Open Access Article
Open Access ArticleStructural elucidation of NASICON (Na3Al2P3O12) based glass electrolyte materials: effective influence of boron and gallium†
Amarnath R. Allu‡
 *a,
Sathravada Balaji‡
*a,
Sathravada Balaji‡ a,
Kavya Illathb,
Chaithanya Hareendranb,
T. G. Ajithkumarb,
Kaushik Biswasa and
K. Annapurna*a
a,
Kavya Illathb,
Chaithanya Hareendranb,
T. G. Ajithkumarb,
Kaushik Biswasa and
K. Annapurna*a
aGlass Division, CSIR-Central Glass and Ceramic Research Institute, 700032, Kolkata, India. E-mail: aareddy@cgcri.res.in; annapurnak@cgcri.res.in; Fax: +91-33-24730957; Tel: +91-33-23223421
bCentral NMR Facility and Physical and Materials Chemistry Division, CSIR-National Chemical Laboratory, 411008, Pune, India
First published on 17th April 2018
Abstract
Understanding the conductivity variations induced by compositional changes in sodium super ionic conducting (NASICON) glass materials is highly relevant for applications such as solid electrolytes for sodium (Na) ion batteries. In the research reported in this paper, NASICON-based NCAP glass (Na2.8Ca0.1Al2P3O12) was selected as the parent glass. The present study demonstrates the changes in the Na+ ion conductivity of NCAP bulk glass with the substitution of boron (NCABP: Na2.8Ca0.1Al2B0.5P2.7O12) and gallium (NCAGP: Na2.8Ca0.1Al2Ga0.5P2.7O12) for phosphorus and the resulting structural variations found in the glass network. For a detailed structural analysis of NCAP, NCABP and NCAGP glasses, micro-Raman and magic angle spinning-nuclear magnetic resonance (MAS-NMR) spectroscopic techniques (for 31P, 27Al, 23Na, 11B and 71Ga nuclei) were used. The Raman spectrum revealed that the NCAP glass structure is more analogous to the AlPO4 mesoporous glass structure. The 31P MAS-NMR spectrum illustrated that the NCAP glass structure consists of a high concentration of Q0 (3Al) units, followed by Q0 (2Al) units. The 27Al MAS-NMR spectrum indicates that alumina exists at five different sites, which include AlO4 units surrounded by AlO6 units, Al(OP)4, Al(OP)5, Al(OAl)6 and Al(OP)6, in the NCAP glass structure. The 31P, 27Al and 11B MAS-NMR spectra of the NCABP glass revealed the absence of B–O–Al linkages and the presence of B3–O–B4–O–P4 linkages which further leads to the formation of borate and borophosphate domains. The 71Ga MAS-NMR spectrum suggests that gallium cations in the NCAGP glass compete with the alumina cations and occupy four (GaO4), five (GaO5) and six (GaO6) coordinated sites. The Raman spectrum of NCAGP glass indicates that sodium cations have also been substituted by gallium cations in the NCAP glass structure. From impedance analysis, the dc conductivity of the NCAP glass (∼3.13 × 10−8 S cm−1) is slightly decreased with the substitution of gallium (∼2.27 × 10−8 S cm−1) but considerably decreased with the substitution of boron (∼1.46 × 10−8 S cm−1). The variation in the conductivity values are described based on the structural changes of NCAP glass with the substitution of gallium and boron.
1. Introduction
The existing technologies used today for electrochemical devices such as batteries are a better alternative to the dwindling fossil fuels in providing energy.1,2 This is relevant, because commercialized lithium ion batteries (LIBs) technology from 1991 is widely used for portable electronic devices.3 Nevertheless, there are increasing needs to replace the existing LIBs because of the increasing cost of production of LIBs and because lithium is scarce.1,4,5 In searching for alternative energy sources, abundant, economical and non-toxic sodium (Na) metal has been considered by the scientific community and continuous efforts have been carried out so far for the development of Na-batteries.5–8 Although the concept of Na-batteries has been proposed and proved several years ago, the topic has rematerialized again. Because of the heavier weight of Na batteries, they exhibit a primary disadvantage of having a low theoretical energy density compared to their lithium counterparts. Recent studies proposed that the output voltage of certain Na-based materials could compete with that of lithium batteries and thus, opened the opportunity to investigate and develop the Na-based materials.5–8 Considerable efforts are presently in progress to improve appropriate electrolyte and electrode materials for practically feasible Na-batteries.7,9,10 Among the various types of electrolyte materials, solid electrolyte materials have attracted significant interest compared to those of the liquid electrolytes because of the protection from leakage, volatilization, or flammability.10–12 However, to create the solid electrolyte batteries, a high ionic conductivity of electrolyte materials (σNa ≳ 10−4 S cm−1) at room temperature (RT) is required.Many studies have already been carried out to develop a suitable RT solid electrolyte material.13–16 In this regard non-oxide chalcogenide glasses (Na2(Ga0.1Ge0.9)2Se4.95 and Na2S–SiS2)15,17 and oxide-based phosphate glasses (P2O5–FeO–Na2O and P2O5–B2O3–Na2O)16,18 are important. Although the non-oxide based electrolyte materials are superior compared to oxide materials, the high cost and poor ambient stability limit their practical implementation.15,16 However, sodium super ionic conductor (NASICON) based phosphate glass and glass-ceramics are better suited as the RT electrolyte materials with Na+ ions as the mobile species.14,19–21 The maximum value of conductivity has been obtained for the Na3.1Zr1.95Mg0.05Si2PO12 which exhibits a high RT ionic conductivity of 3.5 × 10−3 S cm−1.14 In general, NASICON and the relevant solid electrolytes are produced using a conventional solid state reaction process, which includes sintering and calcination over 1100 °C.22 The sintering process usually leads to the formation of voids and may disturb the development of new structured materials to gain high conductivity.
Glass-based electrolyte materials have numerous advantages because of their isotropic properties for ion migration. These advantages are: glasses are easy to mould, having low cost and desired properties can be easily tailored by changing their chemical compositions.23 More importantly, the formation of voids during the sintering and calcination process can be easily avoided if the designed glass composition melts at a low temperature. It is also interesting to note that glasses having higher ionic conductivities than crystalline materials of the same compositions are fairly common. For example, the conductivity of glassy Li3.6P0.4Si0.6O4 at 25 °C is ten times higher than that of its polycrystalline form.24 Moreno-Real et al. have studied the structure and conductivity of glass and crystalline Na3Al2P3O12 material.19 It was also observed that the conductivity of glass (v-Na3Al2P3O12) material is higher than the conductivity of crystalline (c-Na3Al2P3O12) material. It was also found that v-Na3Al2(PO4)3 has no significant grain boundary contributions to the resistance. These examples clearly illustrate the possibility for the development of highly conducting glass-based materials rather than the crystalline materials. However, so far, only a few reports were found highlighting the sodium ionic conductors with the NASICON-type structure containing Al cations.19,25,26 Therefore, the present research reported here, aims at developing the Al-containing NASICON (Na3Al2P3O12) glass for use as a solid electrolyte material by understanding the glass structure and the modifications that occur in the structure with the changes in its chemical composition.
To achieve and to optimise the desired properties from the glass-based materials, it is helpful to have a clear picture of the structure and the role of each cation.27 It is known that to achieve greater conductivity (σ = cμq), in addition to increasing the concentration (c) and mobility (μ) of the charge carriers, understanding the “structure” also plays a crucial role, and it should be considered as one of the key factors in designing the glass-based electrolyte material.28,29 Very recently, glass and glass-ceramic bilayer sealants for solid oxide fuel cell applications have been developed by systematically varying the chemical composition of glasses through an understanding of the structure of glasses.23 Among the various techniques, magic angle spinning-nuclear magnetic resonance (MAS-NMR) and micro-Raman confocal spectroscopies are reliable techniques for structural characterization of glass materials. Solid-state NMR is particularly applicable for probing the coordination numbers of spin active nuclei such as 23Na, 31P and 27Al, network connectivity and, in favourable cases, next-nearest neighbour (NNN) connectivity, whilst Raman and infrared spectroscopy can be utilised to probe the nature of the bond between the metal cation and its surrounding oxygen anion.30,31
In the present study, the NASICON-based glasses with a modified chemical formula of Na2.8Ca0.1Al2P3O12 (NCAP) was considered as a starting glass composition to design and develop the solid-based electrolyte material. The structure of the NCAP glass was studied thoroughly. The NCAP glass composition was further modified using the substitution of boron (B) and gallium (Ga) for phosphorus (P) according to the chemical formula of Na2.8Ca0.1Al2P2.7X0.5O12 (where X = B and Ga). The B and Ga cations were substituted to observe the changes in the structure of the NCAP glass and thereby to utilise them for further improvement in the conductivity of NCAP glass. The 27Al, 11B, 71Ga, 23Na, and 31P MAS-NMR and micro-Raman techniques were utilised to elucidate the structure of the newly designed glasses. The modified glass materials were also characterised, using impedance measurements, to check their applicability as an electrolyte material for batteries.
