Open Access Article
Open Access ArticleA time-of-flight mass spectrometry based strategy to fast screen triterpenoids in Xanthoceras sorbifolia Bunge husks for bioactive substances against Alzheimer's disease†
Weiwei Rong,
Kewen Ding,
Sirui Guo,
Ziyue Yuan,
Qing Li and
Kaishun Bi *
*
National and Local Joint Engineering Laboratory for Key Technology of Chinese Material Medica Quality Control, School of Pharmacy, Shenyang Pharmaceutical University, 103 Wenhua Road, Shenyang 110016, China. E-mail: kaishunbi.syphu@gmail.com
First published on 18th April 2018
Abstract
Xanthoceras sorbifolia Bunge is a folk medicine in China. Recently, the triterpenoids in its husks have attracted more and more attention for potential prevention against Alzheimer's disease. However, current studies on its bioactive substances were still insufficient. To reveal more bioactive substances, an efficient and practical strategy based on high resolution mass spectra coupled with multiple data mining techniques was developed to characterize the barrigenol type triterpenoids in the husks and dosed rat plasma. A total of 50 barrigenol type triterpenoids were identified in the husks, and 6 of these were detected in the rat plasma, which were regarded as bioactive candidates. To find the real bioactive substances, the neuroprotective effect of the candidates was further tested by calculating the PC12 cell viability against amyloid-β-induced cytotoxicity. As a result, three out of the six candidates exhibited obvious neuroprotction against amyloid-β-induced cytotoxicity on PC12 cells, indicating their potential to be bioactive substances against Alzheimer's disease. This study will be a valuable reference of the bioactive substances in Xanthoceras sorbifolia Bunge husks against Alzheimer's disease and the provided strategy can also be applied to the exploration of the effective constituents in other medicines.
1 Introduction
Xanthoceras sorbifolia Bunge (X. sorbifolia), belonging to Sapindaceae, is a kind of indigenous shrub distributed around the north and northeast of China.1,2 Its woods and fruit seeds were used as folk medicines for the treatment of rheumatism and pediatric nocturia.3,4 Recent study found that the husks with the advantages of abundant resource and low cost, were a promising medicinal resource. There were various chemical constituents in the husks, like triterpenoid saponins, coumarins, flavonoids and sterols etc.5–8 Among them, barrigenol type triterpenoid saponins, rich in the husks, were its characteristic constituents and responsible for its multiple bioactivities, such as antitumor activity, anti-inflammatory activity, and especially the potentiality against Alzheimer's disease (AD).8–10 However, current studies on its bioactive substances against AD were insufficient. Most of them mainly focused on a monomer named xanthoceraside because it was rich in the husks and proved to exhibit excellent protection against amyloid-β (Aβ)-induced cognitive disorder in rats.11–18 The neuroprotection of other saponins was rarely reported. For reasonable development and utilization of the husks, sufficient study on its bioactive substances against AD was very necessary. Nevertheless, intensive research on the neuroprotection of every saponin was time-consuming, labor-intensive and high-cost. According to Serum Pharmacochemistry of Traditional Chinese Medicines (SPT) theory, most bioactive constituents were first absorbed into blood after oral administration, and then exerted therapeutic effect after metabolism and distribution.19 Hence, components detected in the dosed plasma were more possible to exert efficacy in practice. Based on this principle, we tried to find an efficient strategy to reveal the bioactive substances.Thus, firstly, chemical profiling of barrigenol type triterpenoids in the husks was carried out. However, as was widely believed, chemical profiling of traditional chinese medicines (TCMs) was very time-consuming for its various components, complex matrices and unavailable analytical standards. Hence, an efficient and reliable analytical method seemed very important. Conventional techniques such as liquid chromatography and capillary electrophoresis in most cases could not satisfy the analysis of TCMs. In recent decades, high resolution mass spectrometry had gained popularity due to its accurate mass measurement, which simplified the process of identification and the analysis time was thus shortened.20,21 Therefore, in this study, an efficient and reliable ultra-high-performance liquid chromatography combined with electrospray ionization quadrupole time-of-flight tandem mass spectrometry (UHPLC/ESI-Q-TOF-MS/MS) method was established to screen and identify the effective components in X. sorbifolia husks. Thousands of high resolution MS and MS/MS data was obtained during chemical profiling. To fast identify target ions from complex interference ions, a powerful data processing tool, PeakView 2.2 software (Sciex, US), was applied to processing the original data automatically based on retention time, accurate mass molecular ion, isotopic pattern, and MS/MS library searching. In general, compounds in the same family were able to yield similar fragment ions or neutral loss molecules after collision induced dissociation (CID). Thus, the modules of the product ion filter (PIF) and neutral loss filter (NLF) within the software were applied to fast screen different kinds of barrigenol type triterpenoids by specifying the values of corresponding fragment ions or neutral loss molecules.22 In this way, a large number of barrigenol type triterpenoid saponins in the husks were identified and a database with their detailed information was created. Then, the dosed rat plasma samples were detected using the same method, and barrigenol type triterpenoids absorbed into the blood were fast identified based on the database in vitro, which could be considered as the bioactive candidates.19 Finally, the neuroprotective effect of the candidates was further tested by Aβ25–35 induced cytotoxicity on PC12 cells to find the real bioactive substances with the potentiality against AD.14,23–25
Up to now, there were few comprehensive studies on chemical profiling of barrigenol type triterpenoids in X. sorbifolia husks. This work not only provided characteristic fragment pathways to fast screen different kinds of barrigenol type triterpenoids in the husks for the first time, but also enriched its bioactive substances studies against AD. In addition, the provided strategy will be very useful for the exploration of effective substances in other medicines.
