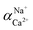Open Access Article
Open Access ArticlePartially reduced graphene oxide and chitosan nanohybrid membranes for selective retention of divalent cations†
Yangyang Wei a,
Jian Wanga,
Hao Lia,
Man Zhao
a,
Jian Wanga,
Hao Lia,
Man Zhao a,
Huifeng Zhanga,
Yipeng Guana,
Hai Huanga,
Baoxia Mi
a,
Huifeng Zhanga,
Yipeng Guana,
Hai Huanga,
Baoxia Mi b and
Yushan Zhang*a
b and
Yushan Zhang*a
aThe Institute of Seawater Desalination and Multipurpose Utilization, State Oceanic Administration, Tianjin 300192, China. E-mail: yszhang_membr@163.com
bDepartment of Civil and Environmental Engineering, University of California, Berkeley, CA 94720, UK. E-mail: mib@berkeley.edu
First published on 11th April 2018
Abstract
A tremendous quantity of brackish water with a high proportion of divalent cations is in great need of water softening. Layer-stacked graphene oxide membranes show potential in membrane processing due to their molecular sieving properties, but show poor selective retention of cations due to unstable interlayer spacing and electrostatic interaction. In this study, a partially reduced graphene oxide (prGO) and chitosan (CS) nanohybrid membrane (prGO–CS) was fabricated to achieve the selective retention of divalent cations by adjusting the configuration and controlling the surface charge. The prGO–CS membrane, which included a CS skin and embedded prGO sheets, showed a performance boost of 98.0% rejection of Mg2+ and 95.5% rejection of Ca2+ when compared with a CS membrane. The membrane showed good water softening performance for brackish water under low operation pressure with a high Na+/Mg2+ selectivity of 33.8. The excellent performance was attributed to the dense structure and positive charge of prGO–CS.
Introduction
The exploitation of a tremendous amount of brackish water in some typically arid areas, such as northwestern China and southern America, is of particular significance in order to alleviate local water scarcity. However, the divalent cations (Mg2+ and Ca2+) in brackish water represent a major hindrance due to their scaling tendencies in practical use. Membrane processing has been applied in brackish water softening, but the selective retention of divalent cations still needs to be improved.Graphene oxide (GO) shows great potential in membrane processing with attributes such as film-forming properties and molecular sieving.1,2 The layer-stacked GO membrane has 2D negatively charged nano-channels between adjacent GO sheets, which are employed for the removal of high valence anions due to their physical sieving behaviour and electrostatic repulsion.3,4 However, the interlayer spacing (d-spacing) of the 2D nano-channels became too large to reject ions due to the swelling effect of the GO membrane in water.5 The reduction of GO was reported to be an effective strategy to regulate the nano-channels,6 although over-reduction may result in low water permeability. In addition, electrostatic attraction between the negatively charged GO and positively charged cations weakens the barrier effect of the “separator” layer for the retention of cations. To overcome these problems, GO-based membranes with a dense structure and desirable charge bearing properties are favoured and achieved via configuration adjustment7–11 and surface charge controlling.12,13 Consequently, we present a strategy of building an interaction between GO and positively charged polymers to gain a dense structure and desirable surface charge simultaneously.
Chitosan (CS), a natural polymer with positively charged amino groups, displays excellent reactivity with GO and ease of film-forming.14,15 CS and GO based membranes were reported to have denser structures than the bare CS membrane and showed an improved performance in ethanol–water separation, proton conductivity and mechanical strength.16,17 Until now, only a few GO–CS desalination membranes have been reported, which focused on the removal of anions and sodium ions. Gao and coworkers reported a negatively charged composite membrane of O-(carboxymethyl)-CS and GO with a nanoporous structure and high rejection of a divalent anion (Na2SO4, 92.9%), but a moderate rejection of a monovalent anion (NaCl, 62.0%).18 Zou and coworkers developed an additional layer of GO and CS on a commercial reverse osmosis membrane (BW30LE).19 The salt rejection of NaCl increased from 88.7% to 95.6%. However, the selective retention of cations was still not investigated for a GO and CS hybrid membrane.
In this work, we propose a facile strategy to tailor partially reduced GO (prGO) and CS hybrid membranes. The partial reduction of GO sheets was undertaken to gain reduced d-spacing and decrease negative charges without losing solution processability. A prGO–CS membrane with a CS skin and embedded prGO sheets showed a positive charge and dense structure, which resulted in a high rejection of Mg2+ and Ca2+ without lowering the water flux. Moreover, the nanohybrid membranes exhibited high Na+/Mg2+ selectivity and demonstrated great promise in brackish water softening under low operating pressures (5 bar or 10 bar).