2. Experimental
The experimental glasses in bulk form were prepared using the melt quenching technique. High purity powders of ammonium dihydrogen phosphate [(NH4)H2PO4], sodium carbonate (Na2CO3), calcium carbonate (CaCO3), aluminium oxide (Al2O3), boric acid (H3BO3) and gallium oxide (Ga2O3) were used to prepare the newly designed glasses. Homogeneous mixtures of batches obtained by hand grinding appropriate quantities of ingredients in agate mortar for about 45 min, were preheated in an alumina crucible for decarbonisation using the following heat treatment schedule: (i) RT to 350 °C at a heating rate (β) of 1 °C min−1 followed by a dwell time of 6 h at 350 °C; (ii) from 350 °C to 600 °C at β = 1 °C min−1 followed by a dwell time of 3 h at 600 °C; and (iii) from 600 °C to 900 °C at β = 4 °C min−1 followed by a dwell time of 1 h at 900 °C. The decarbonized batch mixture was first melted in an alumina crucible and then re-melted again in a platinum crucible to obtain better homogeneity at 1350 °C for 2 h, in an air atmosphere. The glasses in bulk form were obtained by quenching the glass melt into a pre-heated (400 °C) stainless steel mould followed by annealing at temperatures around the glass transition temperature of 350 °C.19 The amorphous nature of the glasses was confirmed using X-ray diffraction (XRD) analysis. The new glass compositions were labelled based on the chemical formula obtained after the substitution of X3+ cations (X = B and Ga) for P5+ cations: Na2.8Ca0.1Al2B0.5P2.7O12 (NCABP) for X = B and Na2.8Ca0.1Al2Ga0.5P2.7O12 (NCAGP) for X = Ga. The detailed chemical compositions (in mol%) of the glasses are provided in Table 1.| NCAP | NCABP | NCAGP | |
|---|---|---|---|
| Na2O | 35.0 | 34.1 | 34.1 |
| CaO | 02.5 | 02.4 | 02.5 |
| Al2O3 | 25.0 | 24.4 | 24.3 |
| P2O5 | 37.5 | 33.0 | 33.0 |
| B2O3 | — | 06.1 | — |
| Ga2O3 | — | — | 06.1 |
Raman spectra for bulk glass samples were obtained using a Horiba LabRam HR 800 Evolution confocal Raman microscope, with a 488 nm argon ion excitation laser source (10 mW) focused with an 100× objective lens (numerical aperture = 0.9) with a spot size of ∼3.14 μm2. The Raman radiation collected was dispersed with a 600 lines mm−1 grating and focused on a Peltier-cooled charge-coupled device (CCD) detector which allowed a spectral resolution of approximately 2 cm−1. All the spectra were recorded in the range of 200 to 1500 cm−1 with an integration time of 10 s and 20 accumulations per spectrum.
All the solid-state NMR experiments were carried out on a Bruker Avance HD 700 MHz spectrometer equipped with a superconducting magnet of 16.5 T. The single pulse 23Na, 27Al, 31P MAS-NMR measurements were carried out in a 2.5 mm Bruker TriGamma probe with Larmor frequency of 185.198 MHz for 23Na, 182.432 MHz for 27Al and 283.418 MHz for 31P. The samples were packed in 2.5 mm zirconia rotors and were spun at 32 kHz. The relaxation delay for 31P, 23Na and 27Al were 1100 s, 1 s and 3 s, respectively, and the number of transients were 16, 256 and 2048, respectively. The 27Al 3-quantum magic-angle spinning NMR (3Q MAS-NMR) spectra were acquired using a Bruker 1.3 mm MAS probe which was packed in a 1.3 mm rotor and spun at 32 kHz. The 64t1 points with 600 transients per t1 point were acquired with a 1 s recycling time. A very high radio frequency power of 200 kHz was used for this experiment. The 11B and 71Ga experiments were acquired using a Hahn echo pulse sequence in a Bruker 1.3 mm MAS probe at a spinning frequency of 60 kHz at Larmor frequencies of 224.5 MHz and 213.477 MHz, respectively. The chemical shifts of 31P, 23Na, 27Al, 11B and 71Ga were compared to 85% of aqueous phosphoric acid, 1 M NaCl solution, 1 M aluminum nitrate solution, 1 M boric acid solution (19.5 ppm), and 1 M gallium nitrate solution, respectively. To extract the NMR parameters, chemical shift, quadrupolar coupling constant and integrated peak area of the individual peaks from the experimental spectra, the DMFIT programme developed by Massiot was used.32
For impedance analysis, all the glass samples were uniformly cut into to 8 × 8 mm2 squares with a thickness of 1.5 mm and polished to optical grade quality and then hand coated with high conductivity (volume resistivity = 0.0174 ohm cm) silver epoxy (Cat. no. 8331-14G, MG Chemicals Ltd., Canada). The silver coated glass samples were cured over night at ∼100 °C in an air circulating oven. The conductivity measurements were carried out in the frequency range of 50 Hz to 1 MHz with 3 V root mean square (rms) using an LCR Hi-Tester model HIOKI-3532-50 (Hioki E. E. Corporation, Japan). A two-probe setup with test leads connected to a Test Fixture HIOKI-9262 was used to acquire the data. The real time data was acquired using HIOKI LCR HiTESTER software version 4.03E. The temperature dependent frequency characteristics of the glass samples were measured from RT to 200 °C with a 25 °C interval using a DPI-1200 high temperature dry calibrator (Divya Process Instruments) with temperature accuracy of ±1 °C.
3. Results
3.1 Raman spectroscopy
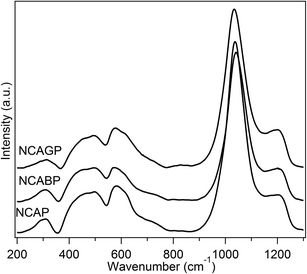
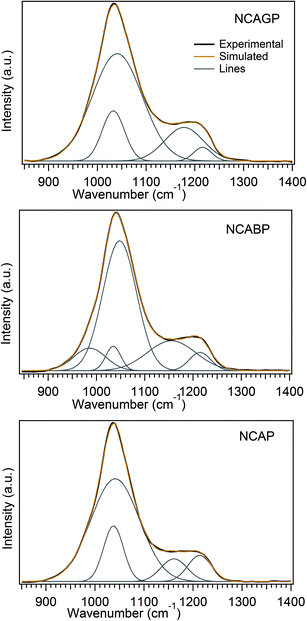
Fig. 1 displays the Raman spectra of all the glasses. The vibrational bands in the Raman spectra for all the glasses were observed in the following three regions: (i) low frequency region (200–650 cm−1), intermediate frequency region (650–850 cm−1), and high frequency regions (850–1400 cm−1). The low frequency region is dominated by five easily identified medium intensity peaks at around 310, 460, 500, 575 and 620 cm−1. The intermediate frequency region contains only one low intensity broad peak at ∼730 cm−1, and the high frequency region is dominated by two high intensity peaks at ∼1042 and ∼1210 cm−1.The Raman spectrum for observed NCAP glass was similar to that of Na3Al2P3O12 (NAP) glass reported by Yifen et al.33 Therefore, the peak obtained at 1040 cm−1 can be unambiguously assigned to the symmetric stretching vibrations of the AlPO4 groups, in which the phosphorus tetrahedral units are surrounded by alumina tetrahedral units as the second nearest neighbour. Nevertheless, a slight discrepancy was found when assigning the peak observed at 1210 cm−1 because of the flat and broad nature of the peak. In order to assign the peak to specific structural units, the deconvolution procedure was used for the high frequency region peak (800–1400 cm−1) with four Gaussian peaks and this is presented in Fig. 2. Tallant and Nelson34 analysed the Raman spectrum for sodium aluminophosphate glasses and attributed the peaks observed at 1161 and 1206 cm−1 to the Q2 tetrahedral units associated with sodium and the Q2 tetrahedral units associated with alumina tetrahedral units, respectively.35 Therefore, the bands observed at 1161 and 1213 cm−1 in the spectrum of NCAP glass were assigned to the Q2 units associated with sodium and alumina, respectively. The peaks observed at 1037 cm−1 may possibly be assigned to the AlPO4 groups in which AlO4− units were charge compensated by Na+ ions. In general, in AlPO4, an excess negative charge on AlO4− units was compensated for by the excess positive charge on the PO4 units.
