heating the sample in thermobalance, the generated gases were analyzed in situ by Quadrupole Mass Spectrometer QMS 422. All PE and its composites were fabricated using a twin screw extruder (Zamak Mercator EHP 2x12). Micro Calorimeter (FAA MICRO Calorimeter) was used to investigate the flammability properties of PE nanocomposites. Samples of about 2.0 mg were heated in air atmosphere (80% of nitrogen and 20% of oxygen) at a constant heating rate of 1 °C s−1 from room temperature to 700 °C. This method allowed to determinate parameters such as heat release rate (W g−1), heat release capacity (J g−1 K−1), total heat release (kJ g−1). The thermal diffusivity of the composites was measured via xenon flash technique using Linseis XFA 300 laser flash apparatus.2.3 Exfoliation of WS2
Few-layered WS2 flakes were synthesized by liquid exfoliation of bulk WS2. First, 1 g of bulk WS2 and cetyltrimethylammonium bromide (0.5 g) were mixed, dissolved in distilled water and sonicated for 3 h. Then, the mixture was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 0.5 h. After that, the solution was washed few times with distilled water and dried 24 h in 60 °C.
000 rpm for 0.5 h. After that, the solution was washed few times with distilled water and dried 24 h in 60 °C.
2.4 Functionalization of WS2
Two samples of WS2 modified by metal oxide nanoparticles (WS2_Ni2O3 and WS2_Fe2O3, respectively) were prepared according to the following procedure: 0.5 g of WS2 and 0.5 g nickel(II) acetate tetrahydrate (product referred to as WS2_Ni2O3) and iron(II) acetate (product referred to as WS2_Fe2O3), respectively, were dispersed in 250 mL of ethanol and sonicated for 3 h. Afterwards, the mixtures were stirred for next 24 h. Finally, the samples were dried in high vacuum at 440 °C for 10 min.2.5 Preparation of nanocomposites
Polyethylene (PE) was used as the polymer matrix. The content of MoS2, MoS2_Ni2O3 and MoS2_Fe2O3, were 0.5%, 2%, 3%, respectively. The composites were prepared by extrusion molding at a temperature of 120 °C.3. Results and discussion
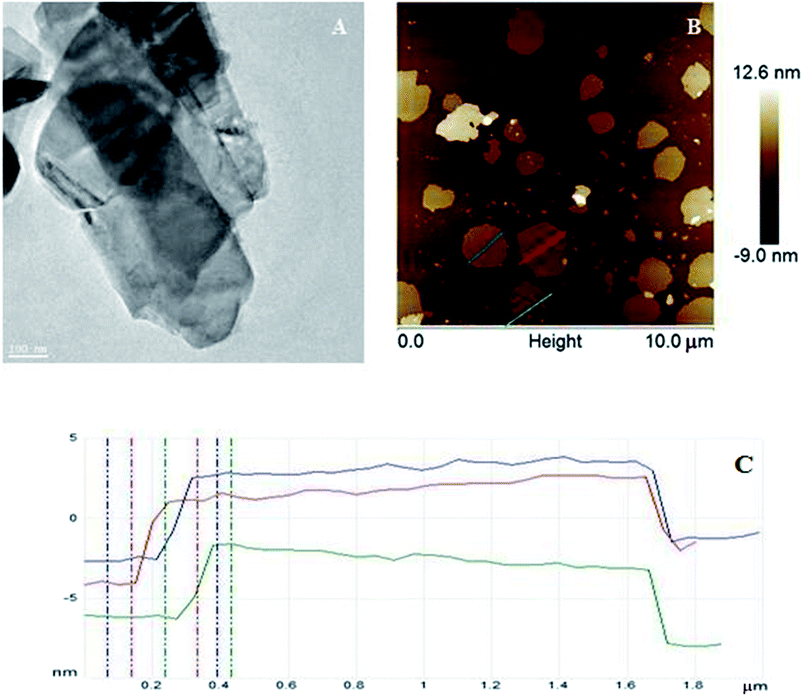