2 Experimental
2.1 Chemicals and materials
Chromatographic-grade methanol, acetonitrile and formic acid were supplied from Fisher Scientific (Fair Lawn, NJ, USA) and distilled water was purchased from Wahaha Co. Ltd (Hangzhou, China). Other analytical-grade reagents and solvents were provided by Shandong Yuwang Industrial Co. Ltd. (Yucheng, China).X. sorbifolia husks were obtained from Chifeng city, Inner Mongolia, China, which was identified by Professor Ying Jia (Department of Traditional Chinese Materia Medica, Shenyang Pharmaceutical University, Shenyang, China). A total of 15 reference standards were used for the identification, 9 (compounds 8, 10, 12–14, 16, 17, 33 and 34) of which were isolated by our library and their chemical structures were unambiguously identified by comparing the experimental NMR and HR-MS data with the previous reports. Other 6 reference standards (compounds 18, 22, 26, 36, 39 and 45) were gifts from Professor Dali Meng and Huiyuan Gao (Department of Traditional Chinese Materia Medica, Shenyang Pharmaceutical University, Shenyang, China). The purity of all the reference standards were over 90% and their structures were listed on Fig. 1.
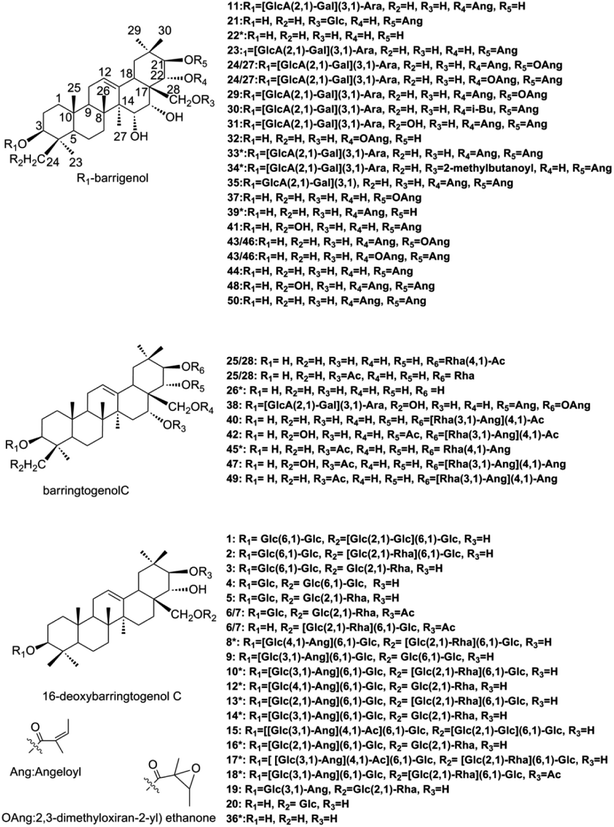 | ||
| Fig. 1 Structures of barrigenol triterpenoids in X. sorbifolia.*: reference standards of barrigenol type triterpenoids. | ||
The PC12 cells were got from BOSTER Biological Technology (Wuhan, China), high glucose-Dulbecco's modified Eagle medium (H-DMEM) was supplied from HYclone (SH30022.01, Lot. B10201637), heat-inactivated fetal bovine serum (FBS) was purchased from Gibco (10099-141 Lot. 1715752) and Aβ25–35 was obtained from Sigma Aldrich (St Louis, MO, USA).
2.2 Preparation of X.sorbifolia husks extract
Dried powdered X. sorbifolia husks (∼100 g) were extracted three times with 1000 ml of 70% ethanol under reflux for 2 h. After filtering, the extraction was combined and concentrated under reduced pressure. The concentrate was redissolved with a concentration of 1 g crude drug per milliliter in water to obtain the solution and stored it at 4 °C for animal oral administration.One milliliter of intragastric administration (1 g ml−1) was diluted with 10 ml of water and passed through a 0.22 μm filter before UHPLC-MS analysis.