The deconvolution analysis of NCABP and NCAGP glasses for the related peak observed at the higher frequency region clearly illustrate that structural changes have occurred with the substitution of B3+ and Ga3+ for the P5+ cation in the NCAP glass structure. The peak appears at 1041 cm−1 for NCAP glass and at 1047 cm−1 for NCABP glass. This indicates that the bond lengths and bond angles linked with Al–O–P bonds in the AlPO4 network structure were slightly rearranged with the addition of boron.36 Furthermore, the presence of an additional peak at 985 cm−1 clearly showed that additional new structural units were formed within the NCABP glass. Anastasopoulou et al.37 assigned the peak appearing at 985 cm−1 to the phosphate units connected to trigonal and tetragonal borate units. Therefore, the peak at 980 cm−1 in NCABP glass was assigned to the borophosphate structural units. Furthermore, it is worth noting that the intensity of the peak at 1037 cm−1, which corresponded to AlPO4 groups in which AlO4− units were charge compensated by Na+ ions, was decreased significantly.
With the Ga substitution, the concentration of Q2 structural units associated with alumina decreased at the expense of Q2 structural units associated with sodium. In addition, the position of the peak observed at 1161 cm−1 for NCAP glass was also shifted towards the higher frequency and positioned at 1177 cm−1 for NCAGP glass. These changes resulted from the modifications in the environment of Q2 structural units and were directly related to the decrease in sodium concentration. Therefore, the band observed at 1177 cm−1 for NCAGP glass can be assigned to the Q2 units associated with Na as well as Ga, which might be in the form of GaO6 modifier cations.38,39 The decrease in the Raman shift of P-non-bridging (or terminal) stretching vibrational modes with the increase in Na content in the system of Na2O–Ga2O3–P2O5 diphosphate glasses was also attributed to the influence of the NaO6 substitution for the GaO6 units.39 The variation in the intensity of the peak at 310 cm−1 in NCAGP glass, which can be assigned to the bending vibrations of the PO4 units with a cation as the modifiers,40 also confirmed the changes in the Na environment.
Considering the vibrations in the middle frequency region, the bands observed in the low frequency region at 620 cm−1 and 500 cm−1 for NCAP glass were attributed to the stretching vibrations of the AlO5 and AlO6 units, respectively.38 With the substitution of boron, the intensity of the peak corresponding to the AlO5 units had decreased and the same was observed for the AlO6 units. These changes were possibly observed because of the rearrangements of the AlPO4 network structure. However, with the substitution of Ga, the intensity of the peak corresponding to the AlO5 units was increased significantly. Whereas, no significant changes were observed in the peak corresponding to the AlO6 units. The bands observed at 575 and 460 cm−1 were assigned to the different bending vibrational modes of the network units.37
3.2 MAS-NMR
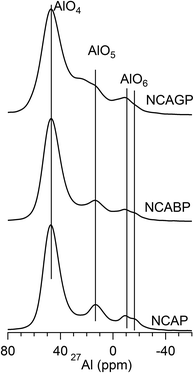
Fig. 3 shows the 27Al MAS-NMR spectra for all the glasses. Each spectrum shows three resonance peaks including the peak at the higher chemical shift side that can be assigned to the AlO4 units. The lowest peak can be assigned to the AlO6 units, and the peak in the middle can be assigned to the AlO5 units.41 These three peaks were distinct and isolated from each other for NCAP and for NCABP glass compositions. However, the peaks corresponding to AlO4 and AlO5 units were overlapped with each other for the NCAGP glasses. From the multiple quantum MAS (MQMAS) spectra shown in Fig. S1 and S2 (ESI†), it was also clear that the AlO4 is clearly resolved into two regions and the AlO6 was also resolved into two regions. Furthermore, the peak typical of AlO6 units splits into two peaks for all the glasses. Irrespective of the chemical composition and based on the quantitative analysis interpretation, all the glasses were enriched with AlO4 units.Fig. 4 shows the 31P MAS-NMR spectra of the glasses studied. The 31P MAS-NMR spectrum for NCAP glass shows two broad peaks, positioned at ∼−12 ppm and ∼−19 ppm, together with two shoulders: one at the higher chemical shift side (−4 ppm) and the other at the lower chemical shift side (−27 ppm). The resolution of two broad peaks decreased with the increase in the substituted cationic radius for P5+ cations and appeared as a single peak for high cationic radius (Ga3+) substitution. However, the spectrum for each glass can be easily deconvoluted into a sum of four/five Gaussian peaks, as shown in Fig. 5. Deconvoluted results are summarised in Table 2. It was clearly observed that the chemical shift values after the deconvolution were more analogous to the values obtained for AlPO4 glass and crystalline materials,42–44 which was in accordance with the Raman experimental results. On the basis of the peak assignments for AlPO4 glass- and crystalline-based materials,42–44 it was suggested that the deconvoluted components obtained can be easily assigned to Q0 species linked to different numbers of aluminium (Al) neighbours. In general, the building units in the aluminophosphate glass network were identified using the Qn (mAl) nomenclature.41,45 The index “n” is the number of phosphorus atoms and “m” is the number of aluminum atoms that are connected to the central phosphorus atom. This notation makes no distinction between AlO4 and AlO6 NNN atoms. Therefore, the deconvoluted curves were assigned to the various Q0 (mAl) species (where the value of m varies from 0 to 4) as follows: the signals with the centre of peaks at around 4–7 ppm and −2 to −3 ppm could be assigned to the isolated phosphate tetrahedral associated with one and two Al atoms (i.e., Q0 (1Al) and Q0 (2Al) species), respectively, the signals around −12 to −10 ppm and ∼−19 ppm, could be attributed to Q0 (3Al) species and Q2 (0Al) species, respectively, and finally, the signal around −24 to −27 ppm could be assigned to Q0 (4Al) species.44,46,47 Table 2 clearly reveals that the majority of the signals were arising mainly because of the Q0 (3Al) species and the Q0 (2Al) species, indicating the higher concentration of Q0 (3Al) species followed by Q0 (2Al) species in all the glasses under study.