Experimental
Materials
Natural graphite (∼200 μm), H2SO4 (≥98%), KMnO4 (≥99%), aqueous H2O2 (30 wt%) and glutaraldehyde (50 wt%) were purchased from Sigma-Aldrich. Chitosan (viscosity > 400 mPa s) was supplied from Aladdin Reagents (Shanghai). Acetic acid, NaCl, MgCl2, CaCl2 and Na2SO4 were purchased from Sinopharm Chemical Reagent. Ultrafiltration polysulfone (PS) membranes with an average pore size of 0.1 μm used as supports were purchased from Shenzhen Jiaquan Water Treatment Science & Technology Co. Ltd. Ultrapure water produced from Merckmillipore was used throughout the study.Synthesis of GO and prGO
A modified Hummers’ method was used to prepare the GO aqueous dispersion from natural graphite.20 150 mL sulfuric acid was added dropwise to a flask with 5 g natural graphite flakes and the mixture was kept stirring for 0.5 h. Then 15 g KMnO4 was added slowly under vigorous stirring. The mixture was heated to 40 °C, stirred for 6 h, and poured into an ice water bath and allowed to cool to room temperature. Then H2O2 (30 wt%) was added dropwise into the mixture until the colour turned bright yellow. GO solution was obtained via centrifugation and alternate washing with HCl solution (1 mol L−1) and water. Finally, prGO was obtained via the thermochemical reduction of the GO aqueous dispersion at 100 °C for 1 h.Fabrication of hybrid membranes based on prGO and CS
The CS membrane was fabricated by a blade-coating method. Chitosan (2 wt%) dissolved in an aqueous acetic acid (2 wt%) solution was blade coated on PS membranes and kept flat in dust-free air for 1 day. Then the CS membrane was immersed into NaOH solution (0.2 mol L−1) at 0 °C for 3 h to remove the residual acetic acid. The membrane was then immersed in a mixture of sulfuric acid (1 wt%) and glutaraldehyde (1 wt%) solution for 1.5 h at 60 °C to completely crosslink the CS matrix. The used aqueous NaOH, sulfuric acid and glutaraldehyde could be utilized repeatedly several times to reduce chemical discharge. Finally, the crosslinked CS membrane was thoroughly washed with water. The prGO–CS membrane was prepared by blade coating the prGO–CS composite on the PS membrane followed by the crosslinking process as above. The prGO–CS composite was synthesized via the chemical reaction between prGO (0.01 wt%) and CS (2 wt%) under mild stirring at room temperature. The GO and prGO membranes were prepared by the pressure-assisted assembly (2 bar) of GO and prGO solution on the substrate of the PS membrane for comparison of surface charge.21 All the prepared membranes above were preserved after being dried at 40 °C for 24 h.Instrumentation
Membrane casting equipment (Elcometer 4340) was used to blade coat the CS casting solution on the PS substrate to prepare the CS membranes. The morphology of the nanosheets (GO and prGO) and the membranes was observed by scanning electron microscopy (SEM, FEI Nova NanoSEM 450) and atomic force microscopy (AFM, AIST-NT SPM Smart SPM™-100). The chemical structures of the nanosheets and the surface chemistry of the membranes were analyzed using attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR, Thermo Scientific Nicolet iS50). An X-ray diffractometer (XRD, Rigaku MiniFlex 600) in Brag–Brentano geometry (θ and 2θ coupled) was used to confirm the crystalline structures of the nanosheets with a Cu Kα X-ray source (λ = 0.154 nm) at 40 kV and 15 mA. The samples were scanned from 5° to 15° (2θ) with a step size of 0.02° and a count time of 0.6 s at each point. Thermogravimetric analysis (TGA) was carried out using a thermogravimetric analyser (NETZSCH TG209) under a nitrogen atmosphere from room temperature to 850 °C at a 10 °C min−1 heating rate. X-ray photoelectron spectroscopy (XPS) was performed using a K-alpha (Thermo Fisher) system to characterize the elementary composition and chemical bonding configuration of the membranes. All binding energies were referenced to the C 1s neutral carbon peak at 284.8 eV. The surface wettability was characterized on a contact angle goniometer (Shanghai zhongchen JC2000D2). The zeta potential was measured on an electrokinetic analyzer (Anton Paar SurPASS™ 3) based on streaming current measurement. The solute concentration in the permeate and feed solution was evaluated respectively by an electrical conductivity meter for single salt feed solution and inductively coupled plasma mass spectrometry (ICP, Thermo Fisher iCAP Q) for mixed salt feed solution.Test of membrane performances for the selective retention of divalent cations