2.3 Animals experiment
Six healthy male Sprague-Dawley rats (220 ± 20 g) were provided by the Experimental Animal Center of Shenyang Pharmaceutical University and bred with free access to food and water in a stable-condition (22 ± 2 °C temperature, 40–60% relative humidity) with a natural light–dark cycle for a week before the experiment to adapt the rats to the environment. Animal study was carried out following the Guideline of Animal Experimentation of Shenyang Pharmaceutical University, and the protocol was approved by the Animal Ethics Committee of the institution.The rats were fasted 12 h with free access to water before oral administration of the husks extract at a dose of 10 g kg−1. 1.0 ml of blood samples were collected from suborbital vein into heparinized tubes before and 2 h after administration and immediately centrifuged at 1000 × g for 10 min. The plasma samples were combined to make plasma pools, and then stored at −80 °C until analysis.
2.4 Pre-processing of plasma samples
The frozen plasma samples were first thawed to room temperature. An aliquot of 1 ml of pooling plasma samples was extracted with 3 ml of ethyl acetate and isopropanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). Then the mixture was vortex-mixed for 3 min and centrifuged at 10
1, v/v). Then the mixture was vortex-mixed for 3 min and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 10 min. The supernatant was evaporated to dryness at 30 °C under a stream of nitrogen, and then redissolved in 100 μl of methanol with vortex-mixing for 3 min. The supernatant was acquired after centrifugation at 10
000 × g for 10 min. The supernatant was evaporated to dryness at 30 °C under a stream of nitrogen, and then redissolved in 100 μl of methanol with vortex-mixing for 3 min. The supernatant was acquired after centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 5 min and 5 μl was injected into the UHPLC/ESI-Q-TOF-MS/MS system for further analysis.
000 × g for 5 min and 5 μl was injected into the UHPLC/ESI-Q-TOF-MS/MS system for further analysis.
2.5 Aβ25–35 preparation and cell culture
The core toxic peptide fragment derived from amyloid precursor protein, Aβ25–35,19 was dissolved in distilled water to obtain 1 mg ml−1 solution. Then the solution was incubated at 37 °C for 7 days to induce the aggregation of Aβ25–35, which exhibited strong neurotoxicity.12The PC12 cells were routinely maintained in H-DMEM supplemented with 10% (v/v) heat-inactivated FBS, 100 IU ml−1 penicillin as well as 100 g ml−1 streptomycin at 37 °C with 5% CO2. Culture medium was changed every other day. First, PC12 cells were seeded into 96-well multiplates (1 × 105 cells per ml). 24 h later, cells were pretreated with 0.005 mg ml−1, 0.01 mg ml−1, 0.05 mg ml−1, 0.1 mg ml−1 test samples and 0.242 × 10−3 mg ml−1 huperzine-A, respectively. After 24 h, Aβ25–35 (20 μM) was added and incubated for an extra 24 h.
2.6 Measurement by MTT
MTT (5 mg ml−1) was added into the Aβ25–35 induced PC12 cells and incubated per well for 4 h. MTT reduction assay was used to reveal the effects of the barrigenol type triterpenoid saponins. The MTT formazan crystals were solubilized by DMSO and spectrophotometrically measured at 490 nm. The percentage of cell viability was calculated as follows,Cell viability (%) = OD test/OD control × 100%.
The results were expressed as mean ± standard deviation. The data was analysed with one-way analysis of variance in SPSS 19.0 software.
2.7 Instrumentation and analytical conditions
UHPLC/ESI-Q-TOF-MS/MS analytical procedure was performed on an AB SCIEX TripleTOF™ 5600 quadrupole-time-of-flight hybrid mass spectrometer (Sciex, Redwood City, CA, USA) coupled with an Agilent 1260 HPLC (Billerica, USA). The chromatographic separation was achieved on a high pressure column prefilter (2.1 × 5 mm, 2.7 μm, USA, Agilent) protected Poroshell 120 SB-C18 (2.1 × 100 mm, 2.7 μm, USA, Agilent) column at 25 °C. The mobile phase system consisted of 0.1% formic acid (A) and 0.1% formic acid in acetonitrile (B). The 50.0 min gradient program was as follows, 10% B → 45% B at 0.01–20.00 min; 45% B → 90% B at 20.01–40.00 min; 90% B at 40.01–43.00 min; 10% B remained for the final 7 min. Efficient and symmetrical peaks were achieved at a flow rate of 0.3 ml min−1 with a sample injection volume of 5 μl.The TOF MS and CID MS/MS data was collected in both positive and negative electrospray ionization mode (ESI+/−) with dynamic background subtraction. The optimized operating parameters were listed as follows, source temperature, 550 °C; ion spray voltage, 5500 V (ESI+)/−4500 V (ESI−); nebulizer gas, 50 psi; heater gas, 50 psi; curtain gas, 30 psi; declustering potential, 80 V (ESI+)/−80 V (ESI−); collision energy, 10 V (ESI+)/−10 V (ESI−). Information dependent acquisition were operated by a TOF-MS survey scan 100–1500 Da (100 ms) and up to 8 dependent TOF-MS/MS scans 50–1500 Da (100 ms) using collision energy of 60 V (ESI+)/−60 V (ESI−) with collision energy spread of ± 15 V. Mass tolerance was set as 50 mDa and continuous recalibration was executed every fifth injection using the calibrant delivery system. Operations and acquisition were all edited in the Analyst TF 1.6 software panel (Sciex, USA).