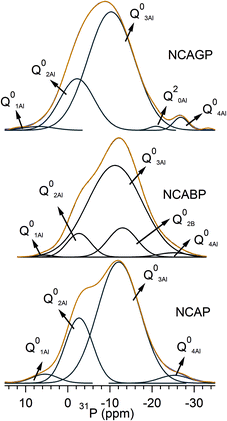 | ||
| Fig. 4 31P MAS-NMR spectra. (Blue color represents the experimental spectrum, orange color represents simulated spectrum and black color represents deconvolution curves). | ||
| NCAP | NCABP | NCAGP | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Peak (ppm) | Area (%) | FWHM (ppm) | Peak (ppm) | Area (%) | FWHM (ppm) | Peak (ppm) | Area (%) | FWHM (ppm) | |
| Q0 (1Al) | 5.3 | 4 | 8.4 | 5.3 | 1 | 4.3 | 4.5 | 3 | 8 |
| Q0 (2Al) | −2.7 | 24 | 7.6 | −2.6 | 8 | 6.6 | −2.3 | 21 | 9 |
| Q0 (3Al) | −12.1 | 68 | 11.6 | −11.2 | 76 | 15.1 | −10 | 71 | 12.7 |
| Q0 (2B) | — | — | — | −12.9 | 13 | 8.0 | — | — | — |
| Q2 (0Al) | — | — | — | — | — | — | −19.3 | 3 | 8 |
| Q0 (4Al) | −24.8 | 4 | 9.9 | −25.0 | 2 | 8.4 | −26.9 | 2 | 3.8 |
The changes in fractions of Q0 (mAl) species upon the substitution of X3+ cations were non-monotonic and depended on the type of X3+ cation in the NCAP glass composition. A significant influence of the X3+ cation radius was observed with the change in chemical shift values, however, systematic changes were observed only for high concentrated Q0 (3Al) species: chemical shift values shifted towards more positive ppm values as the substituted cationic radius for P5+ cation increased. Because of the substitution of B3+ cations for P5+ cations, the chemical shift values of Q0 (1Al), Q0 (2Al) and Q0 (3Al) were shifted towards the de-shield region (positive shift), whereas chemical shift values of Q0 (4Al) shifted towards the shielded region (negative shift). An additional deconvoluted peak at −12.9 ppm was observed in NCABP glass. Raguenet et al.,48 have assigned the peak observed at −11.7 ppm for borophosphate glasses to Q0 (2B) structural units.48,49 Therefore, the peak observed at −12.9 ppm for NCABP glass can be assigned to the isolated phosphorus tetrahedral units linked to boron tetrahedral units, i.e., Q0 (2B) structural units. These results suggest that the substituted B3+ cations show their overall effect on Q0 (2Al) units and decrease their overall concentration at the expense of both Q0 (3Al) and Q0 (2B) units.
With the substitution of Ga3+ cations for P5+ cations, an additional well-resolved peak was observed at −19.3 ppm. This peak was readily attributed to Q2 units interlinked with Na. The relative fraction of Q0 (3Al) was increased. It is interesting to note that the chemical shift value of the Q0 (3Al) species decreased significantly from −12.1 ppm to −10 ppm. This result indicated that some of the AlO4 units, which were linked to Q0 units, might be replaced with GaO4 units. While, the chemical shift values corresponding to Q0 (4Al) species shifted towards more negative ppm values.
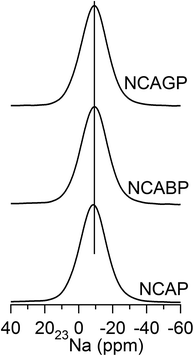
23Na MAS-NMR spectra of all the glasses are shown in Fig. 5. The spectra for NCAP glass displays broad resonance with the peak centre at −9 ppm. The chemical shift was slightly shifted towards lower ppm values with the substitution of B3+ and Ga3+ cations for P5+ cations in NCAP glass.
The 11B MAS-NMR spectrum for NCABP glass is presented in Fig. 6a. The spectrum shows two broad resonance peaks placed at 15.8 ppm and 3.2 ppm. The peak at 15.8 ppm dominates the spectrum and this can be attributed to boron species in three-fold coordination (BO3 units).50,51 Whereas, the peak at 3.2 ppm can be attributed to boron species in four-fold coordination (BO4 units).50,51 The relative proportion of BO3 units and BO4 units, obtained from spectral integration, further revealed that 78% of the boron is placed in three-fold coordination and 22% of the boron is placed in four-fold coordination. Closer observation of the asymmetric nature towards the lower chemical shift values of the peak at 15.8 ppm clearly indicated the presence of two distinct BO3 units [BO3 (ring) and BO3 (non-ring)] in the NCABP glass structure.52 Nevertheless, the presence of a peak intensity at 15.8 ppm mainly indicated the presence of a high concentration of BO3 (ring) type units, which are typically found in borate networks.
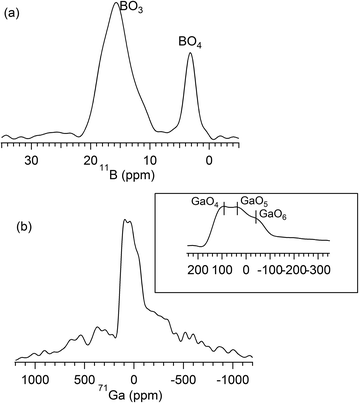 | ||
| Fig. 6 (a) 11B MAS-NMR spectrum for NCABP glass; and (b) 71Ga MAS-NMR spectrum for NCAGP glass. Inset of (b) presents the indication of GaO4, GaO5 and GaO6 chemical shifts. | ||
Fig. 6b shows the 71Ga MAS-NMR spectrum for NCAGP glass. The spectrum is very broad, which is a salient feature because of very strong quadrupolar coupling of the 71Ga nuclei, and is more similar to the spectra reported for the NaPO3–Ga2O3 glasses.53 It has been reported that the 71Ga MAS-NMR spectrum is more analogous to the 27Al MAS-NMR spectrum for glass materials.54 Therefore, the three distinct signals at −38 ppm, 44 ppm and 94 ppm were attributed to GaO6, GaO5, and GaO4 units, respectively, based on the compound network.53 The calculation of the relative proportion of these units was very difficult because of the strong anisotropic quadrupolar broadening.
3.3 Impedance analysis
Fig. 7 shows Nyquist plots for all three glass samples at 100 °C. The plots are fitted to an equivalent circuit as shown in the inset of Fig. 7. A reasonably good fit of the plots revealed a single semi-circle nature, and confirmed that there was a uniform charge transfer process in the prepared glass samples. The frequency response of the impedance of the glass samples fitted to the equivalent circuit is also shown in the inset of Fig. 7. The corresponding fitted values are listed in Table S1 (ESI†). It can be clearly seen that the diameter of the semi-circle increased when Ga and B were substituted for ‘P’ in the NCAP glass indicating the enhanced bulk resistance of the glass. The nature and behaviour of the impedance spectra for the Ga and B substituted glasses were similar at temperatures up to 200 °C in the present case. For all the glasses, the semi-circular nature of the impedance spectra were observed at the operating temperature of 100 °C and above indicating the free mobility of the intact Na+ ions even at lower frequencies in the glass network. The inclined straight line at the lower frequency region was because of the electrode polarization.55 The bulk resistance R, which is equal to Z′ when Z′′ = 0 at the lower frequency region, was used to calculate the dc conductivity, σdc of the samples using the relationship σdc = [t/(R × A)], where t = the thickness of the sample, and A is the applied electrode cross sectional area. At 100 °C, the dc conductivity values of NCAP, NCAGP, and NCABP were found to be 3.13 × 10−8 S cm−1, 2.27 × 10−8 S cm−1 and 1.46 × 10−8 S cm−1, respectively. The dc conductivity is a temperature dependent factor and the variation of conductivity was found to obey the well-known Arrhenius behaviour representing a straight line plot for ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) σdc versus 1/T (where T is temperature) and the slope value of the plot can be used to determine the activation energy Ea for mobile Na+ ions. The activation energy Ea was found to be 65.39 kJ mol−1, 66.79 kJ mol−1 and 71.09 kJ mol−1 for NCAP, NCAGP, and NCABP glasses, respectively. Fig. 8 shows the dc conductivity and activation energy behaviour for Ga and B substituted NCAP glasses. The observed activation energy values were in the range similar to the other Na+ ion conducting glasses reported in the literature.19
σdc versus 1/T (where T is temperature) and the slope value of the plot can be used to determine the activation energy Ea for mobile Na+ ions. The activation energy Ea was found to be 65.39 kJ mol−1, 66.79 kJ mol−1 and 71.09 kJ mol−1 for NCAP, NCAGP, and NCABP glasses, respectively. Fig. 8 shows the dc conductivity and activation energy behaviour for Ga and B substituted NCAP glasses. The observed activation energy values were in the range similar to the other Na+ ion conducting glasses reported in the literature.19
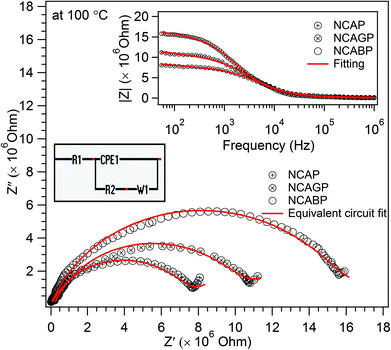 | ||
| Fig. 7 The impedance spectra of the NCAP, NCABP and NCAGP glass samples at 100 °C. (Inset figures shows equivalent circuit (left side) and frequency vs. impedance behavior (right side)). | ||
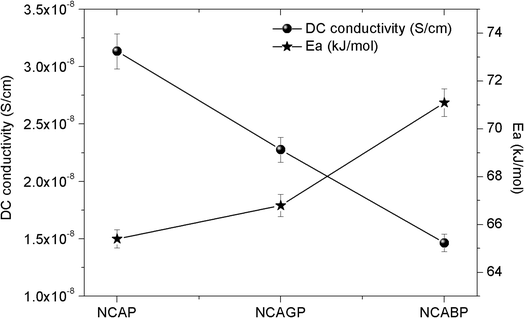 | ||
| Fig. 8 Variation of dc conductivity and activation energy behaviour with the addition of gallium and boron in NCAP glass. | ||
4. Discussion