The morphologies of WS2 after exfoliation investigated by TEM (Fig. 1A) and AFM (Fig. 1B) microscopy. Fig. 1 shows that WS2 was successfully exfoliated from bulk to few nanosheets. Tapping-mode of AFM was used to determinate the size and thickness of exfoliated WS2. The AFM samples were prepared by dropping a few drops of exfoliated WS2 dissolved in ethanol on silica wafer and left to evaporate the solvent. AFM analysis shows that exfoliated WS2 had diameter ∼0.74–1.4 μm and thickness about 4.6–5.9 nm which corresponds to 7–9 layers of WS2.After exfoliation the product was functionalized with nickel and iron compounds. The morphology and structure was characterized by TEM. Fig. 2A and C shows, that metal particles have covered the whole surface of WS2, what proves very good dispersion of metal oxides on WS2 surface. Particle size distribution of iron oxide (Fig. 2D) and nickel oxide (Fig. 2B) was estimated basing on ∼100 of particles pictured in TEM. Detailed analysis reveals very uniform diameters of samples. Particle size distribution of the iron compound and nickel compound is ∼35 and ∼25 nm, respectively.
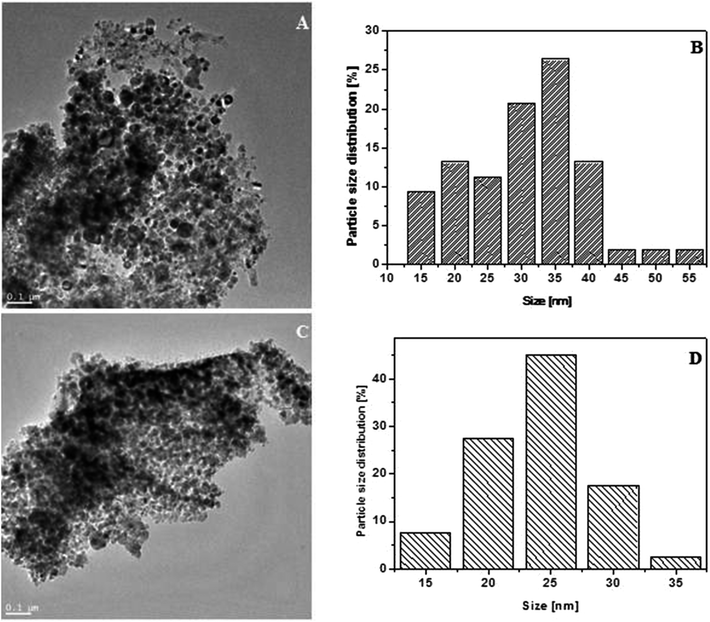 | ||
| Fig. 2 TEM images of WS2_Fe2O3 (A) WS2_Ni2O3 (C) and particle size distribution of WS2_Fe2O3 (B) and WS2_Ni2O3 (D). | ||
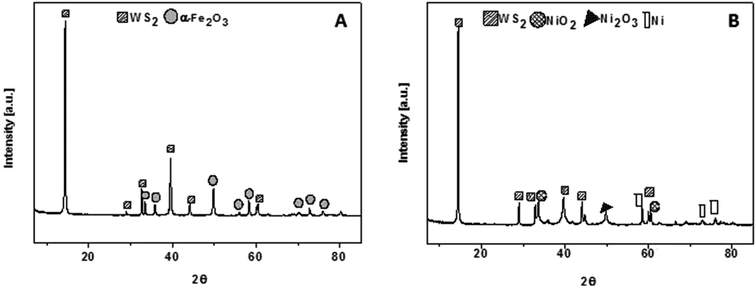
The composition of WS2_Fe2O3 and WS2_Ni2O3 were examined by powder X-ray diffraction, as it is demonstrated in Fig. 3. In both patterns one intense pick at value 2θ 14° is present. This peak is assigned to WS2. Few peaks with lower intensity at 2θ value 29°, 39.5°, 44° and 60° are also associated with pristine WS2. Fig. 3A shows XRD pattern of WS2_Fe2O3. Relatively strong peak is present at 50 °C. This signal belongs to iron(III) oxide. Peaks at 33.5°, 36°, 56°, 58°, 70°, 72° and 75° are consistent with the data for the α-Fe2O3. Fig. 3B presents XRD pattern of WS2_Ni2O3. Besides peaks which correspond to WS2, there are peak, as well a 2θ value 34° and 60° assigned to NiO2. Peak at 49.5° is associated with presence of Ni2O3 phase. There are also peaks of metallic nickel (peaks at 2θ values 58.5°, 72° and 75°). The above analysis proves that WS2 was successfully functionalized with nickel and iron oxides, respectively.