The flux and salt rejection were measured by a lab made cross-flow test device. The effective membrane surface area was 19.6 cm2. Membranes were pressurized at 5 bar for 1 h to achieve a stable water flux. The permeation and rejection were measured using 1 g L−1 single salt solution (MgCl2, CaCl2, Na2SO4 and NaCl) as feeds at 25 °C. Membrane permeation flux (J, L m−1 h−1 bar−1), rejection (R, %), Na+/Mg2+ selectivity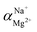 and Na+/Ca2+ selectivity
and Na+/Ca2+ selectivity 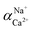 were calculated using eqn (1), (2), (3) and (4) respectively.22
were calculated using eqn (1), (2), (3) and (4) respectively.22
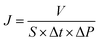 | (1) |
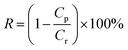 | (2) |
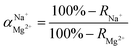 | (3) |
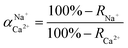 | (4) |
Results and discussion
Characterization of GO and prGO nanosheets
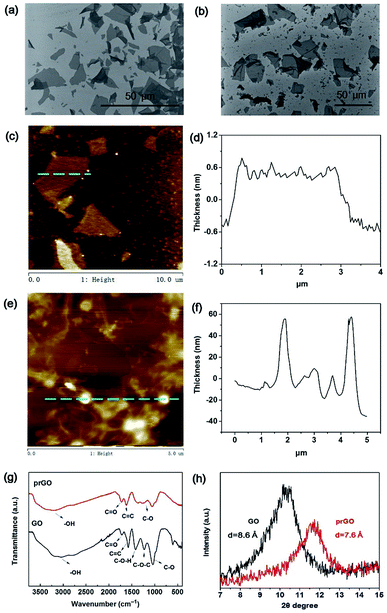
The surface topography of GO and prGO nanosheets was characterized by SEM and AFM (Fig. 1). The size distribution of the GO sheets fell into the range of 5 to 30 μm (Fig. 1a) and the thickness was 1.0 nm (Fig. 1c and d) which confirmed the successful exfoliation from graphite.23 In contrast, the prGO sheets presented a more corrugated morphology (Fig. 1b) and were small sheets curled up into nanoparticles with a diameter of 20–60 nm (Fig. 1e and f). This is because the oxygen-containing groups were partially reduced and the electrostatic repulsion in the prGO nanosheets was weakened after thermal treatment.24 The prGO sheets still showed stable dispersion in water owing to the residual oxygen-containing groups, which provided solution processability in order to fabricate the membranes (Fig. S1†).The chemical structure and elemental composition of GO and prGO were characterized by FTIR, Raman, XRD and XPS spectroscopy and TGA analysis. The FTIR characteristic peaks of GO (Fig. 1g) were the O–H stretching vibration (3230 cm−1), the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration (1725 cm−1) from the carboxyl groups and the C–O–C stretching vibration (1159 cm−1) from the epoxy groups.23 After thermal treatment, the decreased intensities of the C
O stretching vibration (1725 cm−1) from the carboxyl groups and the C–O–C stretching vibration (1159 cm−1) from the epoxy groups.23 After thermal treatment, the decreased intensities of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O–C stretching vibration of prGO demonstrated the partial reduction process.24 Then, XRD was carried out to confirm the interlayer distance of the nanosheets (Fig. 1h) according to the Bragg equation. The dried GO sheets exhibited an intense peak at 10.3° with a d-spacing calculated to be 8.6 Å. Compared to the GO sheets, the prGO sheets showed a weakened peak at 11.7° corresponding to a d-spacing value of 7.6 Å. The decreased d-spacing and shifted peak position of prGO demonstrated the partial reduction of GO and the narrower d-spacing of prGO.25 The Raman spectra (Fig. S2†) of GO and prGO showed the typical D peak (1347 cm−1) and G peak (1592 cm−1) for GO and a peak at 1587 cm−1 for prGO. The corresponding G peak of GO down-shifted from 1592 cm−1 to 1587 cm−1 after the thermal reduction. This resulted from the decrease in isolated double bonds resonating at a high frequency and the recovery of a hexagonal network of carbon atoms from prGO. The intensity ratio ID/IG of prGO (1.03) was lower than that of GO (1.05), which revealed that thermal reduction recovered the graphitic structure and reduced some defects.26 XPS spectra were measured to determine the relative oxidation levels of GO and prGO. The C/O ratios of GO and prGO were 1.85 and 2.13 respectively, which proved there was a lower oxidation level for prGO. Thermal stability and degradation behaviors of the chemical bonds of GO and prGO were evaluated by TGA (Fig. S2†). GO and prGO foams were freeze-dried from aqueous dispersion and used as samples. First, the weight loss of GO (9.50%) and prGO (8.20%) over the temperature range of 25–150 °C was caused by water evaporation in the porous foams. The steep weight loss of GO and prGO between 150 and 235 °C corresponded to the release of CO2 and H2O from the decomposition of the labile groups. A small mass loss between 235 and 950 °C was attributed to the removal of more stable oxygen functionalities. The reduced water evaporation (8.20% for prGO and 9.50% for GO over the temperature range 25–150 °C) and larger residual weight of prGO (53.39% for prGO and 33.42% for GO) demonstrated the better thermostability of prGO, which was credited to fewer oxygen-containing groups.
