Open Access Article
Open Access ArticleModification of a hollow-fibre polyethersulfone membrane using silver nanoparticles formed in situ for biofouling prevention
Jan Dolinaa,
Zuzanna Gončukováa,
Marek Bobákb and
Lukáš Dvořák *a
*a
aInstitute for Nanomaterials, Advanced Technologies and Innovation, Technical University of Liberec, Studentská 2, 461 17 Liberec, Czech Republic. E-mail: lukas.dvorak@tul.cz; Tel: +420 485 353 805
bMembrain s.r.o., Pod Vinicí 87, 471 27 Stráž pod Ralskem, Czech Republic
First published on 18th April 2018
Abstract
Biofouling represents a serious problem limiting the widespread application of membrane technology. Therefore, the aim of this study was to develop and verify a new modification method based on the in situ formation of silver nanoparticles and their incorporation into a membrane polymer to prevent biofouling. The modification method consisted of soaking a commercial hollow-fibre polyethersulfone membrane in a solution of silver ions, diffusion of ions into the membrane polymer, and their reduction using ascorbic acid. Such a modified membrane displayed a lower tendency towards biofouling, exhibiting an about 15% higher permeability compared to an unmodified membrane when filtering actual wastewater treatment plant effluent. The modification also led to the formation of stable silver nanoparticles (mostly in the range of 25–50 nm) homogenously distributed on the surface of the hollow-fibres. This resulted in higher surface hydrophilicity (the water contact angle decreased from 91° to 86°) contributing to the biofouling prevention. The modified membrane also showed high stability, as only 2.1% of the total silver leached after 8 h of filtration. Moreover, no changes in the original membrane cross-section structure or separation properties were observed. Besides the improved antibiofouling properties of the modified membrane, the main advantage of the developed method is its simplicity, short reaction time, absence of high energy-consuming initiation, and the possibility to apply it on site, thus even with commercial membrane modules. It will increase the application potential of membranes in the field of wastewater treatment.
1. Introduction
Membrane bioreactors offer many advantages over current wastewater treatment technologies, including high effluent quality, a smaller footprint, and the possibility of wastewater reclamation; as such, their popularity has increased over the last two decades. However, membrane biofouling, which results in a decrease in hydraulic performance and an increase in operational costs, continues to be the most serious problem limiting the widespread application of this technology.1,2 Since membrane biofouling is a complex problem influenced by a range of different factors (and their combination), a number of issues related to biofouling have already been intensively studied.3,4To date, several methods of minimising membrane biofouling have been developed. These include changes in operational conditions (such as an increase in sludge retention time, increase in hydraulic retention time, decrease in the food-to-microorganism ratio, or wastewater characteristics), all of which affect the biomass characteristics or production of extracellular polymeric substances and, subsequently, interactions with the membrane surface and biofouling.5–7 The ratio of filtration time and flux to time of backpulse/backwashing, including relaxation, also affects membrane biofouling.1 However, while such methods have had a positive impact on biofouling, they offer a partial improvement only. Hence, other more effective approaches are needed, including modifications to the original membrane surface or development and preparation of new membrane materials. Such approaches usually result in an increase in the antimicrobial and hydrophilic characteristics of the membrane surface, resulting in a reduction to mutual hydrophobic interactions between the membrane and microorganisms, thereby mitigating biofouling.8
Polyethersulfone (PES), a polymer material commonly used for membrane preparation, exhibits high temperature, chemical and dimensional stability. As PES is a polar material, it can easily be modified. Several methods for surface modification of PES membranes have been developed.9,10 Since PES displays hydrophobic characteristics with low surface energy, the main goal of such modifications is to obtain a more polar surface, thus reducing surface hydrophobicity. In general, current methods of membrane surface modification can be divided into six main groups. These include coating methods (formation of thin layers subsequently fixed by noncovalent bonding to the original membrane surface, e.g. ref. 11); blending methods (two or more polymers mixed to obtain the required properties, e.g. ref. 12 and 13); composite methods (a membrane prepared from two or more materials differing in physical/chemical properties, e.g. ref. 14); chemical methods (new functional groups directly formed on the original membrane surface, e.g. ref. 15); grafting methods (covalent bonding of suitable monomers or functional groups to the membrane following initiation reactions, e.g. ref. 16 and 17) and combined methods.