3 Results and discussion
3.1 Chemical profiling by UHPLC/ESI-Q-TOF-MS/MS
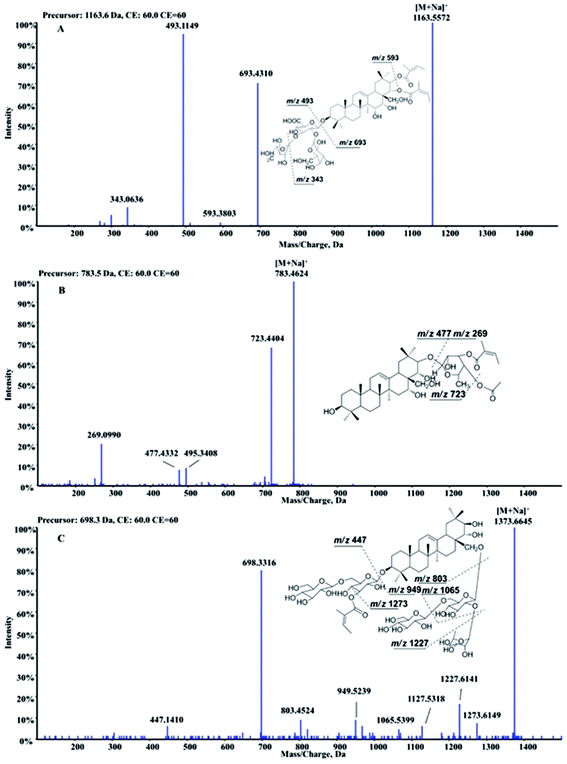
Barrigenol type triterpenoid saponins in X. sorbifolia husks were reported to exhibit multiple bioactivities, including alleviating cognitive deficits, antitumor activities and anti-inflammatory etc.4,10–17 According to the presence of hydroxy at C-15 and/or C-16 and/or C-21 and/or C-22, they can be divided into three groups, known as barringtogenol C, R1-barrigenol and 16-deoxybarringtogenol C.8 PIF and NLF in PeakView® 2.2 software was chosen to fast screen compounds in different types by specifying the values of the corresponding fragment ions or neutral loss molecules.26 The maximum tolerance of mass error was set as 5 ppm. Fragment ions in positive mode were selected to demonstrate the fragment pattern since higher sensitivity and more fragment ions were obtained. A total of 50 triterpenoid saponins were identified preliminarily and their structures were shown in Fig. 1. Among them, 15 triterpenoids were unambiguously identified by comparing with the standards, other 25 triterpenoids were tentatively identified based on the reported information, and the rest 10 were first identified in this study. Information of all the compounds was listed in Table S1† and their XIC spectra were shown in Fig. S1(A and B).†A total of twenty-one R1-barrigenol type triterpenoids were detected in husks extract with the same aglycone structure as shown in Fig. 1. Sugar groups at C-3 as well as angeloyl groups at C-21 and/or C-22 were eliminated easily from the aglycone by CID. As a result, sugar moiety ions such as 493.11 (–GlcA + Gal + Ara) and 343.06 (–GlcA + Gal) were generated in the MS/MS spectra. To illustrate the possible fragmentation pathway in detail, one of the most abundant and widely reported saponins, xanthoceracide (compound 33), was selected as an instance. As shown in Fig. 2(A), the precursor ion [M + Na]+ at m/z 1163.5598 was yielded, indicating a molecular formula of C57H88O23 in ESI(+) mode. Fragment ions at m/z 693.4310 was generated by the loss of the sugar group (–Ara–Gal–GluA) and m/z 593.3803 was further produced by the loss of an angeloyloxy group. In addition, product ions at m/z 493.1149 [Ara + Gal + GluA + Na]+ and m/z 343.0636 [GlcA + Gal–H2O + Na]+ were also observed, indicating the composition of the sugar group at C-3. In the same way, other 14 R1-barrigenol type triterpenoids were identified preliminary,26–30 among which, compounds 22, 34 and 39 were undoubted identified by comparing with the authentic standards. Then PIF and NLF modules were applied to explore unreported compounds. The product ion at m/z 493.11 was taken as an example to illustrate the detailed process. As Fig. S2(A and B)† showed, after filtering process, interference peaks disappeared obviously and target peaks highlighted. After careful analysis, compounds 24 and 27 were picked out particularly since they were not searched in Chemspider online library or previous references. Their MS information indicated that they shared the same molecular formula with compound 31, whereas, their fragment pathways were different (Fig. S2(C and D)).† In the MS/MS spectra of compounds 24 and 27, fragment ion at m/z 709.43 was observed, 116 Da more than m/z 593.38, suggesting the oxidation of one of the angeloyloxy groups. However, the accurate oxidized site was difficult to be confirmed only by the limited mass spectra information. The same strategy offered another four new ones (compounds 32, 37, 43 and 46) and their possible identities were listed in Table S1.†