O and C–O–C stretching vibration of prGO demonstrated the partial reduction process.24 Then, XRD was carried out to confirm the interlayer distance of the nanosheets (Fig. 1h) according to the Bragg equation. The dried GO sheets exhibited an intense peak at 10.3° with a d-spacing calculated to be 8.6 Å. Compared to the GO sheets, the prGO sheets showed a weakened peak at 11.7° corresponding to a d-spacing value of 7.6 Å. The decreased d-spacing and shifted peak position of prGO demonstrated the partial reduction of GO and the narrower d-spacing of prGO.25 The Raman spectra (Fig. S2†) of GO and prGO showed the typical D peak (1347 cm−1) and G peak (1592 cm−1) for GO and a peak at 1587 cm−1 for prGO. The corresponding G peak of GO down-shifted from 1592 cm−1 to 1587 cm−1 after the thermal reduction. This resulted from the decrease in isolated double bonds resonating at a high frequency and the recovery of a hexagonal network of carbon atoms from prGO. The intensity ratio ID/IG of prGO (1.03) was lower than that of GO (1.05), which revealed that thermal reduction recovered the graphitic structure and reduced some defects.26 XPS spectra were measured to determine the relative oxidation levels of GO and prGO. The C/O ratios of GO and prGO were 1.85 and 2.13 respectively, which proved there was a lower oxidation level for prGO. Thermal stability and degradation behaviors of the chemical bonds of GO and prGO were evaluated by TGA (Fig. S2†). GO and prGO foams were freeze-dried from aqueous dispersion and used as samples. First, the weight loss of GO (9.50%) and prGO (8.20%) over the temperature range of 25–150 °C was caused by water evaporation in the porous foams. The steep weight loss of GO and prGO between 150 and 235 °C corresponded to the release of CO2 and H2O from the decomposition of the labile groups. A small mass loss between 235 and 950 °C was attributed to the removal of more stable oxygen functionalities. The reduced water evaporation (8.20% for prGO and 9.50% for GO over the temperature range 25–150 °C) and larger residual weight of prGO (53.39% for prGO and 33.42% for GO) demonstrated the better thermostability of prGO, which was credited to fewer oxygen-containing groups.
Characterization of hybrid membranes based on prGO and CS
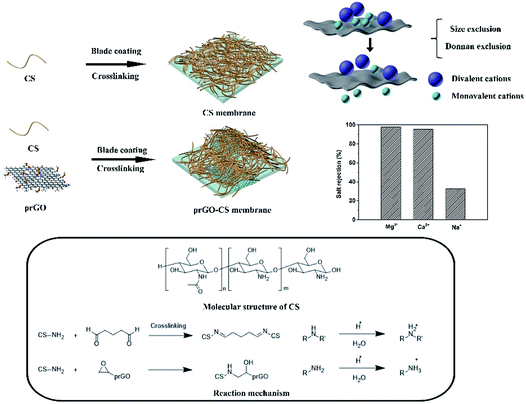
As shown in Scheme 1, the CS membrane was prepared by blade coating a CS dispersion onto a PS membrane and then was crosslinked by glutaraldehyde to form a Schiff base (R–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N).27 The prGO–CS membrane was fabricated by blade coating the prGO–CS composite on a PS membrane with embedded prGO sheets in a CS skin through the ring-opening reaction between the epoxy and amino groups.14 Residual primary amine continued to be crosslinked by glutaraldehyde. The primary amine from CS (CS–NH2) and secondary amine from prGO–CS (CS–NH–CH2–CHOH–prGO) also participated in the protonation process, which endowed membranes with positive charges.
N).27 The prGO–CS membrane was fabricated by blade coating the prGO–CS composite on a PS membrane with embedded prGO sheets in a CS skin through the ring-opening reaction between the epoxy and amino groups.14 Residual primary amine continued to be crosslinked by glutaraldehyde. The primary amine from CS (CS–NH2) and secondary amine from prGO–CS (CS–NH–CH2–CHOH–prGO) also participated in the protonation process, which endowed membranes with positive charges.
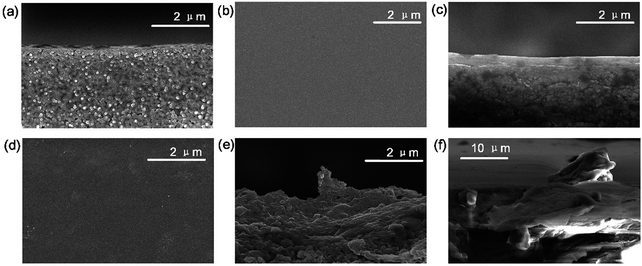
In order to characterize the surface and cross section morphology of the membranes, SEM images are presented. Compared with the smooth morphology of the CS membrane (Fig. 2b and c), angular and wrinkled prGO nanosheets were observed in the prGO–CS membrane (Fig. 2d and e) because of the embedded architecture of the prGO nanosheets in CS. A free-standing prGO–CS membrane was fabricated to further reveal the embedded morphology of prGO in the CS layer on a large scale (Scheme 1 and Fig. 2f), which resulted from π–π self-stacking of the prGO sheets and the chemical reaction in the prGO–CS composite.28
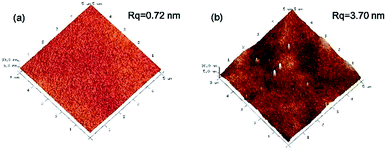
AFM images (Fig. 3) are presented to characterize the surface roughness of the membranes. The CS membrane exhibited an ultra-smooth surface (Rq = 0.72 nm). The roughness of the prGO–CS membrane (Rq = 3.70 nm) was a little higher than the value for the CS membrane. The reason might be that the prGO sheets with sharp bulges were wrapped by CS molecules and then softened.
To characterize the wettability and surface properties of the membranes, photographs of the surface contact angle are presented (Fig. 4). After coating with CS, the surface contact angle of PS decreased from 74.5° to 23.0°, which resulted from the introduction of hydrophilic functional groups (amino, hydroxyl, etc). The surface contact angle of the prGO–CS membrane (25.7°) was slightly larger than that of the CS membrane (23.0°), revealing similar surface properties between CS and prGO–CS (Fig. 4b and c). These similar surface properties were supported by the similar water permeability between the CS and prGO–CS membranes (Fig. 6d).
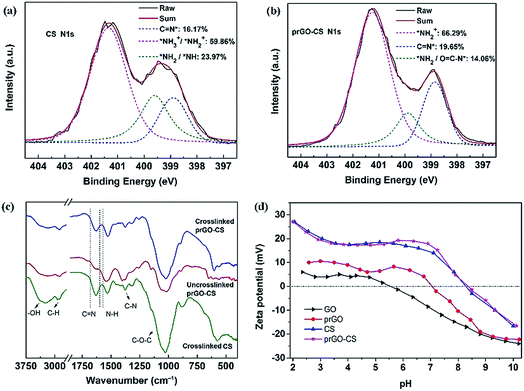
XPS was used to analyze the elementary composition and verify the valence bond structure of the membranes by peak-differentiation-imitating analysis (Fig. S3† and Fig. 5a and b). The prGO–CS membrane had a lower amount of nitrogen than the CS membrane verifying the existence of N-free prGO nanosheets (Fig. S3†). Similarly, the prGO–CS membrane had a lower intensity of C–N and N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O than the CS membrane for the C 1s peak because of the introduction of prGO in CS (Fig. S3†). The prGO–CS membrane had a higher intensity of NH2+/NH3+ and C
O than the CS membrane for the C 1s peak because of the introduction of prGO in CS (Fig. S3†). The prGO–CS membrane had a higher intensity of NH2+/NH3+ and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N than the CS membrane for the N 1s peak (Fig. 5a and b). The increased intensity of NH2+/NH3+ and decreased intensity of NH/NH2 were mainly caused by the ring-opening reaction in prGO–CS and the protonation of the amino groups.29 The increased intensity of C
N than the CS membrane for the N 1s peak (Fig. 5a and b). The increased intensity of NH2+/NH3+ and decreased intensity of NH/NH2 were mainly caused by the ring-opening reaction in prGO–CS and the protonation of the amino groups.29 The increased intensity of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N showed the intensification of the cross-linking reaction by glutaraldehyde. Both of the ring-opening and cross-linking reactions gave the prGO–CS membrane a denser structure than the CS membrane, which was also demonstrated by the desalination performance outlined in the following text.
N showed the intensification of the cross-linking reaction by glutaraldehyde. Both of the ring-opening and cross-linking reactions gave the prGO–CS membrane a denser structure than the CS membrane, which was also demonstrated by the desalination performance outlined in the following text.
The FTIR-ATR spectra (Fig. 5c) were recorded to analyze the chemical structure of the CS and prGO–CS membranes. Free-standing CS and prGO–CS membranes were tested in order to eliminate the influence of the PS membrane. The FTIR characteristic peaks of CS and prGO–CS are the O–H stretching vibration (3261 cm−1), the C–H stretching vibration (2901 cm−1), the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibration (1630 cm−1), the N–H stretching vibration (1530 cm−1) and the C–O–C stretching vibration (1026 cm−1).30 The higher intensity of C
N stretching vibration (1630 cm−1), the N–H stretching vibration (1530 cm−1) and the C–O–C stretching vibration (1026 cm−1).30 The higher intensity of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N in the crosslinked prGO–CS than that in the uncrosslinked one proved the formation of a Schiff base from the amino and aldehyde groups. The higher ratio of N–H to C
N in the crosslinked prGO–CS than that in the uncrosslinked one proved the formation of a Schiff base from the amino and aldehyde groups. The higher ratio of N–H to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N in prGO–CS than that in CS indicated the occurrence of the ring-opening reaction.
N in prGO–CS than that in CS indicated the occurrence of the ring-opening reaction.
The thermal stability and degradation behavior of the CS and prGO–CS free-standing membranes were evaluated by TGA (Fig. S4†). Similar decomposition temperatures of CS and prGO–CS were observed. A major weight loss between 200 °C and 340 °C was likely due to CS decomposition.30 A slow weight loss between 340 °C and 900 °C was due to the partial decomposition of prGO. Compared with prGO–CS, CS showed a little more water evaporation over the temperature range of 25–150 °C, which could be ascribed to the stronger hydrophilicity of CS and is supported by the contact angle data (Fig. 4). This phenomenon also demonstrated the consumption of the hydrophilic groups (amino and hydroxyl groups) during the reaction between CS and prGO and the high chemical stability of the prGO–CS membrane.