Examples of combined methods include the exposure of a commercial PES membrane to a sulfonation reaction, resulting in the formation of new functional groups, followed by immersion in a titanium dioxide solution,18 and attachment of graphene oxide after grafting allylamines to the surface of a PES membrane by UV light.19
In most cases, the surface modification of PES membranes makes use of organic compounds and differing process conditions, often with energy-intensive initiation procedures. Often, however, the processes result in partial blockage of the membrane pores, resulting in lower hydraulic performance together with difficulties in controlling the modification reactions. As such, most processes for modifying PES membranes tend to use inorganic substances, including titanium dioxide, silicon particles, aluminium oxides or silver. As both silver ions and silver nanoparticles have proven antibacterial properties, modification of membranes using silver nanoparticles offers a promising option for mitigating membrane biofouling.20 The antimicrobial properties limit mutual interactions between the microorganisms and the membrane surface while still achieving long-term membrane permeability. Indeed, according to Meng et al.,21 nanotechnology in general has great potential for the modification and development of new membranes.
The above compounds are usually incorporated into the basic polymer (or mixture) prior to membrane preparation. Although the addition of such compounds can result in the required properties on the final membrane surface, the solution used to modify the base polymer matrix may negatively affect the ability to produce hollow-fibres when preparing the PES membrane. For this reason, most studies have focused on the modification or preparation of flat sheet membranes.
Hollow-fibres are the most frequently used membrane type in membrane bioreactors. Their main advantage lies in their very high packing density (surface-to-volume ratio).1 The high surface area allows the application of lower pressures to achieve the required hydraulic performance, which also results in a lower tendency towards biofouling and cake layer formation. A further advantage of hollow-fibre membranes over flat sheet membranes is the possibility of using intensive backpulse or backflush cleaning processes, thereby allowing the cake layer to be removed on a regular basis.22
As most current modification methods are time or/and energy-consuming, the main goal of this study was to develop a new modification method simple in use. The developed method consisted of using silver nanoparticles formed in situ and their incorporation into the membrane polymer. Silver nanoparticles were chosen in this study as they exhibit a strong antimicrobial effect towards a broad spectrum of different microorganisms (Gram-negative and Gram-positive). Moreover, silver nanoparticles are more efficient than other nanoparticles of transition metals and/or their oxides (e.g. CuO, TiO2 or ZnO). To date, most studies dealing with membrane modification have also been conducted on flat sheet membranes; therefore, the developed method was used to modify a commercially available hollow-fibre membrane to verify its efficiency towards the mitigation of membrane biofouling. Following modification, changes in the filtration performance with demineralized water and wastewater treatment plant effluent were evaluated. Silver leaching was also accomplished during the filtration test. Surface and cross-section morphology was characterized by a scanning electron microscope, and surface roughness was assessed by atomic force microscopy. Moreover, the amount of silver on the surface of the modified membrane using an energy dispersive X-ray detector, and the water contact angle to determine membrane hydrophilicity were also assessed.
2. Materials and methods
2.1. Membrane modification method
The developed modification method was based on the diffusion and entrapment of silver in a polyethersulfone (PES) matrix. The individual reagents including their optimal concentrations as well as processing conditions were based on the results of a number of previously performed experiments using PES membranes.The hollow-fibre membrane packed in a tube module supplied by Membrane Solution LLC (USA) was first soaked in a solution of 3.5% (wt) silver nitrate (AgNO3, Sigma-Aldrich), which was circulated through the module for 4 h at room temperature. The membrane was then intensively rinsed three times with demineralised water. Reduction of silver ions, i.e. in situ formation of silver nanoparticles, was conducted by soaking the membrane in a 2% (wt) solution of ascorbic acid (C6H8O6; Sigma-Aldrich) for 2 h. Following silver reduction, the membrane was again rinsed intensively with demineralised water and then heated for 2 h at 70 °C while recirculating the warm demineralized water through the module. The modified membrane was then immediately cooled with fresh demineralised water to laboratory temperature (22–25 °C). The modified membrane was subsequently stored in demineralised water for later use.
The membrane used to verify the efficiency of the developed modification method had an active membrane surface area of 0.7 m2. The hollow-fibres themselves were single capillary with an internal diameter of 0.7 mm, an outer diameter of 1.3 mm, and an out–in flow direction. The membrane had a molecular weight cut-off of 100 kDa.