For barringtogenol C type triterpenoids, functional groups were always substituted at C-21 (–Rha–Ang/–Rha–Ac) and/or C-16/22/28 (–Ac) of the aglycone, where the linkages could be broken easily by CID. Product ions of the aglycone like m/z 477.33 and m/z 495.34 were thus obtained. According to different substituent groups, different fragment ions such as m/z 229.06 refers to [Rha + Ac + Na]+, m/z 269.09 refers to [Rha + Ang + Na]+, m/z 311.10 refers to [Rha + Ang + Ac + Na]+ and m/z 351.14 refers to [Rha + 2Ang + Na]+ were generated. For instance, compound 40 produced the precursor ion at m/z 783.4643 (C43H68O11) as well as a group of characteristic fragment ions at m/z 723.4404, 495.3408 and 477.4332, corresponding to the loss of 60 Da (OAc), 288 Da (Ac + Ang + Rha–H2O) and 306 Da (Ac + Ang + Rha), respectively. Furthermore, product ion at m/z 269.0990 was also generated; suggesting one angeloyl group was substituted on the rhamnose. Based on the above information and previous reports, compound 40 was tentatively identified as 21-O-(4-O-acetyl-3-O-angeloyl)-β-D-fucopyranosyl theasapogenol.27 Its detailed fragment pathway was illustrated in Fig. 2(B). Likewise, compounds 42, 45, 47 and 49 were also tentatively identified.29,30 Particularly, compounds 25 and 28 were highlighted after PIF and NLF since they were not detected in Chemspider online library.
A total of twenty 16-deoxybarringtogenol C type triterpenoids were identified in this study, which shared the same aglycone structure named 16-deoxybarringtogenol C (compound 36). Glycosyl groups consisted of glucose, rhamnose and/or angeloyl group were always substituted at C-3 and/or C-28. Thus, successive or simultaneous losses of sugar groups were observed in their MS/MS spectra. Take compound 10 as an instance (Fig. 2(C)) to explain the fragment pattern. A significant precursor ion [M + 2Na]2+ (m/z 698.3306) in (+) ESI-MS was yielded, suggesting a molecular formula of C65H106O29. Then fragment ions such as m/z 1273.6149, m/z 1227.6141, m/z 1127.5318, m/z 1065.5399, m/z 949.5239 and m/z 821.4626 were produced by the loss of 100 Da (–Ang), 146 Da (–Rha), 246 Da (–Rha–Ang), 308 Da (–Glc–Rha), 424 Da (–2Glc–Ang) and 552 Da (–2Glc–Rha–Ang + H2O), respectively. In addition, fragment ion [2Glc + Ang + Na]+ at m/z 447.1410 was also observed in its MS/MS spectrum, indicating the composition of sugar groups at C-3. Likewise, other nineteen 16-dexoybarringtogenol C type triterpenoids were tentatively identified, among which, compounds 6 and 7 were new.
In conclusion, a practical and reliable UHPLC-ESI-MS/MS method was first established for chemical profiling of barrigenol type triterpenoid saponins in X. sorbifolia husks extract, and a database with detailed information was shown in Table S1,† which was very helpful for further exploration of bioactive substances.