The surface charges of the membranes were found to play an important role in salt rejection based on the well-known Donnan exclusion effect.31 Therefore, the surface charge properties of the membranes in this work were studied by streaming zeta potential measurement (Fig. 5d). The surface charge was affected by the inherent charges, which resulted from protonation and dissociation of the surface functional groups. Due to the existence of oxygen-containing groups (–OH and –COOH), GO showed a negative charge over the pH range of 5.5–10. In contrast, prGO showed an isoelectric point (IEP) of pH 7.0 because of the thermal reduction of the oxygen-containing groups and the rebuilding of the carbon structure. The CS and prGO–CS membranes showed a positive charge over a pH range of 2–8.3, which covers the pH of brackish water with low salinity. Amino groups with lone pair electrons in CS could combine with a hydrogen ion and form positively charged ammonium. According to the Donnan exclusion effect, the positively charged CS and prGO–CS membranes (pH < 8.3) would result in a favorable electro-static repulsion to cations.18 Therefore, it is important to test the desalination performance of the nanohybrid membranes and explore the operating mechanism.
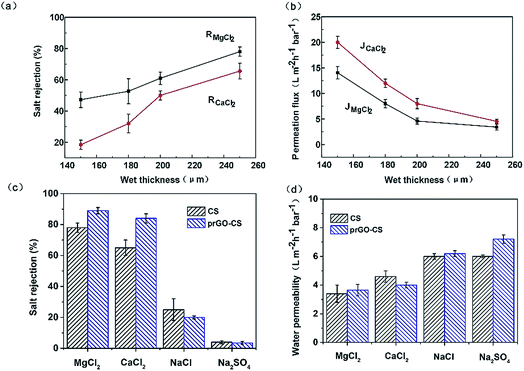
To evaluate the performance of the nanohybrid membranes, water flux and salt rejection of the membranes with different thicknesses of the CS layer and different assembly modes are shown in Fig. 6. As the thickness of the CS layer in wet conditions increased from 150 to 200 μm, salt rejection increased and water flux decreased rapidly (Fig. 6a and b). When the thickness changed from 200 to 250 μm, salt rejection increased slowly and water flux remained stable at around 20 L m−2 h−1 with a downward trend. Thus we studied the operating mechanism of different assembly methods on the premise that the thickness of the CS layer in wet conditions was 250 μm.
All membranes in this study followed a similar order for rejecting salts, i.e. R(MgCl2) > R(CaCl2) > R(NaCl) > R(Na2SO4). On one hand, divalent cations (Mg2+ and Ca2+) had a stronger electrostatic repulsion to the positively charged membrane than the monovalent anion (Na+). The Donnan exclusion effect determined the order of rejected cations, i.e. R(Mg2+) > R(Ca2+) > R(Na+) and the order of rejected anions, i.e. R(Cl−) > R(SO42−). On the other hand, size exclusion determined the order of rejected cations. The hydrated ionic diameter of Mg2+, Ca2+ and Na+ is 8.56 Å, 8.24 Å and 7.16 Å.32 Thus, the observed salt rejection order, i.e. R(Mg2+) > R(Ca2+) > R(Na+), was supported.
The desalination mechanism for the membranes in this work can be summed up as the synergy between size exclusion theory and Donnan exclusion theory.31 For the rejection of Ca2+ and Mg2+, the order was prGO–CS > CS (Fig. 6c). This proved that prGO played an important role in the increased rejection of divalent cations, which was due to the decreased d-spacing of prGO (7.6 Å) and increased compactness of the membrane by chemical bonding between prGO and CS (Fig. 5). In addition, prGO–CS showed a higher zeta potential than the CS membrane over a pH range of 6–8.3 and had a stronger electrostatic repulsion to divalent cations. It is worth mentioning that the water fluxes of the prGO–CS membrane (about 20 L m−2 h−1) were not lower than those of the CS membrane, though they showed a nice performance boost in the removal of Ca2+ and Mg2+. This was probably due to the similar surface properties (Fig. 3 and 4) and water channels created in the prGO–CS composite.
The prGO–CS membrane showed nearly 90% rejection of MgCl2 and nearly 85% rejection of CaCl2 under a low pressure of 5 bar. The salt rejection of the membranes was less than 20% for NaCl and less than 10% for Na2SO4. The comparison of the performance of prGO–CS with several other flat-sheet positively charged membranes is shown in Table 1.33–37 prGO–CS exhibited excellent performance in terms of Na+/Mg2+ selectivity. This indicates that the prGO and CS nanohybrid membranes show great potential for the separation of univalent and divalent cations.
| Membrane | RMgCl2 (%) | RCaCl2 (%) | RNaCl (%) | RNa2SO4 (%) | JMgCl2 (L m−2 h−1 bar−1) | JCaCl2 (L m−2 h−1 bar−1) | Testing condition | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| prGO–CS | 89.3 | 84.3 | 20.3 | 3.5 | 3.7 | 4.0 | 1 g L−1, 5 bar | 7.4 | This work |
| CS | 87.7 (Mg2SO4) | — | 27.8 | — | — | — | 2 g L−1, 4.8 bar | 5.9 | 33 |
| PSDA | 66.1 | 65.8 | 37.2 | 20.1 | 6.0 | 6.0 | 1 g L−1, 5 bar | 1.9 | 34 |
| PEC-NFM-5 | 94.0 | — | 37.9 | 6.2 | 2.2 | — | 1 g L−1, 6 bar | 10.4 | 35 |
| DA/PEIc | 89.3 | — | 27.3 | — | 5.5 | — | 1 g L−1, 8 bar | 6.8 | 36 |
| PAN/PEIc | 92.8 | — | 61.3 | — | 1.6 | — | 2 g L−1, 10 bar | 5.4 | 36 |
| HACC | 83.1 | — | 13.7 | 12.5 | 3.6 | — | 1 g L−1, 3 bar | 5.1 | 37 |
The prGO–CS membrane in this work showed a decreased rejection of divalent cations (70% rejection for MgCl2 and CaCl2) using high-concentration solutions (0.1 mol L−1). This phenomenon was due to the weakened Donnan exclusion effect. The degree of compactness of the membrane is the determining factor that affects rejection performance based on the synergistic mechanism. The method of increasing the crosslinking reaction time was tried to further explore the desalination mechanism. The prGO–CS membrane with the longer crosslinking reaction time showed a high rejection of divalent cations (90% rejection for MgCl2 and 85% rejection for CaCl2) when using high-concentration solutions (0.1 mol L−1), although the flux decreased (less than 1 L m−2 h−1 bar−1). The experimental results demonstrated that there is synergy between the size exclusion theory and Donnan exclusion theory for prGO–CS membranes.
In order to simulate practical application in brackish water, we designed a cross-flow filtration experiment in 3000 ppm TDS mixed salt feed solution.38 As shown in Table 2, the prGO–CS membrane showed a rejection of 98.0% towards MgCl2, rejection of 95.5% towards CaCl2 and a high Na+/Mg2+ selectivity of 33.8, which demonstrated its promise in brackish water softening. This is attributed to the dense structure, controllable positive charge and water channels in the prGO and CS nanohybrid membranes.
Conclusions
In summary, a facile method of configuration adjustment and surface charge control was developed to fabricate prGO and CS nanohybrid membranes to block divalent cations and allow monovalent cations through. prGO sheets were synthesized to deliver both reduced d-spacing and good solution processability. The prGO–CS membranes with a CS skin and embedded prGO sheets showed a desalination performance improvement of nearly 98.0% rejection of MgCl2 and nearly 95.5% rejection of CaCl2, which was attributed to the dense structure and positive charge of prGO–CS. The high Na+/Mg2+ selectivity of the prGO–CS membrane demonstrates a great prospect for brackish water softening and shows potential applications in classification and concentration, such as descaling for circulation cooling water systems, demineralizing and concentrating for whey protein and the removal of BOD/COD for devices under low system pressure.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Key Research and Development Program of China (2016YFE0102400), Special Fund for Basic Scientific Research Business of Central Public Research Institutes (K-JBYWF-2016-G13, K-JBYWF-2015-T12, K-JBYWF-2017-T12, and K-JBYWF-2016-T12), National Natural Science Foundation of China (No. 21606059) and Regional Demonstration Project of Marine Economic Innovation and Development (BHSF2017-12).Notes and references
- P. Sun, K. Wang and H. Zhu, Adv. Mater., 2016, 28, 2287–2310 CrossRef CAS PubMed.
- R. K. Joshi, P. Carbone, F. C. Wang, V. G. Kravets, Y. Su, I. V. Grigorieva, H. A. Wu, A. K. Geim and R. R. Nair, Science, 2014, 343, 752–754 CrossRef CAS PubMed.
- L. Huang, M. Zhang, C. Li and G. Shi, J. Phys. Chem. Lett., 2015, 6, 2806–2815 CrossRef CAS PubMed.
- G. Liu, W. Jin and N. Xu, Chem. Soc. Rev., 2015, 44, 5016–5030 RSC.
- S. Zheng, Q. Tu, J. J. Urban, S. Li and B. Mi, ACS Nano, 2017, 11, 6440–6450 CrossRef CAS PubMed.
- Y. H. Xi, Z. Liu, J. Ji, Y. Wang, Y. Faraj, Y. Zhu, R. Xie, X. J. Ju, W. Wang, X. H. Lu and L. Y. Chu, J. Membr. Sci., 2018, 550, 208–218 CrossRef CAS.
- L. Qiu, X. Zhang, W. Yang, Y. Wang, G. P. Simon and D. Li, Chem. Commun., 2011, 47, 5810 RSC.
- Y. Han, Z. Xu and C. Gao, Adv. Funct. Mater., 2013, 23, 3693–3700 CrossRef CAS.
- B. Mi, Science, 2014, 343, 740–742 CrossRef CAS PubMed.
- X. Xu, F. Lin, Y. Du, X. Zhang, J. Wu and Z. Xu, ACS Appl. Mater. Interfaces, 2016, 20, 12588–12593 Search PubMed.