The Raman and MAS-NMR results clearly illustrate the presence of isolated phosphorus tetrahedral units surrounded by alumina tetrahedral units as NNN in the NCAP glass structure. More precisely, the NCAP glass structure can be illustrated with the high concentration of Q0 (3Al) units (68%) followed by Q0 (2Al) units (24%). It has been reported that the addition of alumina into the sodium phosphate glasses directly influences the P–O–P crosslinks by replacing the P–O–P bonds with P–O–Al bonds and the network structure disrupts because of the following disproportion reaction: Q2 (0Al) → Q1 (mAl) → Q0 (mAl).41,45,46 Therefore, the presence of Q2 (0Al) type structural units in the NCAP glass structure cannot be neglected. Despite the confirmation of Q2 units from the high-frequency region of the Raman spectrum (as shown in Fig. 2), the indication of chemical shift values corresponding to Q2 units was not identified from the 31P MAS-NMR spectrum. This could be because of the overlap of a similar range of resonance frequencies (−20 to −25 ppm) for Q0 (4Al) and Q2 units.41Noting that the Na–O–Al bonds or non-bridging oxygens (NBOs) for Al tetrahedra do not exist, the AlO4 species bounded by isolated PO4 units should not be considered as anionic. In general, the stability of the structural units can be understood based on the total bond strength, expressed in terms of valence units (VU = charge/coordination number), of the oxygen associated with the structural unit. Each alumina cation in its four-fold coordination contribute 0.75 VU to the next nearest oxygen, whereas the phosphorus ion in its isolated tetrahedral form contributes 1.25 VU to the next nearest oxygen, resulting in perfectly charge-balanced oxygen atoms within the P(4)–O–Al(4) bridges. This suggests that AlO4 units surrounded by Q0 units may not require charge compensation from Na+ cations. Here that some of the modifier cations are required for compensating the charge of AlO6 units, which are part of the Q0 (3Al) units.56 Nevertheless, the concentration of the AlO6 units (∼7.5%) obtained (Table 3), which are not in the proportion required for Q0 (3Al) units, which requires 19% of AlO6, however, from the 27Al MAS-NMR spectra it was revealed that Q0 (3Al) units were fully surrounded by AlO4 units. It is worth mentioning that the existing AlO6 units which are fully associated with Q2 type tetrahedral units as proposed by Brow et al.,41 may not require an additional charge compensator. Because phosphorus in Q2 type tetrahedral contributes 1 VU to each bridging oxygen (BO) and 1.5 VU to each NBO and the required 0.5 VU can be directly provided by the AlO6 units. Overall, the MAS-NMR and Raman spectra suggest that the NCAP glass structure is more analogous to the structure of AlPO4 mesoporous glass. However, the available sodium cation in NCAP glass composition disturbs the fully polymerized Q0 (4Al) structure, which is characteristic of the AlPO4 structure, into the depolymerized structure and this leaves the Q0 (3Al) and Q0 (2Al) units (Table 2). The changes in the chemical shift and the fraction of Q0 (mAl) species (Table 2) clearly indicates the significant influence of B3+ and Ga3+ cations on the further rearrangement of the NCAP glass structure. The discussion in the next section explains the influence of Ga and B on the structure of NCAP glass and the consequence in achieving high electrical conductivity, a critical requirement for electrolyte materials.
| Glass | Al(OAl)4 | Al(OP)4 | Al(OP)5 | Al(OAl)6 | Al(OP)6 | Parameters |
|---|---|---|---|---|---|---|
| NCAP | 60 | 50 | 26 | 16 | −2.5 | Position (ppm) |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 549 549 |
4200 | 7083.09 | 7600 | 7600 | CQ (quad) | |
| 20 | 56 | 8 | 8 | 8 | Integrated area (%) | |
| NCABP | 60 | 50 | 25 | 16 | −3 | Position (ppm) |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
4200 | 7000 | 7600 | 7000 | CQ (quad) | |
| 17 | 62 | 9 | 6 | 6 | Integrated area (%) | |
| NCAGP | 61 | 51 | 29 | 17 | −1 | Position (ppm) |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 570 570 |
5000 | 6974 | 8468 | 6518 | CQ (quad) | |
| 19 | 54 | 12 | 10 | 5 | Integrated area (%) |
4.1 Influence of gallium
Quantitative analysis of Q0 (mAl) units requires Q0 (2Al) and Q0 (3Al) units to be slightly depolymerized with the addition of Ga. It has been found that both Ga and Al in oxide-based glasses often behave as network intermediates.54 It was observed in crystalline phosphates that the 31P chemical shift increases by ≈4 ppm for each replacement of Al3+ by Ga3+ cations because of the decrease in the P–O–Al bond angle with the substitution of Al by Ga.57 Likewise, the 31P MAS-NMR chemical shift increases on an average of 1 ppm for 5% substation of Ga for Al in AlPO4 glass systems.58 Therefore, the decrease in chemical shift of Q0 (3Al) species illustrates that one Al site is substituted by a Ga site. The changes in the Raman spectrum for NCAGP glass suggests that some of the Na+ cations are also replaced with Ga. Nelson and Exarhos have explained the variation in the symmetric stretch Raman frequency of the P–O− bonds with respect to the interaction between metal cation and NBO and postulated the following: increasing the O–P–O (intra tetrahedral angle) bond angle, which decreases with decrease in cation radius, decreases the stretching vibrations of the PO2 tetrahedral.59 In the present study, the shift in frequency of the Q2 units, which are connected with octahedral sodium, towards a higher frequency confirms the substitution of Ga for Na. The chemical shift corresponding to the Q2 (GaO6) units should appear at around −7 ppm in the 31P MAS-NMR spectrum.60 Because of the overlap of this chemical shift range with the broad chemical shift range of the Q0 (3Al) units, the presence of the Q2 (GaO6) units were not identified from the analysis of the 31PMAS-NMR spectra. Nevertheless, the increase in full width at half maximum (FWHM) of the peak corresponding to the Q0 (3Al) units from 11.6 ppm to 12.7 ppm confirms the contribution of the Q2 (GaO6) units in the total concentration of the Q0 (3Al) units. However, it has been observed that increasing the concentration of Ga increases the Q0 (3Ga) units in the NaPO3 glass system.53 This clearly demonstrates that the added Ga3+ cations in the NCAP glass system behave like Al3+ cations and might lead to the increase in the concentration of the Q0 (3Al) units in the NCAGP glass system.Deconvolution analysis of the 27Al MAS-NMR spectra [Fig. S1 and S2 (ESI†)] reveals the presence of five different sites of alumina in the NCAP and NCAGP glass structure. Results obtained after the deconvolution are presented in Table 3. In general, the 27Al MAS-NMR chemical shift of AlO4 tetrahedra units depends highly on the neighbouring atom in the second coordination sphere. The 27Al MAS-NMR chemical shift for the AlO4 units moves towards zero ppm with increasing electronegativity of the neighbouring tetrahedral. Therefore, the 27Al MAS-NMR chemical shift appears at around 50 ppm for the Al(OP)4 units, at around 60 ppm for Al(OSi)4 and at around 80 ppm for Al(OAl)4 units.61,62 Nevertheless, interesting behaviour was observed in the presence of the AlO6 octahedron in the second coordination sphere of the AlO4 tetrahedron: the chemical shift moves towards the shielded region and appeared at around 60 ppm. This unusual increase in chemical shift compared to that of Al(OAl)4 is attributed to the increase in average Al–O bond length and an increase in coordination of the shared oxygen (four) between the AlO4 and AlO6 units.61 On the contrary, it was observed that substitution of AlO4 for PO4 in the second coordination sphere of AlO6 moves the chemical shift towards the deshielded region because of the low electronegativity of AlO4.61,62 In agreement with this, the 27Al MAS-NMR chemical shifts appearing in the range of 1–12 ppm may generally be assigned to the Al(OAl)6 units. The presence of deconvolution peaks at around 60 ppm and 15 ppm in the 27Al MAS-NMR spectrum for NCAP glass reveals that the NCAP glass consists of an AlO4 tetrahedron surrounded by the AlO6 octahedron units. Therefore, the peaks appeared after the deconvolution are assigned to the following structural units: the peak at around 60 ppm is attributed to the AlO4 tetrahedra connected to the AlO6 octahedra units, the peak at around 50 ppm is assigned to Al(OP)4 units, the peak at around 26 ppm is assigned to Al(OP)5 units, the peak at around 16 ppm is allocated to Al(OAl)6 units, and the peak at around −2.5 ppm is attributed to Al(OP)6 units.61 Nevertheless, the deconvolution peaks obtained from the 27Al MAS-NMR spectra for NCAGP glass appeared in the more deshielded region compared to that of NCAP glass. This shift was attributed to the effect of partial substitution of Ga for Al in AlO6 sites, which are attached to the AlO4 tetrahedron, and Ga for P in PO4 units, which were connected in Al(OP)5 and Al(OP)6 units.