3.2 Detection of barrigenol type triterpenoid saponins in rat plasma after oral administration of X. sorbifolia husks
TCMs usually exhibit advantages on the treatment of chronic and complicated disease for its characteristic of multi-components, -targets and -pathways. However, it is too difficult to illustrate the molecular mechanism and effective substances thoroughly due to the diversity of its components. Intensive study on the efficacy of every chemical component was time-consuming and unreasonable since TCMs exert therapeutic effects depended on the contribution of multiple constituents rather than individual ones. To solve the problem, SPT theory was proposed in early 1990s,31 providing an efficient approach to discovery bioactive constituents which really worked in practice. It stated that most bioactive constituents in TCMs were firstly absorbed into blood after oral administration, and then exerted therapeutic effects after a series of complicated distribution and metabolism. Therefore, constituents in the dosed plasma are more possible to work in practice.Current study on the bioactive substances of the husks against AD was insufficient. To discover the bioactive substances more efficiently, our attention was mainly focused on the constituents absorbed into blood. Their spectral information was collected by UHPLC/ESI-Q-TOF-MS/MS and analysed with PeakView® 2.2 software at the same condition with Section 3.1. The compounds present in the dosed plasma and absent in the blank samples were selected. The prototype compounds could be fast identified by comparison with the information of database established in vitro. Eventually, only 6 prototype compounds, including four 16-deoxybarringtogenol C type triterpenoids, one barringtogenol C type triterpenoid and one R1-barrigenol type triterpenoid were identified in dosed plasma. XIC spectra of all the 6 compounds were shown in Fig. S1(C–F).† Neither phase I nor phase II saponins metabolites were detected in the plasma samples after oral administration of the husks, although a lot of attempts were tried in the pre-experiments, including different pre-treatment methods (precipitation protein by methanol or acetonitrile, liquid–liquid extraction by ethyl acetate and isopropanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), and solid phase extraction by Waters Oasis) and detection of plasma samples at different time points (0.5 h, 1 h, 2 h, 4 h, 6 h, 8 h and 12 h). The results indicated that some of the barrigenol type triterpenoid saponins probably exerted efficacy as prototype components through absorbing into blood, while, others were probably to work in other ways, which needed further study. The speculation was consistent with the characteristics of TCMs, known as multi-components, targets and pathways.
1, v/v), and solid phase extraction by Waters Oasis) and detection of plasma samples at different time points (0.5 h, 1 h, 2 h, 4 h, 6 h, 8 h and 12 h). The results indicated that some of the barrigenol type triterpenoid saponins probably exerted efficacy as prototype components through absorbing into blood, while, others were probably to work in other ways, which needed further study. The speculation was consistent with the characteristics of TCMs, known as multi-components, targets and pathways.
3.3 Effect on Aβ-induced PC12 cells damage
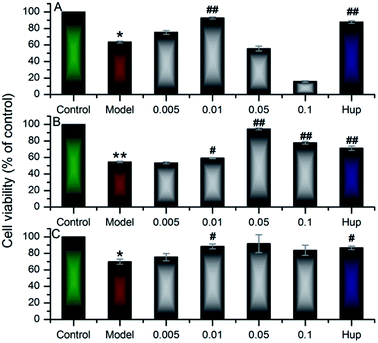
In the work, up to 50 barrigenol type triterpenoid saponins were tentatively identified, which could be divided into three types, known as R1-barrigenol type triterpenoid saponin, 16-deoxybarringtogenol C type triterpenoid saponin and barringtogenol C saponin.8,32 Among them, only one R1-barrigenol type triterpenoid saponin (compound 33), rich in the husks, had been reported as the effective substance against AD.14–18 There are few reports on other saponins for the difficulty of their effective separation and complete identification. As shown in Fig. 1, the structures of saponins in the same type were very similar, and their sugar chains usually contain 3 to 5 sugar residues, suggesting their high structural complexity, which resulted in the difficulty to obtain their standards. Fortunately, more than compound 33, some 16-deoxybarringtogenol C type triterpenoid saponins were also isolated and identified successfully in our previous study,9 two of which (compound 10 and 17) were detected in the dosed rat plasma, implying their potentiality to work in practice according to SPT theory. Since little information was available on their neuroprotection, their neuroprotective effect was further tested by Aβ25–35 induced cytotoxicity on PC12 cells. It was worth mentioning that compared to compound 33, the number and site of sugar groups and angeloyl groups on compound 10 and 17 were different. Therefore, we speculated the neuroprotection of compound 10 and 17 were probably different with the reported compound 33. To verify our speculation, the neuroprotective effect of compound 33 was also tested to compare to compound 10 and 17.The test of Aβ25–35 induced PC12 cells was widely applied to fast screen the compounds with potential neuroprotective effect.14,23–25 After 24 h incubating with the 3 saponins (compounds 10, 17 and 33), the viability of PC12 cells was tested by MTT analysis. Fig. 3 demonstrated that the viability of the model groups, exposure to 20 μM Aβ25–35 for 24 h, significantly degenerated compared with the control groups (p < 0.05), confirming the successful establishment of the model.