2.2. Filtration tests
Following modification, the filtration performance of the modified membrane was tested against an unmodified (original) PES hollow-fibre membrane as a reference. Two filtration tests were performed using an upgraded LabUnit M10 laboratory-scale cross-flow filtration unit provided by Alfa Laval (Sweden). The filtration unit was equipped with KOBOLD Messring GmbH digital flowmeters (accuracy of ±2.5%; Germany) and AHLBORN GmbH digital pressure sensors (accuracy of ±0.5%; Germany) for continuous data measurement and recording. All of the filtration tests were performed at laboratory temperature.To evaluate potential changes in the removal of the organic compounds caused by modification of the membrane, chemical oxygen demand (COD) was determined in the membrane module feed (actual WWTP effluent) and permeate. The COD concentration was measured in duplicate using the Hach-Lange (Germany) cuvette test (LCK 414).
2.3. Silver leaching test
Samples for the evaluation of silver leaching were taken after 8 h of the filtration test with demineralized water, which was performed at a TMP of 0.5 bar and a cross-flow volume velocity of approximately 1.0 L min−1. This corresponded to the total volume of permeate of 1200 L per square meter of the membrane.The concentration of released silver was measured by inductively coupled plasma atomic emission spectroscopy (ICP-OES) using an Optima 2100 DV spectrophotometer (Perkin Elmer, USA).
2.4. Surface and cross-section morphology
The samples used for the analysis of cross-section morphology were cut with a fine razor blade, since breaking followed by soaking in liquid nitrogen was not possible due to the very high material stability of the hollow-fibres. After cutting, the samples were relaxed for 2 h under ambient conditions and then placed into the holder of the scanning electron microscope (JEOL Ltd., Japan).The surface morphology of the hollow-fibres was evaluated followed by water evaporation and placing onto a standard pin stub mount provided by Tescan (Czech Republic).
A Carl Zeiss ULTRA Plus scanning electron microscope (SEM) (Carl Zeiss Microscopy GmbH, Germany) was employed to visualise the membrane surface and cross-section morphology at different magnifications. Before the SEM analysis, the membrane samples were first covered with a 2 nm layer of platinum. The SEM analysis was undertaken under an acceleration voltage of 2 kV. The SEM was equipped with a secondary electron detector in order to analyse the chemical composition of the membrane surface. The amount of silver on the membrane surface was determined using a built-in energy dispersive X-ray (EDX) detector (Oxford instruments, UK) with no coating of samples by platinum to avoid the reduction of chemical contrast.
Atomic force microscopy was used to assess membrane surface roughness. First, the samples were fixed on a glass plate and placed into the holder of a Nanowizard 3 microscope (JPK Instruments, Germany). The samples were measured in contact mode using a cantilever with a 1–2 nm sharp tip of a silicone probe (SHOCONA-SS, Applied NanoStructures, Inc., USA) to scan a 10 × 10 μm portion of the membrane surface. Following analysis, JPK data processing software provided by JPK Instruments (Germany) was employed to calculate roughness parameters. To minimise the influence of curvature, the surface area analysed was set at 2 × 2 μm. A three-dimensional structural profile of the membrane surface was also formed using this JPK data processing software.
2.5. Water contact angle
The water contact angle, which provides information on the surface wettability (i.e. hydrophobicity or hydrophilicity) of the modified and unmodified membranes, was determined using a See System E portable computer-based instrument (Advex Instruments, Czech Republic). Measurements were carried out at laboratory temperature and a relative humidity of 45%. A 2 μl of demineralised water was carefully dropped onto the membrane surface and, immediately after drop stabilisation, the captured image (period of 0.2 s) was analysed and the contact angle between the water and the membrane surface was calculated. The measurement was conducted 10 times at different sites on the membrane surface to minimise experimental error.3. Results and discussion
3.1. Membrane permeability
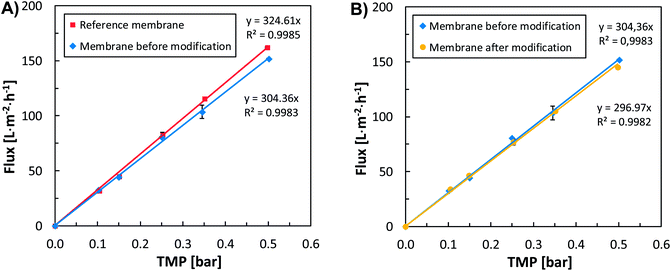
The first filtration test (with demineralized water) examined the dependence of flux on TMP over a range of 0.1 to 0.5 bar for a reference membrane and a membrane before modification (identical membranes provided by the supplier). The results showed only negligible differences in flux for the individual membranes (Fig. 1A). As both of the tested membranes were ultrafiltration membranes, both could be considered identical in terms of their flow characteristics.Unlike other methods currently applied for membrane modification, modification through the in situ formation of silver nanoparticles, as developed in this study, did not lead to any change in flow characteristics (Fig. 1B). The average flux at different TMPs before and after modification was basically the same (Fig. 1B). In comparison, Rahimpour et al.,23 who synthesised two types of PES membrane modified with titanium dioxide nanoparticles (one prepared through entrapment and the other by coating and UV irradiation), observed a lower initial flux in the titanium dioxide entrapped membranes compared with the initial flux of an untreated PES membrane. In further tests, however, both membranes exhibited enhanced antibiofouling properties and long-term flux stability compared to the neat PES membrane.23
In the second filtration test, actual effluent from a municipal WWTP was used as a feed to demonstrate the efficiency of the developed method to mitigate biofouling. While the absolute permeability values for the reference membrane were slightly higher than the modified membrane (Fig. 2A), the normalised permeability (K/K0) was higher for the modified membrane (Fig. 2B). This disproportion could be explained by a slightly different concentration of suspended solids in the feed used on both membranes, as the feed comprised actual WWTP effluent. In fact, the concentration of suspended solids throughout the test with the reference membrane was 10.7 mg L−1, compared to a concentration of 15.0 mg L−1 during the test with the modified membrane.