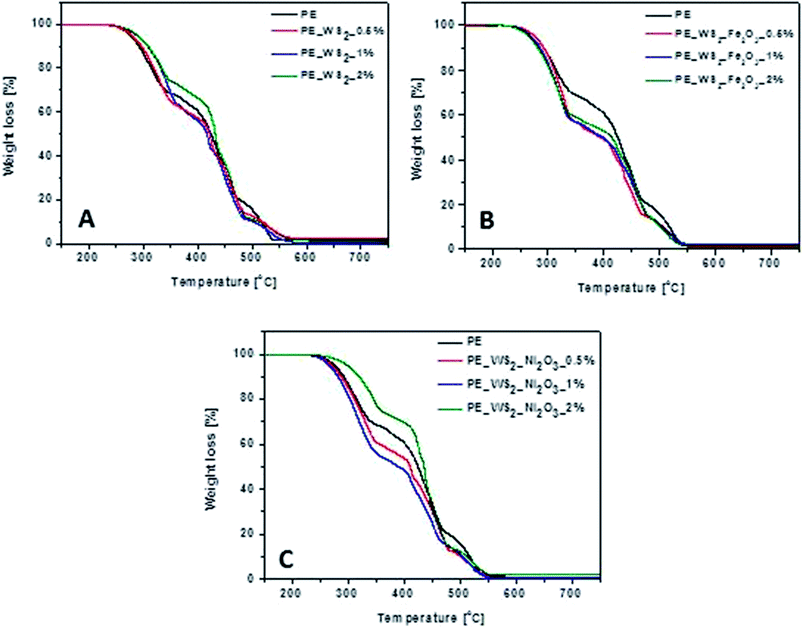
TGA was performed to investigate the general thermal stability of PE and WS2 nanocomposites. Fig. 4 shows thermograms of PE and PE_WS2 nanocomposites. The corresponding TGA data are presented in Table 1. The temperature where the weight loss is 10 wt% is denoted as T0.1 and the temperature there half of the sample was loss is T0.5. Char yield is the weight percent obtained at the end of pyrolysis. Thermal stability of composites has decreased for most samples. The most significant drop was observed for composite PE_WS2_Ni2O3_2% for which the temperatures of T0.1 and T0.5 has increased by 27 °C and 11 °C, respectively, compared to neat PE. The position of PE and WS2_Fe2O3 has not satisfactory influenced thermal stability of the nanocomposites. The lowest decrease of T0.1 and T0.5 was obtained for the composites with 2 wt% (11 °C lower than in pristine PE). T0.1 and T0.5 has increase by 8 °C and 16 °C, respectively, in the PE_WS2 with 2 wt% of WS2. Additionally, char yield increased of ∼7% in composite PE_WS2_0.5% composite with PE_WS2_Ni2O3 consisting 2 wt% exhibit the best value and it is increased ∼51% compared with PE. For materials of PE_WS2_Fe2O3 the best value was obtained for 1 wt% and the value of char yield has not changed. Char yield and carbon monoxide generation of nanocomposites with metal oxides functionalized few layered WS2 are lower than the neat PE. We suppose that inorganic oxides assist in conversion of CO to CO2 and they limit generation of soot.25