As shown in Fig. 3, compound 33 (xanthoceracide) exhibited a significant decrement on Aβ25–35 induced PC12 cell death at a concentration of 0.01 mg ml−1, whereas, it exhibited a strong cytotoxicity at a concentration of 0.1 mg ml−1, which was consistent with previous research.4,14 As a kind of 16-deoxybarringtogenol C type triterpenoid saponin, compound 10 exhibited significant neuroprotective effect at the concentration between 0.01 mg ml−1 and 0.1 mg ml−1, and the best neuroprotective effect was shown at the concentration of 0.05 mg ml−1. Similarly, the other 16-deoxybarringtogenol C type triterpenoid saponin, compound 17, also exhibited significantly neuroprotection at the concentration of 0.01 mg ml−1. At the higher concentration (0.1 mg ml−1), compounds 10 and 17 both exhibited much weaker cytotoxicity than compound 33. This phenomena could be explained by the presence of sugar groups at C-28 as well as the absence of angeloyl groups at C-21 and/or C-22.4,8,32 The result suggested that the three compounds were all the bioactive substances of the husks against AD, but compound 10 and 17 belonging to 16-deoxybarringtogenol C type triterpenoid saponins showed weaker cytotoxicity than compound 33 at higher concentration. The neuroprotection of compound 10 and 17 was reported for the first time, which will be an important reference for the further development of the husks.
4 Conclusions
In this study, a practical and efficient strategy was developed to reveal the bioactive substances of X. sorbifolia husks, systemically. A total of 50 barrigenol type triterpenoids were found in husks extract by an UHPLC-ESI-MS/MS method, 6 of which were detected in the dosed plasma as bioactive candidates based on the SPT theory. The neuroprotection of the candidates was further tested by Aβ25–35 induced cytotoxicity on PC12 cells. As a result, three candidates, known as compounds 10, 17 and 33, exhibited significant neuroprotection against Aβ25–35 induced cytotoxicity on PC12 cells, indicating their potentiality against AD. The neuroprotection of compounds 10 and 17 was reported for the first time. This work provided a significant reference for the study of effective constituents in medicines.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
This work was supported by the Key Technologies of Common Quality Evaluation of New Drugs (Grant No. 2015010201) and National Natural Science Foundation of China (Grant No. U1508220).References
- S. Zhang, Y. G. Zu, Y. J. Fu, M. Luo, W. Liu, J. Li and T. Efferth, Bioresour. Technol., 2010, 101, 2537–2544 CrossRef CAS PubMed.
- Z. Y. Yao, J. H. Qi and L. M. Yin, Renewable Sustainable Energy Rev., 2013, 24, 57–65 CrossRef CAS.
- J. Li, Y. G. Zu, Y. J. Fu, Y. C. Yang, S. M. Li, Z. N. Li and M. Wink, Innovative Food Sci. Emerging Technol., 2010, 11, 637–643 CrossRef CAS.
- W. Xiao, Y. Wang, P. Zhang, N. Li, S. Jiang, J. H. Wang, J. Huang and X. Li, Eur. J. Med. Chem., 2013, 60, 263–270 CrossRef CAS PubMed.
- N. Li, Y. Wang, X. Z. Li, H. Zhang, D. Zhou, W. L. Wang, W. Li, X. R. Zhang, X. Y. Li, Y. Hou and D. L. Meng, Bioorg. Med. Chem. Lett., 2016, 26, 5018–5023 CrossRef CAS PubMed.
- Y. Zhang, J. N. Ma, C. L. Ma, Z. Qi and C. M. Ma, Chin. J. Nat. Med., 2015, 13, 873–880 CAS.
- G. S. Wan, X. B. Wang, L. J. Wu and H. Y. Gao, Chin. Tradit. Herb. Drugs, 2013, 13, 1842–1851 Search PubMed.
- D. Wang, D. Su, X. Z. Li, D. Liu, R. G. Xi, H. Y. Gao and X. B. Wang, RSC Adv., 2016, 6, 27434–27446 RSC.
- J. H. Ling, L. L. Liu, X. Y. Wang, Z. Y. Li, R. Liu, Q. Li, Y. Wang, B. Z. Yang, X. H. Chen and K. S. Bi, J. Pharm. Biomed. Anal., 2011, 55, 259–264 CrossRef CAS PubMed.
- Y. J. Li, J. K. Xu, P. Xu, S. J. Song, P. Liu, T. Y. Chi, X. F. Ji, G. Jin, S. M. Qiu, Y. P. Hou, C. Zheng, L. L. Wang, D. L. Meng and L. B. Zou, Neurosci. Lett., 2016, 629, 208–214 CrossRef CAS PubMed.