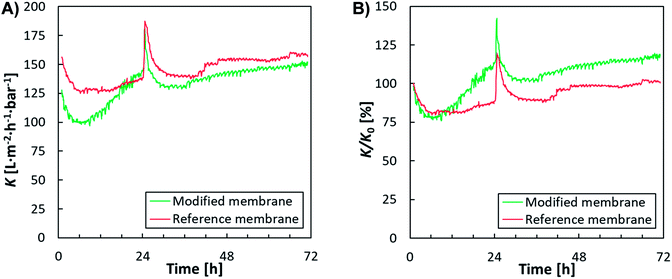 | ||
| Fig. 2 Permeability over time: (A) absolute values (K) for the modified and reference membranes, (B) normalised permeability (K/K0), after eliminating the influence of different feed characteristics. | ||
To better compare the permeability between these two membranes, normalised permeability values (permeability [K/K0] obtained 60 minutes after the stabilising process) against the initial permeability of each membrane were plotted (Fig. 2B). Following a sharp initial decrease in permeability caused by unstable process conditions and the formation of a filtration layer on the membrane surface, the permeability increased in the modified membrane over time (Fig. 2). This suggests that (a) the compounds responsible for the fouling were not fixed to the surface, and (b) that the modified membrane showed a lowered tendency towards biofouling compared to the reference (unmodified) membrane. Moreover, after membrane cleaning, i.e. following an intensive reversal flow of permeate in the opposite direction to filtration in the module performed after 24 h of testing, the modified membrane displayed an increased recovery of permeability.
A rapid increase in permeability difference (dK/Kref) was also shown by the modified membrane when comparing the permeability difference to a reference membrane plotted over time (Fig. 3). At the start of the experiment, the modified membrane showed 5% lower permeability than the reference membrane; however, after approximately 6 h, the difference in permeability (dK/Kref) began to decrease rapidly and, after 12 h, the permeability values were comparable. At this point, the permeability values for the modified membrane were always higher than those for the reference membrane. The greatest difference in dK/Kref values (approximately 20%) was recorded after 24 h, i.e. just before cleaning the membrane module. During further phases of the test, dK/Kref values for the modified membrane were approximately 15% higher than those for the reference membrane.
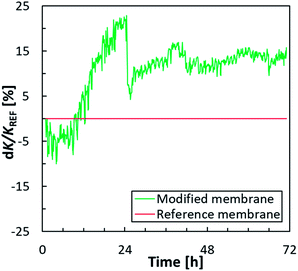 | ||
| Fig. 3 Difference in permeability (dK/Kref; in %) between the modified membrane and a reference (unmodified) membrane during a filtration test with WWTP effluent. | ||
These results suggest that the modification of the membrane through the developed in situ formation of silver nanoparticles had no negative effect on membrane filtration performance. On the contrary, both the permeability of the modified membrane and its anti-biofouling properties improved due to the modification procedure.
The separation properties of the membranes were evaluated based on the retention of organic substances (expressed as COD). The reference and modified membranes exhibited comparable separation properties, with COD retention for the reference membrane reaching 27.2% while the modified membrane reached 30.5%. Retention of COD was caused by the capture of suspended solids and soluble substances with a molecular weight greater than 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 daltons (molecular weight cut-off guaranteed by the supplier) on the membrane surface.
000 daltons (molecular weight cut-off guaranteed by the supplier) on the membrane surface.