| Sample | T0.1 [°C] | T0.5 [°C] | Char yield [%] |
|---|---|---|---|
| PE | 292 | 424 | 1.89 |
| PE_WS2_0.5% | 296 | 422 | 2.68 |
| PE_WS2_1% | 307 | 417 | 0.76 |
| PE_WS2_2% | 308 | 432 | 1.64 |
| PE_WS2_Fe_0.5% | 292 | 394 | 0.74 |
| PE_WS2_Fe_1% | 285 | 397 | 1.89 |
| PE_WS2_Fe_2% | 281 | 413 | 1.48 |
| PE_WS2_Ni_0.5% | 287 | 410 | 0.55 |
| PE_WS2_Ni_1% | 281 | 389 | 0.57 |
| PE_WS2_Ni_2% | 319 | 435 | 2.02 |
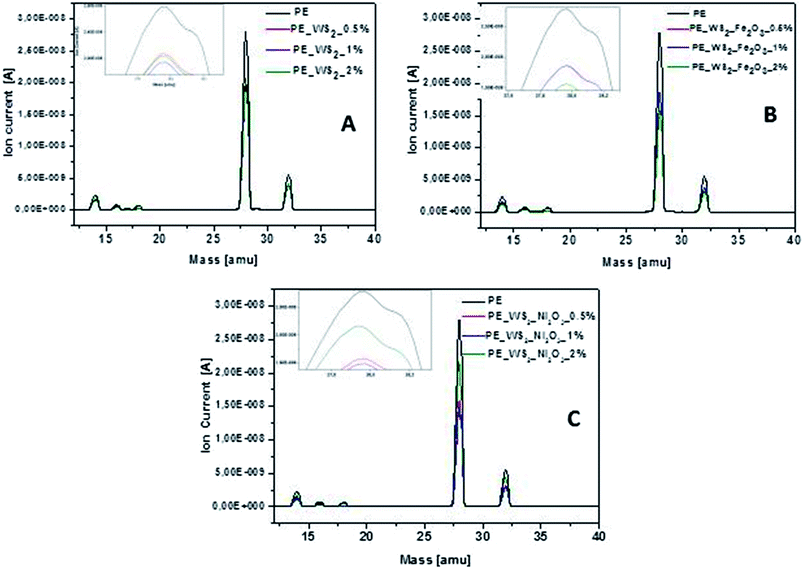
During the thermogravimetric analysis, mass spectrometer was coupled and in situ gas analysis was carried out. The emission of toxic gases is considered as important parameter for flame-retardant materials. Mass spectra of all samples (Fig. 5) exhibit very significant peak at position of 28 amu, which corresponds to CO emission. The lowest value of carbon oxide emission was obtained for composite consisting PE_WS2_Ni2O3 with 2 wt% compared to the polyethylene the value of emission decreases by 47%. Good efficiency of reduced CO emission was also obtained for PE_WS2_Fe2O3_2% (∼44% compared to pristine polyethylene). For composites with exfoliated WS2 the amount of filler has not significantly influenced the reduction CO emission. This value has decreased from 30% (for 2 wt%) to 35% (for 1 wt%) compared to polyethylene.