- Y. Qi, L. B. Zou, L. H. Wang, G. Jin, J. J. Pan, T. Y. Chi and X. F. Ji, J. Pharmacol. Sci., 2013, 122, 305–317 CrossRef CAS.
- X. F. Ji, T. Y. Chi, Q. Xu, X. L. He, X. Y. Zhou, R. Zhang and L. B. Zou, J. Evidence-Based Complementary Altern. Med., 2014, 2014, 969342 Search PubMed.
- P. Lu, T. Mamiya, L. L. Lu, A. Mouri, T. Ikejima, H. C. Kim, L. B. Zou and T. Nabeshima, Psychopharmacology, 2012, 219, 181–190 CrossRef CAS PubMed.
- P. Liu, L. B. Zou, Q. Jiao, T. Y. Chi, X. F. Ji, Y. Qi, Q. Xu and L. H. Wang, Neurosci. Lett., 2013, 543, 115–120 CrossRef CAS PubMed.
- T. Y. Chi, L. H. Wang, X. F. Ji, L. Shen and L. B. Zou, J. Asian Nat. Prod. Res., 2013, 15, 1013–1022 CrossRef CAS PubMed.
- T. Y. Chi, L. H. Wang, C. Qu, B. Z. Yang, X. F. Ji, Y. Wang, T. Okuyama, O. Shihito and B. Zou, J. Asian Nat. Prod. Res., 2009, 11, 1019–1027 CrossRef CAS PubMed.
- G. Jin, L. H. Wang, X. F. Ji, T. Y Chi, Y. Qi, Q. Jiao, Q. Xu, X. Y. Zhou, R. Zhang and L. B. Zou, Neurosci. Lett., 2014, 573, 58–63 CrossRef CAS PubMed.
- D. L. Meng, L. Shang, X. H. Feng, X. F. Huang and X. Che, Int. J. Pharm., 2016, 506, 184–190 CrossRef CAS PubMed.
- X. J. Wang, A. H. Zhang, H. Sun, Y. Han and G. L. Yan, TrAC, Trends Anal. Chem., 2016, 76, 86–94 CrossRef CAS.
- Q. Liang, H. Liu, H. T. Xing, Y. Jiang, T. Y. Zhang and A. H. Zhang, RSC Adv., 2016, 6, 29863–29868 RSC.
- L. F. Lin, H. M. Lin, M. Zhang, X. X. Dong, X. B. Yin, C. H. Qu and J. Ni, RSC Adv., 2015, 5, 107623–107636 RSC.
- X. Zhang, C. J. Liang, J. T. Yin, Y. P. Sun and L. T. Zhang, RSC Adv., 2018, 8, 11813–11827 RSC.
- J. Nie, Y. Tian, Y. Zhang, Y. L. Lu, L. S. Li and J. S. Shi, PeerJ, 2016, 4, e2739 Search PubMed.
- H. X. Zhang, Y. Z. Cao, L. X. Chen, J. J. Wang, Q. H. Tian, N. Wang, Z. J. Liu, J. Li, N. Wang, X. K. Wang, P. Y. Sun and L. H. Wang, Carbohyd. Polym., 2015, 117, 879–886 CrossRef CAS PubMed.
- C. M. Wang, M. Y. Liu, F. Wang, M. J. Wei, S. Wang, C. F. Wu and J. Y. Yang, Pharmacol., Biochem. Behav., 2013, 106, 57–67 CrossRef CAS PubMed.
- Z. L. Li, X. Li, L. H. Li, N. Li, M. Yu and D. L. Meng, Planta Med. Lett., 2005, 71, 1068–1070 CrossRef CAS PubMed.
- G. S. Wan, X. B. Wang, L. J. Wu and H. Y. Gao, Chin. Herb. Med., 2013, 44, 1842–1851 CAS.
- W. Li, X. Li, D. L. Meng, P. Zhang and Z. L. Li, J. Asian Nat. Prod. Res., 2007, 9, 7–11 CrossRef CAS PubMed.
- Y. J. Chen, T. Takeda and Y. Ogihara, Chem. Pharm. Bull., 1985, 33, 127–134 CrossRef CAS.
- Y. J. Chen, T. Takeda and Y. Ogihara, Chem. Pharm. Bull., 1985, 33, 1387–1394 CrossRef CAS.
- X. J. Wang, China J. Chin. Mater. Med., 2006, 31, 789–792 Search PubMed.
- D. Wang, D. Su, B. Yu, C. M. Chen, L. Cheng, X. Z. Li, R. G. Xi, H. Y. Gao and X. B. Wang, Fitoterapia, 2017, 116, 51–60 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra01765d |
| This journal is © The Royal Society of Chemistry 2018 |