3.2. Silver leaching
The membrane modified through the developed method also showed high stability, with only 2.1% of the total amount of silver leached after 8 h of testing, i.e. when passing 1200 L of demineralized water through the square meter of the membrane. As the main portion of the silver nanoparticles is typically released during the first hours of filtration, the results are in agreement with other studies.24–26 For example, Biswas et al.25 modified a sulfonated PES membrane using silver nanoparticles, and they also observed only 1.7% silver release to the permeate after passing 180 L of water after 3 h of filtration. Moreover, the release of silver in our study was lower than in other published studies. For instance, Zhang et al.27 reported 3.7% of leached silver from a silver nanocomposite PES ultrafiltration membrane after filtration of 6500 L per square meter of membrane. A similar amount of silver leached, i.e. 4.3% of the total amount of silver present on the sulfonated PES membrane, was observed in a study by Biswas et al.25 following filtration of 5000 L water per square meter of membrane.3.3. Cross-section morphology
To evaluate possible changes caused by the modification procedure, the cross-section morphology of the modified (Fig. 4A) and reference membranes (Fig. 4B) under different magnifications was examined using a SEM. Both images (Fig. 4A and B) showed a dense skin layer supported by a finger-like structure, typical for ultrafiltration membranes. As such, there was no evidence for any structural effects on the cross-section morphology caused by the in situ formation of silver nanoparticles on the modified membrane.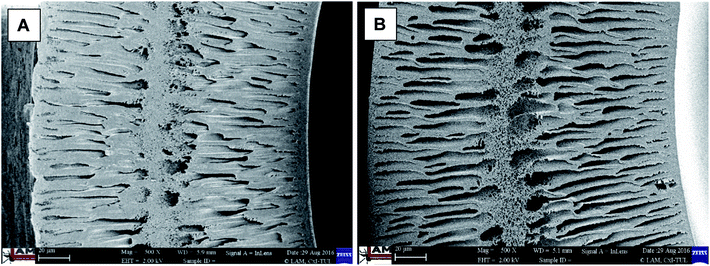 | ||
| Fig. 4 Cross-section morphology of (A) the modified membrane, and (B) the reference membrane; 500× magnification. | ||
Under higher magnification, the presence of silver nanoparticles on the surface and in the active surface layer of the modified membrane was clearly visible (Fig. 5A). Furthermore, compared with the reference membrane (Fig. 5B) there was clear evidence of the presence of silver nanoparticles inside the polymer matrix (from the surface up to the macrovoid structure) of the modified membrane (Fig. 5A).
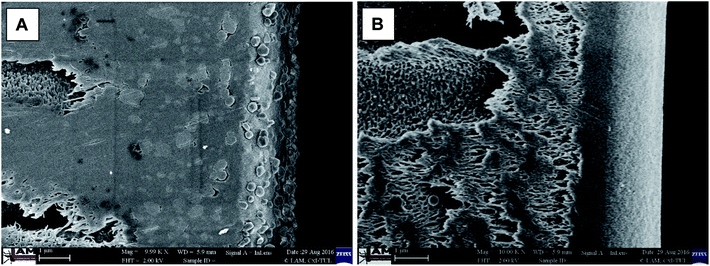 | ||
Fig. 5 Cross-section morphology of (A) the modified membrane, with silver nanoparticles clearly visible, and (B) the reference membrane; 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× magnification. 000× magnification. | ||
The performed modification resulted in the incorporation of silver nanoparticles into the active membrane layer as well as into the dense inner pore structure. As silver nanoparticles exhibit hydrophilic properties, they can be beneficial for the mitigation of biofouling also on the surface of membrane pores.
3.4. Surface morphology
When comparing the surface morphology of the modified and reference membrane at a magnification of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×, white spots on the surface of the modified membrane (Fig. 6A), and there absence on the reference membrane (Fig. 6B), clearly indicated the presence of silver nanoparticles. Moreover, the silver nanoparticles were clearly distributed homogeneously on the surface (apart from the inner pore structure, Fig. 5A), with no agglomerates visible.
000×, white spots on the surface of the modified membrane (Fig. 6A), and there absence on the reference membrane (Fig. 6B), clearly indicated the presence of silver nanoparticles. Moreover, the silver nanoparticles were clearly distributed homogeneously on the surface (apart from the inner pore structure, Fig. 5A), with no agglomerates visible.