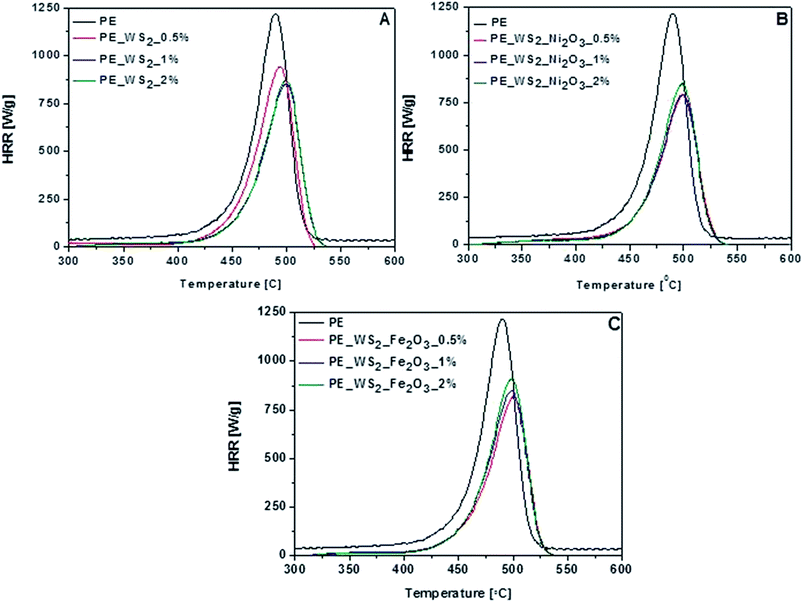
For assessment flammability properties of polyethylene-based nanocomposites microcalorimeter was used. The microscale combustion calorimeter (MCC) is a small-scale instrument that measures the heat release of a material by oxygen consumption calorimetry. Using this technique, the samples are exposed to a fast heating rate to mimic fire-type conditions. During MCC measurement several parameters are obtained, such as total heat release (THR), heat release rate (HRR) and heat release capacity (HRC). These parameters are crucial for assessing the fire risk of materials. The results of the MCC testing are summarized in Fig. 6 and Table 2.
| Sample | pHRR [W g−1] | THR [kJ g−1] | HRC [J g−1 K−1] |
|---|---|---|---|
| PE | 1216 | 47 | 1179 |
| PE_WS2_0.5% | 939 | 43.4 | 950 |
| PE_WS2_1% | 852 | 39.9 | 858 |
| PE_WS2_2% | 868 | 40.1 | 871 |
| PE_WS2_Fe2O3_0.5% | 819 | 38.8 | 824 |
| PE_WS2_Fe2O3_1% | 849 | 40.3 | 851 |
| PE_WS2_Fe2O3_2% | 910 | 41.1 | 907 |
| PE_WS2_Ni2O3_0.5% | 851 | 39.7 | 856 |
| PE_WS2_Ni2O3_1% | 799 | 38.3 | 796 |
| PE_WS2_Ni2O3_2% | 792 | 39 | 797 |
The HRR curves of nanocomposites derived from MCC are plotted in Fig. 6. The pHRR value has been often regarded as the most accurate indicator of flame. As it is shown, the addition of small amount of fillers leads to significant decrease of pHRR value in all composites. Compared to the PE, values of pHRR of composites with PE_WS2 and PE_WS2_Fe2O3 have decreased with decreasing loading of fillers. The best improvement was obtained for composites consisting of 0.5 wt% of WS2_Fe2O3 and it was about 33%. For composites consists PE_WS2 pHRR has decreased significantly compared with pristine PE. The largest decrease was observed for the sample containing 2 wt% of the filler. The value pHRR has decreased by 28%. Diez-Pascual et al. explains these improvements that WS2 could behave like a mass transport barrier which prevent the escape of volatile products generated during the burning and also hinders access to the matrix.26 The addition of fillers increase the temperature of maximum value peak HRR, which indicates the improvement in flame retardancy of nanocomposites. The most interesting value was obtained for composite the PE_WS2_Ni2O3 containing 2 wt% of metal oxide. The peak heat release rate of PE_WS2_Ni2O3 was reduced to 792 W g−1, and the total heat release was decreased to 39 kJ g−1. For almost each composite the temperature of pHRR increased by at least 10 °C. Interesting results was obtained also for PE_WS2_Ni2O3 containing 1 wt% and 2 wt% the temperature has increased by 12 °C.
As is known to all, incorporation of 2D layered nanofillers usually increase the thermal stability of a polymer matrix due to the physical barrier effect which retards the diffusion of degradation products, gases and heat. MoS2 nanosheets must present better physical barrier effects compared to pristine PE. A lot of attention is devoted to MoS2 and its composite from PE,27 PS,28 PVA.29 However, composites with WS2 have much better fire retardancy and thermal stability properties. Compared data with MoS2 dispersed in the PE matrix, WS2 shows a decrease of flame retardancy. For the composites contained 2% nanofillers Ni2O3. The pHRR for composites with WS2 and MoS2 decreased ∼35% and 30%, respectively. The flame retardance analysis, it is reasonably speculated that independent WS2 nanosheets in the PE matrix act as nano-barriers to restrain the permeation of heat and oxygen and inhibit the effusion of volatile toxic materials. In comparison to the composites containing carbon nanotubes as fillers they did not exhibit flame retardant properties as good as WS2. Polyethylene with 2 wt% of CNT's decrease pHRR value about 24.5% compared to neat PE, but that nanocomposite reaches mu higher char yield.30,31
To measure the thermal conductivity of composites xenon flash method was used. The measurement was carried out along the thickness direction of each sample. The samples were coated with a thin layer of graphite to facilitate the absorption of the laser light at the surface of the sample. Measurements were conducted in the vacuum (1.0 × 10−2 bar). Three laser shots were applied to each sample at a room temperature. The final result for each parameter was presented as the average value of three partial measurements. The results are shown in the Table 3. Compared to the value of pristine polyethylene, thermal conductivity increased for all composites. The addition of small amount of filler brings the increase value of thermal conductivity about 100% for all the composites. Furthermore, the addition of 0.5 wt% of WS2_Fe2O3 into PE exhibits the most significant increase in thermal conductivity. Compared to neat PE the value has increased about ∼240%. The most significant difference of value for composites containing WS2_Ni2O3 was obtained for 2 wt% and it was 290% higher than for pure PE. The interesting value was obtained for composite PE_WS2_1%. In this case, the improvement of thermal conductivity was 230% compared to pristine PE.
| Sample | Thermal conductivity [W m−1 K−1] |
|---|---|
| PE | 186 |
| PE_WS2_0.5% | 370 |
| PE_WS2_1% | 524 |
| PE_WS2_2% | 507 |
| PE_WS2_Ni2O3_0.5% | 427 |
| PE_WS2_Ni2O3_1% | 510 |
| PE_WS2_Ni2O3_2% | 550 |
| PE_WS2_Fe2O3_0.5% | 454 |
| PE_WS2_Fe2O3_1% | 389 |
| PE_WS2_Fe2O3_2% | 433 |
4. Conclusion
In this study, the aqueous phase exfoliated WS2 nanosheets were successfully functionalized with metal oxides (nickel and iron) and incorporated into polyethylene matrix by extruder melting. Adding small amount of layered nanofillers improved the thermal stability and fire resistance of composites significantly. MCC, TGA and thermal conductivity measurements indicate that the reduction of flammability is dependent on the content of WS2 fillers. The best improvement in thermal degradation was obtained for PE_WS2_Ni2O3_2% for which the temperature T0.1 and T0.5 increased by 27 °C and 11 °C, respectively, compared to neat PE. Significant decrease in value of peak heat release rate and CO emission was obtained for PE_WS2_Ni2O3_0.5% which was 33% and 44%, respectively. Thermal conductivity of this composite has increased by 144% compared to pristine polyethylene. The reduction of fire hazard was attributed to the physical barrier effect of WS2. Therefore, it is believed that new flame-retardant nanocomposites can find important and practical applications, such as wall insulation, cable ropes, pipes or casings.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for the financial support of National Science Centre Poland OPUS 10 UMO-2015/19/B/ST8/00648.References
- Y. Yan, C. Zhang and W. Gu, et al. Facile Synthesis of Water-Soluble WS2 Quantum Dots for Turn-On Fluorescent Measurement of Lipoic Acid, J. Phys. Chem. C, 2016, 120, 12170 CAS
.