The 27Al MAS-NMR spectra for NCAGP glass indicates that the addition of Ga3+ also increased the fraction of AlO5/AlO6 units (Table 3). The similar increasing tendency of AlO5/AlO6 concentration was also observed with the increase in the concentration of Ga in the AlPO4 glass structure.58 Hee et al. studied the effect of progressive addition of Ga2O3 on the local glass structure of NaPO3, and reported that Ga attains the GaO6, GaO5, and GaO4 sites only in the medium concentration range of 12–25 mol% Ga2O3, which is much higher than the concentration in the present study.60 The 71Ga MAS-NMR spectra for NCAGP glass, which contains 6 mol% of Ga2O3, clearly reveals that GaO4, GaO5 and GaO6 structural units are present in the NCAGP glass structure. This indicates that the Ga added competes well with the alumina intermediate cations, which generally occupy all possible polyhedral sites in the glass network structure, and might lead to the generation of a mixed-intermediate effect in the NCAGP glass system. Further experimental studies are required to examine the mixed-intermediate effect.
4.2 Influence of boron
Because of the ability of boron atoms to form BO4 and BO3 (ref. 49 and 52) units, the influence of the addition of boron on the NCAP structure should be dissimilar compared to that of the Ga addition. It has been stated that if boron is substituted for aluminium as a second neighbour of phosphorus, the 31P chemical shift must change to be more negative.50 The observed chemical shift at −12.9 ppm, indicative of the Q0 (2B) units, might have occurred because of the substitution of boron for aluminium in the Q0 (2Al) structural units, which are significantly decreased compared to that of NCAP glass. Nevertheless, the chemical shift (3.2 ppm) for the BO4 units clearly shows that the BO4 sites are surrounded by fewer phosphate tetrahedral units.48,51 It can be clearly understood that because of the larger tendency of the Al3+ to occupy the next nearest sites of P5+ compared to that of B3+, at high concentrations of Al2O3 in sodium alumina borophosphate glasses, most of the boron atoms convert from the BO4 species to the BO3 species.63 The available high concentration of the BO3 units (in the form of rings), which was revealed from the 11B MAS-NMR spectra, clearly illustrate that the available BO4 units in NCABP glass are mostly connected to the BO3 rings and most of the boron is not connected with the phosphorus. It should be noted at this point that the formation of the BO3 rings together with the BO4 and PO4 units in the NCABP glass structure might lead to the formation of small domains (in nm size) of borophosphate and borate networks, which are possibly connected through the B3–O–B4–O–P chains. The formation of such small domains in borophosphate and borosilicate glasses are well documented.64,65The same chemical shift of four-, five- and six-coordinated aluminium compared with NCAP glass further confirms that the Al–O–B linkages are absent in NCABP glasses. The increase in intensity of the Raman vibrational band corresponding to AlO6 units at 500 cm−1 and the change in chemical shift of the 27Al MAS-NMR spectra for the Al(OP)6 units (Table 3), further suggests that the ionic character between Al and O might be decreased through the decrease of the average Al–O bond length in the Al(OP)6 unit.31,61 This indicates the rearrangement of Q0 (3Al) and Q0 (2Al) units with the addition of boron. This activity could be explained based on the formation of borate and borophosphate domains in NCABP glass. In general, the excess of phosphorus is primarily responsible for the formation of the Al(OP)5 and Al(OP)6 units in aluminophosphate glasses.41,58 Nevertheless, the formation of borate and borophosphate domains decreases the excess phosphorus available for the Al(OP)5 and Al(OP)6 units, and thus, increases the probability for the formation of the Al(OP)4 units because of the larger tendency of Al3+ to occupy the next nearest sites of P5+. Deconvolution of the 27Al MAS-NMR spectra for NCABP glass also illustrates that the Al(OP)4 structural units are increased with the addition of boron. It has also been reported that the addition of boron to the aluminophosphate glasses significantly influenced the coordination of Al and AlO4 units which have been increased compared to AlO6 units.66,67 This is agrees with the 27Al MAS-NMR results observed for NCABP glass, where the concentration of AlO4 units increases with the addition of boron (Table 3). This might be responsible for increasing the concentration of Q0 (3Al) units in NCABP glass.
4.3 Ion conductivity
On the basis of the previous analysis, the structural changes associated with the Na+ ionic conductivities in the NCAP glass can be correlated. In general, all the phosphorus Q0 (mAl) species present in the network structure should be electrically neutral to neutralize the total glass. Therefore, the charges located on each species can be calculated on the basis of band valence consideration, and this is more analogous to the models proposed by Ren and Eckert53 for alumina phosphate glasses. Considering the AlPO4 structure, which contains an equal number of the AlO4 and PO4 units, the four oxygen atoms linking the Q0 type phosphate unit and the Al tetrahedral unit are locally neutral by contributing 1.25 VU from phosphorus and 0.75 VU from the aluminum atom, and therefore require no additional charges. Implementing a similar model to the species present in the NCAP glass structure, Q0 (3Al), Q0 (2Al), Q0 (1Al) and Q0 (0Al) structural units require an additional charge of 0.75, 1.5, 2.25 and 3 VU, respectively. In the present case, highly mobile Na+ provide the required additional charges for the Q0 (mAl) units. Nevertheless, the high concentration of the Q0 (3Al) units requires a smaller number of sodium cations (0.75 VU for each Q0 (3Al) unit). The continuous network structure of the Q0 (3Al) units leads to supression of the hopping sites required for long-range motion of the mobile sodium ions. In addition, the existence of several environments for alumina, as is revealed from the broad signals of 27Al MAS-NMR spectra, also suggests the discontinuity of the electric field limiting conduction process in the NCAP glass structure. These results support the fact that lower mobile ion conductivity was achieved from NCAP glass that from any other types of NASICON glasses.68The changes in the environment of the Na+ cation in the NCABP and NCAGP glasses are also accounted for from the observation of changes in 23Na MAS-NMR spectra. In general, the 23Na MAS-NMR chemical shift is highly sensitive to the coordination number and the Na–O bond length. The 23Na chemical shift is correlated well with the Na–O bond length because the chemical shift decreases with the increasing average bond length. Apparently, the increase in coordination number leads to an increase in the Na–O bond length and thereby increasing the interaction.69 Therefore, the decrease in the 23Na chemical shift value clearly suggests a strong interaction of Na+ cations with the Q0 (mAl) species in boron and Ga containing glasses, than in the NCAP glass. In support of this, the experimental conductivity results revealed that the electrical conductivity was decreased with the addition of Ga and was decreased even further with the addition of boron. The significant decrease in the conductivity of NCABP glass could be because of the formation of borate and borophosphate domains.50 The conductivity of NCAGP glass was slightly decreased because of the possible mixed intermediate effect. In addition, the formation of a high concentration of AlO5/AlO6 units, which generally compensates the charge of the Q0 (mAl) units instead of the Na+ cation, when compared to that of NCAP glasses also increases the distance between the hopping centres for Na+ ion mobility.68 The previous results suggest that decreasing the Q0 (3Al) units and increasing the charge compensation centres in the network structure might increase the conductivity of the NCAP glass. Therefore, further experimental studies are required to enhance the ionic conductivity at RT for the NCAP glass to be useful for Na-ion batteries.