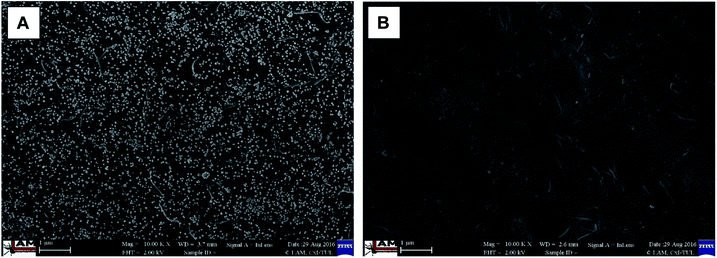 | ||
Fig. 6 Scanning electron microscopy images of the surface of (A) the modified membrane, and (B) the reference membrane; 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× magnification. 000× magnification. | ||
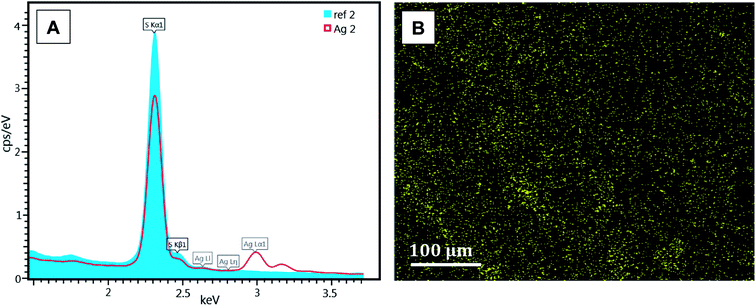
Comparison of EDX spectra for the reference and modified membranes also clearly indicates the presence of silver on the modified membrane (Fig. 7A; ratios of individual elements [mass and molar ratios] shown in Table 1). The EDX analysis confirmed that silver nanoparticles were homogeneously distributed (Fig. 7B), with a mass ratio of silver of 3.6% on the surface of the modified membrane.
| Elements | Reference membrane | Modified membrane | ||
|---|---|---|---|---|
| Mass ratio [%] | Molar ratio [%] | Mass ratio [%] | Molar ratio [%] | |
| Carbon (C) | 69.74 | 77.57 | 67.77 | 77.84 |
| Oxygen (O) | 23.48 | 19.61 | 21.73 | 18.74 |
| Sulphur (S) | 6.78 | 2.82 | 6.87 | 2.95 |
| Silver (Ag) | — | — | 3.63 | 0.46 |
The size of the silver nanoparticles present on the surface of the modified membrane was also assessed as it has been shown that the size of nanoparticles affects their antimicrobial activity.28 The mean diameter of the silver nanoparticles detected on the modified membrane was 43 ± 18 nm (n = 20). According to Panáček et al.,28 smaller silver nanoparticles of around 25 nm exhibit the strongest antimicrobial activity, while large nanoparticles of around 50 nm have a reduced effect. This is due to the higher surface/volume ratio of smaller silver nanoparticles facilitating the release of silver ions and/or production of reactive oxygen species.29 The particle size distribution analysis performed in this study indicated that approximately 10% of the silver nanoparticles had a diameter of <25 nm, and only 15% of were larger than 50 nm. As most of the nanoparticles ranged from 25–50 nm, the membrane could be expected to display strong antimicrobial properties. This also corresponds with the results of the filtration tests, where a lowered tendency toward biofouling was observed in the modified membrane.
In addition to the size of the silver nanoparticles, their antimicrobial activity may also be influenced by their shape.30 In this study, the silver nanoparticles present on the surface of the modified membrane were clearly spherical (Fig. 8). According to Pal et al.,30 spherical silver nanoparticles exhibit average antimicrobial activity compared to truncated triangular nanoparticles, which show strong activity, and rod-shaped nanoparticles, which display an inferior antimicrobial effect.