- H. R. Gutierrez, N. Perea-Lopez and A. L. Elías, et al., Extraordinary Room-Temperature Photoluminescence in Triangular WS2 Monolayers, Nano Lett., 2013, 13, 3447 CrossRef CAS PubMed
.
- M. Chhowalla, H. Suk Shin and G. Eda, et al., The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets, Nat. Chem., 2013, 5, 263 CrossRef PubMed
.
- J. N. Coleman, M. Lotya and A. O'Neill, et al., Two-Dimensional Nanosheets Produced by Liquid Exfoliation of Layered Materials, Science, 2011, 331(6017), 568 CrossRef CAS PubMed
.
- M. Sahu, L. Narashimhan and O. Prakash, et al., Noncovalently Functionalized Tungsten Disulfide Nanosheets for Enhanced Mechanical and Thermal Properties of Epoxy Nanocomposites, ACS Appl. Mater. Interfaces, 2017, 9, 14347 CAS
.
- N. Peimyoo, J. Shang and W. Yang, et al., Thermal conductivity determination of suspended mono-and bilayer WS2 by Raman spectroscopy, Nano Res., 2015, 8(4), 1210 CrossRef CAS
.
- S. Z. Butler, S. M. Hollen and L. Y. Cao, et al., Progress, Challenges, and Opportunities in Two-Dimensional Materials Beyond Graphene, ACS Nano, 2013, 7, 2898 CrossRef CAS PubMed
.
- Y. Cui, R. Xin and Z. Yu, et al., High Performance Field-Effect Transistor Based on Multilayer Tungsten Disulfide, Adv. Mater., 2015, 27, 5230 CrossRef CAS PubMed
.
- F. Cheng, A. D. Johnson and Y. Tsai, et al., Enhanced Photoluminescence of Monolayer WS2 on Ag Films and Nanowire-WS2-Film Composites, ACS Photonics, 2017, 1421–1430 CrossRef CAS
.
- Q. H. Wang, K. Kalantar-Zadeh and A. Kis, et al., Electronics and optoelectronics of two-dimensional transition metal dichalcogenides, Nat. Nanotechnol., 2012, 7, 699 CrossRef CAS PubMed
.
- H. Zeng, G.-B. Liu and J. Dai, et al., Optical signature of symmetry variations and spin-valley coupling in atomically thin tungsten dichalcogenides, Sci. Rep., 2013, 3, 1608 CrossRef PubMed
.
- D. Jariwala, V. K. Sangwan and L. J. Lauhon, et al., Emerging Device Applications for Semiconducting Two-Dimensional Transition Metal Dichalcogenides, ACS Nano, 2014, 8, 1102 CrossRef CAS PubMed
.
- W. Zhao, Z. Ghorannevis and L. Chu, et al., Evolution of Electronic Structure in Atomically Thin Sheets of WS2 and WSe2, ACS Nano, 2013, 7, 791 CrossRef CAS PubMed
.
- R. J. Smith, P. J. King and M. Lotya, et al., Large-Scale Exfoliation of Inorganic Layered Compounds in Aqueous Surfactant Solutions, Adv. Mater., 2011, 23, 3944 CrossRef CAS PubMed
.
- K. Kang, S. Xie and L. Huang, et al., High-mobility three-atom-thick semiconducting films with wafer-scale homogeneity, Nature, 2015, 520, 656 CrossRef CAS PubMed
.
- S. Xu, D. Li and P. One-Pot Wu, et al., Synthesis of Monolayer MoS2/WS2 Quantum Dots as Bioimaging Probes and Efficient Electrocatalysts for Hydrogen Evolution Reaction, Adv. Funct. Mater., 2015, 25, 1127 CrossRef CAS
.
- L. Cheng, W. Huang and Q. Gong, et al., Ultrathin WS2 Nanoflakes as a High-Performance Electrocatalyst for the Hydrogen Evolution Reaction, Angew. Chem., Int. Ed., 2014, 53, 7860 CrossRef CAS PubMed
.
- Y. Cui, R. Xin and Z. Yu, et al., High-Performance Monolayer WS2 Field-Effect Transistors on High-κ Dielectrics, Adv. Mater., 2015, 27, 5230 CrossRef CAS PubMed
.
- Q. Mahmood, M. G. Kim and S. Yun, et al., Unveiling Surface Redox Charge Storage of Interacting Two-Dimensional Heteronanosheets in Hierarchical Architectures, Nano Lett., 2015, 15, 2269 CrossRef CAS PubMed
.
- L. Cheng, J. Liu and X. Gu, et al., PEGylated WS2 Nanosheets as a Multifunctional Theranostic Agent for in Vivo Dual-Modal CT/Photoacoustic Imaging Guided Photothermal Therapy, Adv. Mater., 2014, 26, 1886 CrossRef CAS PubMed
.
- B. Mortazavi and G. Cuniberti, Mechanical Properties of Polycrystalline Boron–Nitride Nanosheets, RSC Adv., 2014, 4, 19137 RSC
.
- K. Liu, Q. Yan and M. Chen, et al., Elastic Properties of Chemical-Vapor-Deposited Monolayer MoS2, WS2, and Their Bilayer Heterostructures, Nano Lett., 2014, 14, 5097 CrossRef CAS PubMed
.
- Q. Wu, Q. Zhang and L. Zhao, et al., A novel and facile strategy for highly flame retardant polymer foam composite materials: Transforming silicone resin coating into silica self-extinguishing layer,, J. Hazard. Mater., 2017, 336, 222 CrossRef CAS PubMed
.
- K. Zhou, S. Jiang and C. Bao, et al., Preparation of poly(vinyl alcohol) nanocomposites with molybdenum disulfide (MoS2): structural characteristics and markedly enhanced properties, RSC Adv., 2012, 2, 11695 RSC
.
- W. E. Horn, Inorganic Hydroxides and Hydroxycarbonates: Their Function and Use as Flame-Retardant Additives, Fire Retardancy of Polymeric Materials, Marcel Dekker, 2000 Search PubMed
.
- A. M. Díez-Pascual and M. Naffakh, Inorganic Nanoparticle-Modified Poly(Phenylene Sulphide)/Carbon Fiber Laminates: Thermomechanical Behaviour, Materials, 2013, 6, 3171 CrossRef PubMed
.
- K. Wenelska and E. Mijowska, Preparation, thermal conductivity, and thermal stability of flame retardant polyethylene with exfoliated MoS2/MxOy, New J. Chem., 2017, 41, 13287–13292 RSC
.
- K. Zhou, Q. Zhang, J. Liu, B. Wang, S. Jiang, Y. Shi, Y. Hu and G. Zhou, Synergetic effect of ferrocene and MoS2 in polystyrene composites with enhanced thermal stability, flame retardant and smoke suppression properties, RSC Adv., 2014, 4, 13205–13214 RSC
.
- K. Zhou, S. Jiang, C. Bao, L. Song, B. Wang, G. Tang, Y. Hu and G. Zhou, Preparation of poly(vinyl alcohol) nanocomposites with molybdenum disulfide (MoS2): structural characteristics and markedly enhanced properties, RSC Adv., 2012, 2, 11695–11703 RSC
.
- K. Wenelska, X. Chen, B. Zielińska, R. J. Kaleńczuk, P. K. Chu, T. Tang and E. Mijowska, Mechanism of MxOy nanoparticles/CNTs for catalytic carbonization of polyethylene and application to flame retardancy, J. Appl. Polym. Sci., 2017, 134(34), 45233 CrossRef
.
- J. Gong, R. Niu, J. Liu, X. Chen, X. Wen, E. Mijowska, Z. Sun and T. Tang, Simultaneously improving the thermal stability, flame retardancy and mechanical properties of polyethylene by the combination of graphene with carbon black, RSC Adv., 2014, 2014(4), 33776–33784 RSC
.
| This journal is © The Royal Society of Chemistry 2018 |