5. Conclusions
In summary, the structural and electrical properties of sodium super ionic conductor (NASICON) based phosphate glasses having the chemical formula Na2.8Ca0.1Al2P3O12 (NCAP) were evaluated thoroughly using Raman, MAS-NMR, and impedance spectroscopy techniques. Furthermore, the influence of boron and gallium on the structural and electrical properties of NCAP glass were also studied. The NCAP glass network structure consists of a high concentration of the Q0 (3Al) units followed by the Q0 (2Al) units, as confirmed from the Raman and 31P MAS-NMR spectra. A high concentration of the Q0 (3Al) units leads to suppression of the hopping sites required for long-range motion of the mobile sodium ions, which is also responsible for the low conductivity achieved compared to the other NASICON based glasses. The 27Al MAS-NMR spectra further indicate that aluminium plays a major role in constructing the NCAP structure. The existence of alumina in five different sites might impede the electric field, which further limits the mobility of the Na+ in the NCAP glass structure. 11B and 31P MAS-NMR spectra for NCABP glass indicate that boron cations in the NCABP glass structure interacted with phosphorus cations and created the borate and borophosphate domains by forming B3–O–B4–O–P4 linkages. Formation of domains and the increased fraction of the Q0 (3Al) units significantly reduced the conductivity of NCABP glass compared to that of NCAP glass. Gallium competed with the aluminium cations and partially occupied the possible four, five and six coordination sites for alumina in NCAP glass, as revealed from the 27Al and 71Ga MAS-NMR spectrum for NCAGP glass. A slight increase in the Q0 (3Al) units and disturbance in the arrangement of alumina occupied sites slightly reduced the conductivity of the NCAGP glass. Results obtained from the present study indicate that reducing the overall concentration of the Q0 (3Al) units and limiting the distribution of alumina sites might improve the Na-ion conductivity in the NCAP glass.Conflicts of interest
There are no conflicts to declare.Acknowledgements
ARA, SB, KB and KA gratefully acknowledge the support and encouragement of Dr K. Muraleedharan, Director, CSIR-CGCRI and Dr Ranjan Sen, Head, Glass Division, CSIR-Central Glass & Ceramic Research Institute. ARA would like to thank to Prof. Gaddam Vijaya Prakash for his approval for carrying out further studies on Na3Al2P3O12 glass compositions. Useful suggestions and discussions from Dr S. Mahanty are greatly acknowledged. The use of the Bruker AV 700 MHz NMR spectrometer in the Central NMR Facility, CSIR-NCL, purchased under the Twelfth Five Year Plan Project CSC0405 is gratefully acknowledged. ARA acknowledges the support of Dr R. N. Basu, Head, Fuel Cell and Battery Division, CSIR-CGCRI for doing conductivity measurements.References
- Y. Sun, Chem. Soc. Rev., 2017, 46, 3529–3614 RSC.
- J. B. Goodenough, ACS Catal., 2017, 7, 1132–1135 CrossRef CAS.
- Y. Nishi, J. Power Sources, 2001, 100, 101–106 CrossRef CAS.
- J. Tang, A. D. Dysart and V. G. Pol, Curr. Opin. Chem. Eng., 2015, 9, 34–41 CrossRef.
- K. Kubota and S. Komaba, J. Electrochem. Soc., 2015, 162, 2538–2550 CrossRef.
- Z. Zhang, Q. Zhang, C. Ren, F. Luo, Q. Ma, Y.-S. Hu, Z. Zhou, H. Li, X. Huang and L. Chen, J. Mater. Chem. A, 2016, 4, 15823–15828 CAS.
- F. Lalere, V. Seznec, M. Courty, R. David, J. N. Chotard and C. Masquelier, J. Mater. Chem. A, 2015, 3, 16198–16205 CAS.
- L. P. Wang, L. Yu, X. Wang, M. Srinivasan and Z. J. Xu, J. Mater. Chem. A, 2015, 3, 9353–9378 CAS.
- K. Vignarooban, R. Kushagra, A. Elango, P. Badami, B.-E. Mellander, X. Xu, T. G. Tucker, C. Nam and A. M. Kannan, Int. J. Hydrogen Energy, 2016, 41, 2829–2846 CrossRef CAS.
- W. Zhou, Y. Li, S. Xin and J. B. Goodenough, ACS Cent. Sci., 2017, 3, 52–57 CrossRef CAS PubMed.
- J.-J. Kim, K. Yoon, I. Park and K. Kang, Small Methods, 2017, 1, 1700219 CrossRef.
- I.-H. Chu, C. S. Kompella, H. Nguyen, Z. Zhu, S. Hy, Z. Deng, Y. S. Meng and S. P. Ong, Sci. Rep., 2016, 6, 33733 CrossRef CAS PubMed.
- Y. S. Zhu, L. L. Li, C. Y. Li, L. Zhou and Y. P. Wu, Solid State Ionics, 2016, 289, 113–117 CrossRef CAS.
- S. Song, H. M. Duong, A. M. Korsunsky, N. Hu and L. Lu, Sci. Rep., 2016, 6, 32330 CrossRef CAS PubMed.
- S. K. Kim, A. Mao, S. Sen and S. Kim, Chem. Mater., 2014, 26, 5695–5699 CrossRef CAS.
- Y. Ni, R. Zheng, X. Tan, W. Yue, P. Lv, J. Yang, D. Song, K. Yu and W. Wei, J. Mater. Chem. A, 2015, 3, 17558–17562 CAS.
- D. E. Watson and S. W. Martin, J. Non-Cryst. Solids, 2017, 471, 39–50 CrossRef CAS.
- R. Christensen, G. Olson and S. W. Martin, J. Phys. Chem. B, 2013, 117, 16577–16586 CrossRef CAS PubMed.
- L. Moreno-Real, P. Maldonado-Manso, L. Leon-Reina, E. R. Losilla, F. E. Mouahid, M. Zahir and J. Sanz, J. Mater. Chem., 2002, 12, 3681–3687 RSC.
- H.-P. Hong, Mater. Res. Bull., 1976, 11, 173–182 CrossRef CAS.
- J. B. Goodenough, H.-P. Hong and J. A. Kafalas, Mater. Res. Bull., 1976, 11, 203–220 CrossRef CAS.
- I. Lisenker and C. R. Stoldt, Front. Energy Res., 2016, 4, 13 Search PubMed.
- A. A. Reddy, U. Tulyaganov, V. V Kharton and J. M. F. Ferreira, J. Solid State Chem., 2015, 19, 2899–2916 CAS.
- K. Kanehori, K. Matsumoto, K. Miyauchi and T. Kudo, Solid State Ionics, 1983, 9–10, 1445–1448 CrossRef CAS.
- S. Li, J. Cai and Z. Lin, Solid State Ionics, 1988, 28–30, 1265–1270 CrossRef.