- J. Abraham, K. S. Vasu, C. D. Williams, K. Gopinadhan, Y. Su, C. T. Cherian, J. Dix, E. Prestat, S. J. Haigh, I. V. Grigorieva, P. Carbone, A. K. Geim and R. R. Nair, Nat. Nanotechnol., 2017, 12, 546–550 CrossRef CAS PubMed.
- Q. Nan, P. Li and B. Cao, Appl. Surf. Sci., 2016, 387, 521–528 CrossRef CAS.
- X. Song, R. S. Zambare, S. Qi, B. N. Sowrirajalu, A. P. James Selvaraj, C. Y. Tang and C. Gao, ACS Appl. Mater. Interfaces, 2017, 47, 41482–41495 Search PubMed.
- L. Shao, X. Chang, Y. Zhang, Y. Huang, Y. Yao and Z. Guo, Appl. Surf. Sci., 2013, 280, 989–992 CrossRef CAS.
- S. P. Dharupaneedi, R. V. Anjanapura, J. M. Han and T. M. Aminabhavi, Ind. Eng. Chem. Res., 2014, 53, 14474–14484 CrossRef CAS.
- A. Shirdast, A. Sharif and M. Abdollahi, J. Power Sources, 2016, 306, 541–551 CrossRef CAS.
- S. Sayyar, E. Murray, S. Gambhir, G. Spinks, G. G. Wallace and D. L. Officer, J. Occup. Med., 2016, 68, 384–390 CAS.
- J. Wang, X. Gao, J. Wang, Y. Wei, Z. Li and C. Gao, ACS Appl. Mater. Interfaces, 2015, 7, 4381–4389 CAS.
- H. M. Hegab, Y. Wimalasiri, M. Ginic-Markovic and L. Zou, Desalination, 2015, 365, 99–107 CrossRef CAS.
- Z. Xu, H. Sun, X. Zhao and C. Gao, Adv. Mater., 2013, 25, 188–193 CrossRef CAS PubMed.
- W.-S. Hung, Q.-F. An, M. De Guzman, H.-Y. Lin, S.-H. Huang, W.-R. Liu, C.-C. Hu, K.-R. Lee and J.-Y. Lai, Carbon, 2014, 68, 670–677 CrossRef CAS.
- L. Ouyang, R. Malaisamy and M. L. Bruening, J. Membr. Sci., 2008, 310, 76–84 CrossRef CAS.
- D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, ACS Nano, 2010, 4, 4806–4814 CrossRef CAS PubMed.
- H. Zhao, S. Chen, X. Quan, H. Yu and H. Zhao, Appl. Catal., B, 2016, 194, 134–140 CrossRef CAS.
- Q. Zheng, W. H. Ip, X. Lin, N. Yousefi, K. K. Yeung, Z. Li and J.-K. Kim, ACS Nano, 2011, 5, 6039–6051 CrossRef CAS PubMed.
- L. Zou, J. Membr. Sci., 2016, 515, 204–211 CrossRef.
- D. A. Musale and A. Kumar, Sep. Purif. Technol., 2000, 21, 27–37 CrossRef CAS.
- C.-H. Tsou, Q.-F. An, S.-C. Lo, M. De Guzman, W.-S. Hung, C.-C. Hu, K.-R. Lee and J.-Y. Lai, J. Membr. Sci., 2015, 477, 93–100 CrossRef CAS.
- T. Sugama and M. Cook, Prog. Org. Coat., 2000, 38, 79–87 CrossRef CAS.
- S. Sayyar, E. Murray, S. Gambhir, G. Spinks, G. G. Wallace and D. L. Officer, J. Occup. Med., 2016, 68, 384–390 CAS.
- N. Hilal, H. Al-Zoubi, N. A. Darwish, A. W. Mohamma and M. Abu Arabi, Desalination, 2004, 170, 281–308 CrossRef CAS.
- E. R. Nightingale Jr, J. Phys. Chem., 1959, 63, 1381–1387 CrossRef.
- D. A. Musale and A. Kumar, J. Appl. Polym. Sci., 2000, 77, 1782–1793 CrossRef CAS.
- Z. Zhao, A. Wu, S. Luan and C. Zhang, J. Polym. Res., 2015, 22, 168 CrossRef.
- F.-Y. Zhao, Q.-F. An, Y.-L. Ji and C.-J. Gao, J. Membr. Sci., 2015, 492, 412–421 CrossRef CAS.
- M. Li, J. Xu, C. Y. Chang, C. Feng, L. Zhang, Y. Tang and C. Gao, J. Membr. Sci., 2014, 459, 62–71 CrossRef CAS.
- R. Han, J. Zeng, Y. Wang, Q. Chang, X. Zhang and J. Zhou, Desalin. Water Treat., 2014, 52, 5790–5795 CrossRef CAS.
- W. Fang, L. Shi and R. Wang, J. Membr. Sci., 2013, 430, 129–139 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Solubility of GO and prGO in water, Raman spectra and TGA plots of GO and prGO, XPS spectra, XPS survey spectra and C 1s spectra of membranes, and TGA plots of CS and prGO–CS. See DOI: 10.1039/c8ra01916a |
| This journal is © The Royal Society of Chemistry 2018 |