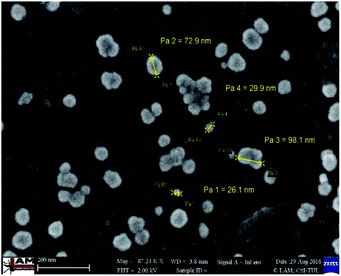 | ||
Fig. 8 Size of the silver nanoparticles present on the surface of the modified membrane; 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× magnification. 000× magnification. | ||
3.5. Surface roughness
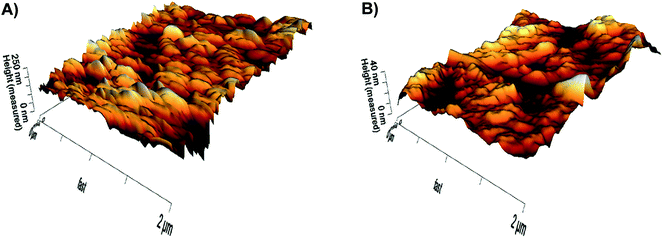
The surface of the modified membrane was ‘rougher’ than that of the reference membrane, with the average roughness (Ra) increasing from 4 nm for the reference (original) to 24 nm for the modified membrane (Fig. 9A and B; Table 2). Furthermore, the peak-to-valley roughness (Rt) was much higher in the modified membrane (modified – 150 nm; reference – 29 nm). This increase in surface roughness for the modified membrane was a direct consequence of the presence of silver nanoparticles. Although some researchers have reported that membranes with low roughness exhibit greater antifouling capabilities (e.g. ref. 7 and 8), the membranes modified in our study through the developed method displayed a lower decline in permeability when filtering actual WWTP effluent. Such a finding corresponds to the results of Yan et al.,31 who reported that membranes with increased surface roughness were more permeable (higher flux) due to their greater filtration area. Nevertheless, in our study, the antimicrobial properties and increasing hydrophilicity (see Section 3.6) of the silver nanoparticles themselves more likely mitigated biofouling than the increased surface roughness caused by the silver nanoparticles.| Parameters | Modified membrane | Reference membrane |
|---|---|---|
| Average roughness Ra [nm] | 24 | 4 |
| Peak-to-valley roughness Rt [nm] | 150 | 29 |
| Max. height [nm] | 79 | 11 |
| Max. depth [nm] | −71 | −18 |
3.6. Water contact angle
Following modification, the water contact angle decreased from 91.6° in the reference membrane (illustrating the hydrophobic nature of the basic PES polymer) to 86.1° in the modified membrane (Table 3), thereby confirming a slight increase in surface hydrophilicity. The observed changes were a direct result of the presence of silver nanoparticles, which are known to be hydrophilic. This finding is also in agreement with other studies (e.g. ref. 32) where increased hydrophilicity was also observed for silver-modified membranes.| Membrane | Average water contact angle [°] | Standard deviation [°] |
|---|---|---|
| Modified | 86.1 | 1.6 |
| Reference | 91.6 | 3.3 |
4. Conclusions
A new modification method consisting of the in situ formation of silver nanoparticles was developed and verified in this study. The modification method was based on the diffusion of silver ions, followed by their reduction and thermal stabilisation. To verify the efficiency of the developed modification method, a commercially available hollow-fibre PES ultrafiltration membrane was used.The developed modification method resulted in stable silver nanoparticles firmly anchored to the membrane surface and in the active layer (confirmed by SEM and EDX), leading to a membrane with improved antibiofouling properties. SEM analysis also confirmed no negative impact of the in situ formation of silver nanoparticles on membrane cross-section morphology. A decrease in the water contact angle indicated that the modification method resulted in a more hydrophilic character of the modified membrane surface than the reference membrane. As a result, the modified membrane displayed an approximately 15% higher permeability when filtering actual effluent from a municipal wastewater treatment plant.
The simplicity of the developed method, the short reaction time, and the ability to be performed on site resulting in a lower tendency toward biofouling represent its great potential for real application.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The result was created with the financial support of the Technological Agency of the Czech Republic within the framework of the project No. TE02000077 “Smart Regions – Buildings and Settlements Information Modelling, Technology and Infrastructure for Sustainable Development” using the infrastructure of the Membrane Innovation Centre. The projects LO1201 “National Programme for Sustainability I”, and the OPR&DI project “Centre for Nanomaterials, Advanced Technologies and Innovation CZ.1.05/2.1.00/01.0005” are also hereby acknowledged.References
- S. Judd, The MBR book: principles and applications of membrane bioreactors for water and wastewater treatment, Elsevier, Oxford, 2011 Search PubMed.
- L. Yu, Y. Zhang, B. Zhang, J. Liu, H. Zhang and C. Song, J. Membr. Sci., 2013, 447, 452–462 CrossRef CAS.
- A. Boyle-Gotla, P. D. Jensen, S. D. Yap, M. Pidou, Y. Wang and D. J. Batstone, J. Membr. Sci., 2014, 467, 153–161 CrossRef CAS.
- F. Meng, B. Shi, F. Yang and H. Zhang, Bioprocess Biosyst. Eng., 2007, 30, 359–367 CrossRef CAS PubMed.
- M. Dalmau, H. Monclús, S. Gabarrón, I. Rodriguez-Roda and J. Comas, Bioresour. Technol., 2014, 171, 103–112 CrossRef CAS PubMed.
- L. Dvořák, M. Gómez, M. Dvořáková, I. Růžičková and J. Wanner, Bioresour. Technol., 2011, 102, 6870–6875 CrossRef PubMed.