- B. E. Francisco, C. R. Stoldt and J.-C. M'Peko, J. Phys. Chem. C, 2015, 119, 16432–16442 CAS.
- M. de Oliveira, T. S. Gonçalves, C. Ferrari, C. J. Magon, P. S. Pizani, A. S. S. de Camargo and H. Eckert, J. Phys. Chem. C, 2017, 121, 2968–2986 CAS.
- I. R. Evans, J. S. O. Evans, H. G. Davies, A. R. Haworth and M. L. Tate, Chem. Mater., 2014, 26, 5187–5189 CrossRef CAS.
- E. R. Losilla, M. A. G. Aranda, M. A. Paris and A. R. West, Chem. Mater., 1998, 10, 665–673 CrossRef CAS.
- A. A. Reddy, D. U. Tulyaganov, G. C. Mather, S. Rodr, S. Das, M. J. Pascual, F. Mun, J. Senker and J. M. F. Ferreira, J. Phys. Chem. C, 2015, 119, 11482–11492 CAS.
- A. R. Allu, S. Balaji, D. U. Tulyaganov, G. C. Mather, F. Margit, M. J. Pascual, R. Siegel, W. Milius, J. Senker, D. A. Agarkov, V. V Kharton and J. M. F. Ferreira, ACS Omega, 2017, 2, 6233–6243 CrossRef CAS.
- D. Massiot, F. Fayon, M. Capron, I. King, S. Le Calvé, B. Alonso, J.-O. Durand, B. Bujoli, Z. Gan and G. Hoatson, Magn. Reson. Chem., 2002, 40, 70–76 CrossRef CAS.
- J. Yifen, J. Dehua, C. Xiangsheng, B. Beiya and H. Xihuai, J. Non-Cryst. Solids, 1986, 80, 147–151 CrossRef.
- D. R. Tallant and C. Nelson, Phys. Chem. Glasses, 1986, 27, 75 CAS.
- A. Moguš-Milanković, A. Gajović, A. Šantić and D. E. Day, J. Non-Cryst. Solids, 2001, 289, 204–213 CrossRef.
- G. B. Rouse, P. J. Miller and W. M. Risen, J. Non-Cryst. Solids, 1978, 28, 193–207 CrossRef CAS.
- M. Anastasopoulou, K. C. Vasilopoulos, D. Anagnostopoulos, I. Koutselas, D. K. Papayannis and M. A. Karakassides, J. Phys. Chem. B, 2017, 121, 4610–4619 CrossRef CAS PubMed.
- A. Belkébir, J. Rocha, A. P. Esculcas, P. Berthet, B. Gilbert, Z. Gabelica, G. Llabres, F. Wijzen and A. Rulmont, Spectrochim. Acta, Part A, 1999, 55, 1323–1336 CrossRef.
- A. Belkébir, J. Rocha, A. P. Esculcas, P. Berthet, B. Gilbert, Z. Gabelica, G. Llabres, F. Wijzen and A. Rulmont, Spectrochim. Acta, Part A, 2000, 56, 435–446 CrossRef.
- Y. M. Lai, X. F. Liang, S. Y. Yang, J. X. Wang and B. T. Zhang, J. Mol. Struct., 2012, 1013, 134–137 CrossRef CAS.
- R. K. Brow, R. J. Kirkpatrick and G. L. Turner, J. Am. Ceram. Soc., 1993, 76, 919–928 CrossRef CAS.
- Z. Zhao, S. Xu, M. Y. Hu, X. Bao and J. Z. Hu, J. Phys. Chem. C, 2016, 120, 1701–1708 CAS.
- S. Delsarte, P. Grange, T. Chen, P. Grobet and P. Jacobs, J. Phys. Chem. B, 2003, 107, 6504–6510 CrossRef CAS.
- L. Zhang, A. Bögershausen and H. Eckert, J. Am. Ceram. Soc., 2005, 88, 897–902 CrossRef CAS.
- R. K. Brow, J. Non-Cryst. Solids, 2000, 263&264, 1–28 CAS.
- J. M. Egan, R. M. Wenslow and K. T. Mueller, J. Non-Cryst. Solids, 2000, 261, 115–126 CrossRef CAS.
- L. Zhang and H. Eckert, J. Mater. Chem., 2004, 14, 1605–1615 RSC.
- B. Raguenet, G. Tricot, G. Silly, M. Ribes and A. Pradel, J. Mater. Chem., 2011, 21, 17693–17704 RSC.
- G. Tricot, K. Ben Tayeb, L. Koudelka, P. Mosner and H. Vezin, J. Phys. Chem. C, 2016, 120, 9443–9452 CAS.
- N. Mascaraque, A. Durán and F. Muñoz, J. Non-Cryst. Solids, 2011, 357, 3212–3220 CrossRef CAS.
- F. Muñoz, L. Montagne, L. Pascual and A. Durán, J. Non-Cryst. Solids, 2009, 355, 2571–2577 CrossRef.
- K. Januchta, R. E. Youngman, A. Goel, M. Bauchy, S. L. Logunov, S. J. Rzoska, M. Bockowski, L. R. Jensen and M. M. Smedskjaer, Chem. Mater., 2017, 29, 5865–5876 CrossRef CAS.
- J. Ren and H. Eckert, J. Phys. Chem. C, 2014, 118, 15386–15403 CAS.
- S. M. Bradley, R. F. Howe and R. A. Kydd, Magn. Reson. Chem., 1993, 31, 883–886 CrossRef CAS.
- I. M. Hodge, M. D. Ingram and A. R. West, J. Electroanal. Chem. Interfacial Electrochem., 1976, 74, 125–143 CrossRef CAS.
- S. Wegner, L. Van Wu and G. Tricot, J. Non-Cryst. Solids, 2008, 354, 1703–1714 CrossRef CAS.
- S. K. Kulshreshtha, O. D. Jayakumar and V. Sudarsan, J. Phys. Chem. Solids, 2004, 65, 1141–1146 CrossRef CAS.
- J. He, P. Ma, G. Zhang, R. Li and L. Zhang, RSC Adv., 2016, 6, 99149–99157 RSC.
- B. N. Nelson and G. J. Exarhos, J. Chem. Phys., 1979, 71, 2739–2747 CrossRef CAS.
- P. Hee, R. Christensen, Y. Ledemi, J. E. C. Wren, M. Dussauze, T. Cardinal, E. Fargin, S. Kroeker and Y. Messaddeq, J. Mater. Chem. C, 2014, 2, 7906–7917 RSC.
- D. Muller, W. Gessner, A. Samoson, E. Lippmaa and G. Scheler, J. Chem. Soc., Dalton Trans., 1986, 1277–1281 RSC.
- R. Hussin, D. Holland and R. Dupree, J. Non-Cryst. Solids, 2002, 298, 32–42 CrossRef CAS.
- K. Januchta, R. E. Youngman, A. Goel, M. Bauchy, S. J. Rzoska, M. Bockowski and M. M. Smedskjaer, J. Non-Cryst. Solids, 2017, 460, 54–65 CrossRef CAS.
- Y. Yu and M. Eden, RSC Adv., 2016, 6, 101288–101303 RSC.
- A. Saitoh, G. Tricot, P. Rajbhandari, S. Anan and H. Takebe, Mater. Chem. Phys., 2015, 149–150, 648–656 CrossRef CAS.
- Y. Z. Khimyak and J. Klinowski, J. Mater. Chem., 2002, 12, 1079–1085 RSC.
- L. van Wüllen, S. Wegner and G. Tricot, J. Phys. Chem. B, 2007, 111, 7529–7534 CrossRef PubMed.
- D. R. Clayton, D. Lepage, P. N. Plassmeyer, C. J. Page and M. C. Lonergan, RSC Adv., 2017, 7, 7046–7051 RSC.
- A. M. George, S. Sen and J. F. Stebbins, Solid State Nucl. Magn. Reson., 1997, 10, 9–17 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: 27Al 3Q MAS-NMR and deconvolution of 27Al MAS-NMR spectra. Impedance analysis and fitted data for the equivalent circuit. See DOI: 10.1039/c8ra01676c |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2018 |