- Z. Q. Tang, W. Li, J. Zhou, H. Y. Yu, L. Huang, M. G. Yan, J. S. Gu and X. W. Wei, Sep. Purif. Technol., 2009, 64, 332–336 CrossRef CAS.
- T. H. Bae and T. H. Tak, J. Membr. Sci., 2005, 264, 151–160 CrossRef CAS.
- L. C. Branco, J. G. Crespo and C. A. Afonso, Chem.–Eur. J., 2002, 8, 3865–3871 CrossRef CAS.
- G. Pearce, Filtr. Sep., 2007, 44, 36–38 Search PubMed.
- L. J. Mu and W. Z. Zhao, Appl. Surf. Sci., 2009, 255, 7273–7278 CrossRef CAS.
- Y. Su, C. Li, W. Zhao, Q. Shi, H. Wang, Z. Jiang and S. Zhu, J. Membr. Sci., 2008, 322, 171–177 CrossRef CAS.
- J. Liu, C. Tian, J. Xiong and L. Wang, J. Colloid Interface Sci., 2017, 494, 124–129 CrossRef CAS PubMed.
- Z. P. Zhao, Z. Wang and S. C. Wang, J. Membr. Sci., 2003, 217, 151–158 CrossRef CAS.
- N. Nady, M. C. R. Franssen, H. Zuilhof, M. S. M. Eldin, R. Boom and K. Schroën, Desalination, 2011, 275, 1–9 CrossRef CAS.
- H. Y. Yu, Z. K. Xu, H. Lei, M. X. Hu and Q. Yang, Sep. Purif. Technol., 2007, 53, 119–125 CrossRef CAS.
- P. Krystosiak, W. Tomaszewski and E. Megiel, J. Colloid Interface Sci., 2017, 498, 9–21 CrossRef CAS PubMed.
- T. H. Bae, I. C. Kim and T. M. Tak, J. Membr. Sci., 2006, 275, 1–5 CrossRef CAS.
- E. Igbinigun, Y. Fennell, R. Malaisamy, K. L. Jones and V. Morris, J. Membr. Sci., 2016, 514, 518–526 CrossRef CAS.
- A. L. Ahmad, A. A. Abdulkarim, B. S. Ooi and S. Ismail, Chem. Eng. J., 2013, 223, 246–267 CrossRef CAS.
- F. Meng, S. R. Chae, A. Drews, M. Kraume, H. S. Shin and F. Yang, Water Res., 2009, 43, 1489–1512 CrossRef CAS PubMed.
- S. Judd, Filtr. Sep., 2002, 39, 30–31 CrossRef CAS.
- A. Rahimpour, S. S. Madaeni, A. H. Taheri and Y. Mansourpanah, J. Membr. Sci., 2008, 313, 158–169 CrossRef CAS.
- J. Dolina, O. Dlask, T. Lederer and L. Dvořák, Chem. Eng. J., 2015, 275, 125–133 CrossRef CAS.
- P. Biswas and R. Bandyopadhyaya, J. Colloid Interface Sci., 2017, 491, 13–26 CrossRef CAS PubMed.
- L. Tang, K. A. Huynh, M. L. Fleming, M. Larronde-Larretche and K. L. Chen, J. Colloid Interface Sci., 2015, 451, 125–133 CrossRef CAS PubMed.
- M. Zhang, R. W. Field and K. Zhang, J. Membr. Sci., 2014, 471, 274–284 CrossRef CAS.
- A. Panáček, L. Kvitek, R. Prucek, M. Kolar, R. Vecerova, N. Pizurova, V. K. Sharma, T. Nevečná and R. Zbořil, J. Phys. Chem. B, 2006, 110, 16248–16253 CrossRef PubMed.
- J. R. Morones, J. L. Elechiguerra, A. Camacho, K. Holt, J. B. Kouri, J. T. Ramírez and M. J. Yacaman, Nanotechnology, 2005, 16, 2346 CrossRef CAS PubMed.
- S. Pal, Y. K. Tak and J. M. Song, Appl. Environ. Microbiol., 2007, 73, 1712–1720 CrossRef CAS PubMed.
- L. Yan, Y. S. Li, C. B. Xiang and S. Xianda, J. Membr. Sci., 2006, 276, 162–167 CrossRef CAS.
- K. Zodrow, L. Brunet, S. Mahendra, D. Li, A. Zhang, Q. Li and P. J. J. Alvarez, Water Res., 2009, 43, 715–723 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |