Open Access Article
Open Access ArticleAlleviation of cadmium phytotoxicity to wheat is associated with Cd re-distribution in soil aggregates as affected by amendments
Shanshan Lia,
Meng Wang *b,
Zhongqiu Zhaoa,
Xiaoyue Lia,
Yun Hanb and
Shibao Chen*b
*b,
Zhongqiu Zhaoa,
Xiaoyue Lia,
Yun Hanb and
Shibao Chen*b
aSchool of Land Science and Technology, China University of Geosciences, Beijing 100083, P. R. China
bKey Laboratory of Plant Nutrition and Fertilizer, Ministry of Agriculture/Institute of Agricultural Resources and Regional Planning, Chinese Academy of Agricultural Sciences, No. 12 Zhongguancun South Street, Beijing 100081, P. R. China. E-mail: wangmeng@caas.cn; chenshibao@caas.cn; Fax: +86 01082106543; Tel: +86 01082106722
First published on 11th May 2018
Abstract
Soil aggregates exert a significant influence on the retention and bioavailability of Cd in soil. This study investigated how applications of various soil amendments affected soil aggregation and Cd phytotoxicity. A staple crop, wheat (Triticum spp.), was grown in Cd-polluted soil amended with either clay mineral (CM), rock mineral (RM), humic substances (HS), biochar (BC) or iron-based biochar (Fe-BC). Results indicate that addition of soil amendments promoted the formation of large soil aggregates (0.2–2 mm and 0.02–0.2 mm) with greater mass loading of Cd (total Cd or DTPA-extractable Cd). Moreover, significant negative correlations between the mass loading of Cd in large aggregates and Cd accumulation in wheat tissues were observed. The effectiveness in mitigating Cd phytotoxicity was dependent on the type of amendment applied. Among them, addition of HS was most effective with the highest total Cd accumulation observed in the soil fraction of 0.2–2 mm (138.1% of the control) and lowest Cd concentration observed in wheat grain (56.9% of the control). The results suggest that the re-distribution of Cd among soil aggregates was the likely factor that controlled the quantity of plant available Cd in the soil-plant system.
1. Introduction
The accumulation, mobility and availability of heavy metals in soil environments strongly correlate with the properties of soil aggregates.1 Soil aggregates are a basic physical and functional unit of soil. Soil physical, chemical and biological components generally interact in a non-linear manner yet display clear properties of co-evolution and self-organization.2–4 Fine soil particles typically have more clay minerals, organic matter, large surface area and a greater presence of Fe/Mn/Al oxides. These characteristics facilitate metal accumulation by the particles to gradually form organic mineral aggregates which promote co-precipitation, occlusion, adsorption and complexation in the soil environment.5,6 Due to its small size, fine soil particles are often found in deep soils, surface/ground water and air, as well as plant tissues.6 The fine particulates can be harmful, especially when carrying heavy metals that are toxic when they enter plant and animal systems, and pose serious ecological and health risks. Therefore, understanding how we can manipulate soil aggregate distribution can help us better manage the amount of plant-available toxic heavy metals in polluted soils.Cadmium is generally considered a highly toxic element; its negative effects on plant development and growth has been well documented.7,8 Wheat grain products are the primary source of dietary Cd-intake for humans in China.9 The most efficient route to control the risk of elevated Cd exposure may be the remediation of Cd-polluted agricultural soils. Among the technologies for soil remediation (e.g. excavation, landfilling, electroremediation and phytoremediation), in situ stabilization by applying soil amendments has been regarded as one of the most promising approaches due to its low cost, its ease of implementation and most importantly, it does not disrupt tillage practices.10,11 Generally, the goal of applying amendments into contaminated soils is to reduce the mobility and toxicity of heavy metals by accelerating the processes of complexation, adsorption or redox reactions, and promoting surface precipitation reactions, metal binding, and fixation inside mineral particles.12 To date, various emerging amendments for soil remediation have been broadly studied in the lab or field. These amendments include industrial wastes (red mud, slag, fly ash, steel slags), iron oxides or hydroxides, clay minerals (zeolites, sepiolite, apatite), nano-materials (nano-hydroxyapatite particles, stabilized iron sulfide nanoparticles), activated carbon and composted biosolids.13–15 In recent years, many efforts have been made to evaluate the potential of these amendments to immobilize Cd in soils by investigating their remediation efficiencies or demonstrating their immobilization mechanisms.16
Though soil aggregation has been confirmed to strongly correlate with the fate of heavy metals in the soil-plant system, how the effects of amendment addition on soil particle distribution and heavy metal partitioning among different sized aggregates is unclear. Only recently has Cui et al. reported that the application of apatite, lime and charcoal redistributed Cu and Cd into soil aggregates and decreased the plant-available Cu and Cd concentrations.1 Therefore, the relation between alleviation of Cd phytotoxicity by soil amendments and the distribution of Cd into soil aggregate fractions is worthy of study. The objectives of this study were: (i) to investigate the response of soil aggregates after applying soil amendments into Cd-polluted soil; (ii) to evaluate the effect of soil amendments on alleviation of Cd phytotoxicity to wheat; and (iii) to determine potential correlations between Cd distribution in aggregates and Cd accumulation in wheat tissues.
2. Materials and methods
2.1 Characterization of soil and soil amendments
The naturally Cd-polluted soil samples used for laboratory experiments were collected from the Eastern Baoding (38°76′N, 115°47′E), Hebei province, the North China Plain, which is a major grain producing area of China. The soil in this site has been subjected to Cd contamination due to agricultural irrigation, as sewage effluents originating from the industrial wastewater and domestic sewage have been used for irrigation purposes since 1995, long-term fertilization and pesticide applications also contribute to the intensification of soil Cd pollution. The soil in this area is mainly cinnamon soil (argosols) according to the traditional soil genesis classification in China. Five soil cores (6 cm diameter) were taken from the plot (100 m2) as a composite sample from a 0–20 cm depth. Soil material was air-dried, thoroughly mixed and passed through a 2 mm mesh sieve. Several physiochemical properties of the soil and Cd content were determined according to Chinese standard methods given in Table 1. Soil pH of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 (w/w) soil–CaCl2 water suspension was measured with a pH meter (Denver Instrument UB-7 pH/mV Meter, ultraBASIC). Soil organic matter content was determined by the potassium dichromate external-heating method. Cation-exchange capacity (CEC) was measured using the barium chloride method. The total concentration of Cd in soil was determined using a PerkinElmer 1100B atomic absorption spectrometer. Cadmium tests were conducted on the soil samples collected; its concentration significantly exceeded more than four times the concentration limit (0.3 mg kg−1) listed in the Environmental Quality Standard for Soils of China, GB15618-1995 (Table 1).
2.5 (w/w) soil–CaCl2 water suspension was measured with a pH meter (Denver Instrument UB-7 pH/mV Meter, ultraBASIC). Soil organic matter content was determined by the potassium dichromate external-heating method. Cation-exchange capacity (CEC) was measured using the barium chloride method. The total concentration of Cd in soil was determined using a PerkinElmer 1100B atomic absorption spectrometer. Cadmium tests were conducted on the soil samples collected; its concentration significantly exceeded more than four times the concentration limit (0.3 mg kg−1) listed in the Environmental Quality Standard for Soils of China, GB15618-1995 (Table 1).
| Treatment | pH (water/soil = 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
CEC (cmol+ kg−1) | Organic matter content (%) | Cd background value (mg kg−1) |
|---|---|---|---|---|
| Before planting | ||||
| 7.89 ± 0.06 | 16.70 ± 0.48 | 1.12 ± 0.02 | 1.32 ± 0.021 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| After planting | ||||
| CK | 8.06 ± 0.02 | 16.59 ± 0.55 | 1.05 ± 0.01 | 1.31 ± 0.034 |
| CM | 7.94 ± 0.07 | 18.09 ± 0.64 | 1.81 ± 0.02 | 1.30 ± 0.011 |
| RM | 7.98 ± 0.06 | 18.35 ± 0.32 | 1.58 ± 0.02 | 1.30 ± 0.026 |
| HS | 7.92 ± 0.07 | 18.21 ± 0.51 | 3.04 ± 0.40 | 1.31 ± 0.021 |
| BC | 8.33 ± 0.02 | 17.75 ± 0.44 | 2.89 ± 0.35 | 1.31 ± 0.024 |
| Fe-BC | 8.01 ± 0.03 | 17.34 ± 0.28 | 2.73 ± 0.58 | 1.30 ± 0.013 |
Numerous natural mineral and organic compounds, waste products of agriculture and industry etc. including phosphate compounds, clay minerals, organic composts, metal oxides, biochar, humus etc. can be used as soil amendments. These amendments that are traditionally considered as sources of soil nutrients, because they have the ability of increasing cation exchange capacities, intensifying microbiological activities, improving physical properties (e.g. structure, water holding capacity) and fertility of soils13–15 In this study, five different amendments were prepared and compared: clay mineral (CM), rock mineral (RM), humic substances (HS), biochar (BC), and iron-based biochar material (Fe-BC). The CM contained a mixture of attapulgite, triple superphosphate and humus. The RM consisted of zeolite, triple superphosphate and humus. The HS were prepared by mixing hydroxyapatite and humus. Biochar was prepared by a method of high-temperature (up to 800 °C) carbonization of rice hulls, and an iron-containing compound (Fe(NO3)2) was added in the process of biochar production to prepare the iron-based biochar material (Fe-BC). These amendments were passed through a 0.047 mm mesh sieve before use. They were also characterized by physical and chemical methods similar to what was carried out for the polluted soil samples. Specifically, the mean grain size was analysed using a laser particle analyser (HORIBA LA-950, Japan) and the surface area was measured by the single point Brunauer, Emmett and Teller N2 sorption procedure (BET-N2: Quantachrome Instruments, U.S.). The basic properties of soil amendments are displayed in Table 2.
| Amendments | pH (water/soil = 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
BET surface area (m2 g−1) | Particle mean size (μm) | CEC (cmol+ kg−1) | Organic carbon (g kg−1) | Cd(mg kg−1) |
|---|---|---|---|---|---|---|
| CM | 7.95 ± 0.06 | 58.70 ± 2.76 | 7.29 ± 1.27 | 125.60 ± 8.19 | 58.62 ± 3.12 | ND |
| RM | 8.13 ± 0.09 | 21.27 ± 1.90 | 14.60 ± 1.71 | 210.30 ± 10.23 | 36.73 ± 1.95 | ND |
| HS | 7.92 ± 0.04 | 4.63 ± 0.52 | 35.90 ± 3.80 | 176.70 ± 7.65 | 420.21 ± 23.14 | ND |
| BC | 9.28 ± 0.06 | 36.51 ± 1.21 | 33.30 ± 2.27 | 115.10 ± 1.88 | 67.38 ± 25.89 | ND |
| Fe-BC | 8.02 ± 0.02 | 36.74 ± 0.98 | 23.02 ± 1.54 | 114.20 ± 1.31 | 89.49 ± 26.67 | ND |
2.2 Experimental design
A pot experiment was conducted in a semi-closed greenhouse under a normal diel light/dark cycle at the Chinese Academy of Agricultural Sciences in Beijing, China between March and June, 2017. Each sample of 7 kg of soil was ground to pass through a 2 mm mesh sieve and placed into a plastic pot. The six treatments in this study included soil without application of an amendment (control) and soil with application of 3% (w/w) of one of the five amendments. Additionally, 1.75 g urea, 1.05 g KH2PO4 and 0.28 g KCl were dissolved in deionized water and evenly mixed with the soil as a basal fertilizer. Once all of the experimental units had received their additives, each pot was incubated in a 25 °C greenhouse for two weeks with soil moisture maintained at 70% of the field water-holding capacity. The wheat seeds (variety Liao chun 18) were sterilized by soaking in 5% hydrogen peroxide for 20 min and rinsing with distilled water. Sterile seeds were then placed in a culture dish containing two pieces of wet filter paper at 28 °C for 48 h. Germinated seeds were sown into each pot and then thinned to five seedlings after emergence from the soil surface. The pots were arranged in a randomized block design with three replicates for each treatment. During wheat growth, each pot was irrigated every three days with distilled water to maintain soil moisture at approximately 60–70% of water holding capacity. The weeds in all pots were removed by hand when their growth were observed, and the pulled-out weeds were kept in each pot to avoid the loss of Cd which might has been taken by weed. After 90 days of growth, the plant samples were collected from each pot. They were washed with tap water and then rinsed 3–4 times with deionized water. The plant samples were then oven-dried (60 °C) until a constant weight was reached. Dried biomass were ground using a stainless steel mill and passed through a 0.25 mm sieve. The soil samples in each pot were air-dried, ground and passed through a 2 mm mesh sieve for further analysis.2.3 Soil aggregate preparation and sample analysis
The physical procedure for soil aggregate separation was adopted from Manna et al. with modifications.19 The air-dried soil was wet sieved to obtain different aggregate size fractions: macroaggregates (0.2–2 mm), large microaggregates (0.02–0.2 mm), silt-sized particles (0.02–0.002 mm) and clay-sized particles (<0.002 mm). In brief, each equivalent of 50 g dry mass of the soil sample was then transferred to a nest of sieves with mesh sizes of 0.2 mm (top), 0.02 mm (middle) and 0.002 mm (bottom). The soil-water suspension was dispersed by shaking the sieve 3 cm vertically for 30 times in two minutes. Subsequently, the macro- and micro-aggregate fractions retained on each sieve were collected. The smaller fractions (0.02–0.002 mm and <0.002 mm) were collected by centrifuging suspensions for 10 min at 3000 rpm. All fractions were then oven-dried at 40 °C for 24 h and weighed to obtain the mass proportion of each fraction relative to the bulk soil.The “total” (strong acid-extractable) Cd concentration in the bulk soil and different sized soil fractions under each treatment was measured by digesting approximately 0.2 g of air-dried soil with 4.5 ml HCl (37%), 1.5 ml HNO3 (65%) and 1 ml H2O2 (30%) in a Teflon bomb placed in a microwave digestion apparatus (Milestone MLS 1200 Mega). A similar procedure was used to digest plant materials but only with HNO3. The concentration of DTPA-extractable Cd is usually considered as an indicator of the available metal pool in soils. The DTPA extraction method is suitable for neutral and alkaline soils because the DTPA method was originally developed for the extraction of soluble metals from the neutral and calcareous soils with insufficient transition metals.17 More recently, DTPA-extractable metal has been broadly used as a chemical indicator for plant-available fraction of metals in soil.1,18 The available Cd in the samples was measured via chemical extraction with diethylenetriamine pentaacetate (DTPA). The mixture of 0.005 mol L−1 DTPA, 0.01 mol L−1 CaCl2 and 0.1 mol L−1 triethanolamine at pH 7.30 was added into soil samples with a soil-to-solution ratio (w/v) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. After shaking for 2 h, the suspensions were processed by centrifugation and filtration by a 0.22 μm membrane before analysis. Similarly, the ammonium bicarbonate–diethylenetriaminepentaacetic acid (DTPA) (AB-DTPA) soil test could also accurately indicate the bioavailable contents of heavy metals in soil especially in neutral and alkaline soils. The Cd concentrations in both the digests and extracts of plant and soil samples were determined using a PerkinElmer 1100B atomic absorption spectrometer.
5. After shaking for 2 h, the suspensions were processed by centrifugation and filtration by a 0.22 μm membrane before analysis. Similarly, the ammonium bicarbonate–diethylenetriaminepentaacetic acid (DTPA) (AB-DTPA) soil test could also accurately indicate the bioavailable contents of heavy metals in soil especially in neutral and alkaline soils. The Cd concentrations in both the digests and extracts of plant and soil samples were determined using a PerkinElmer 1100B atomic absorption spectrometer.
2.4 Statistical analysis
To quantify the transport of Cd from the soil to plant tissues, the bioconcentration factor (BCF) of soil Cd per plant was calculated as:
 | (1) |
The mass loading of Cd (%) of individual aggregate fraction (MLFi) was calculated using the following equation:
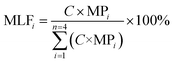 | (2) |
All data were subjected to statistical analysis using SPSS version 16.0. Means and standard deviations were calculated for triplicates. One-way analysis of variance (ANOVA) was used to assess differences among different treatments at p < 0.05. Pearson correlation coefficients (R value) with the respective probabilities (p) were calculated to indicate significance between parameters.
3. Results and discussion
3.1 Soil aggregates
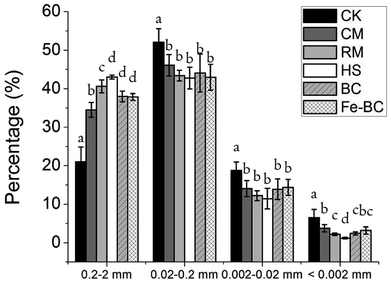
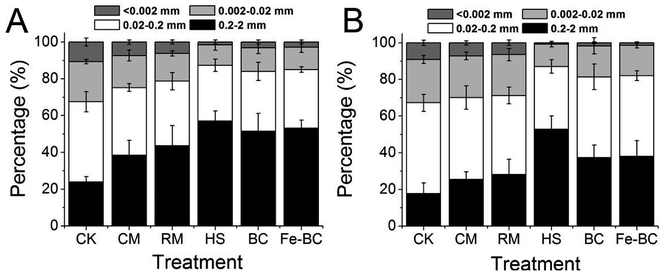 | ||
| Fig. 2 Mass loading values of total Cd (A) and DTPA–Cd (B) in different size fractions of soil aggregates. | ||
Application of amendments altered the mass loading of Cd in different size fractions of soil aggregates (Fig. 2A). The addition of amendments may have promoted the formation of Cd-containing large aggregates from aggregation of Cd-containing small aggregates or translocation of Cd from small-sized aggregates (<0.002 mm and 0.002–0.02 mm) to large ones (0.2–2 mm and 0.02–0.2 mm). The negatively charged substances or minerals and organic matter in the amendments have physical and chemical properties that aid co-precipitation, adsorption, complexation or electrostatic attraction of soil Cd. Thus, soil Cd forms complexes with these substances and with stronger affinity to bond with small soil particles.23,24 In addition, with greater concentrations of organic components and minerals present in the soil, the microaggregates containing metal ions are more likely to interact with these reactive amendments to form more stable macroaggregates.27,28 Notably, the increase of Cd mass loading in the size fraction of large aggregates and decrease of it in the size fraction of small aggregates could be beneficial for preventing toxic heavy metals to transfer from soil into plant tissues. Because fine soil aggregates have a higher affinity for heavy metals than large aggregates and a greater ability to move from one place to another because of their smaller size, they are more likely to be transported into plant tissues along with the heavy metals.6 Addition of HS resulted in significantly higher values of Cd mass loading in large aggregates (0.2–2 mm and 0.02–0.2 mm) and the next highest values were observed in the Fe-BC and BC treatments, which indicate that these amendments were more effective in stabilizing Cd into large soil aggregates than the other amendments (Fig. 2). Those amendments were likely more effective because of the physical and chemical characteristics. As previously mentioned, humic substances have negative charges that can enhance adsorption of Cd through electrostatic interactions and/or complexation reactions.23,24 Moreover, humic substances are able to bond with soil mineral particles to form large aggregates; mineral precipitation or the sorption of chemical species are usually responsible for the sequestration of heavy metals.24,29 In addition, the lowest available Cd concentration in HS treatment is also attributed to its relatively high CEC and soil OC (Table 1), as a significantly negative correlation between soil available Cd and CEC as well as OC was reported.30,31 Similarly, electrostatic attraction also occurs between anions like Cd and the negatively charged surface of BC and its functional groups (e.g. phenol hydroxyl, carboxyl, and carbonyl groups). Fe-BC not only plays a critical role in adsorbing Cd but also creates strong bonds with small soil particles to form stable macroaggregates.22,32
The concentration of DTPA-extractable heavy metals (including Cu, Zn, Fe, Mn, and Cd) is usually considered as an indicator of the available metal pool in soils. One of the main study objectives was to determine the efficacy of soil amendments in reducing Cd availability and inhibiting its transfer into plants; therefore, we quantified how much soil Cd would be (un)available after treatment based on DTPA-extracted concentrations of Cd. All treatments were effective in decreasing the level of DTPA-extractable Cd (Table 3). The lowest concentration of DTPA-extractable Cd was again observed in the HS treatment, where the concentration was 50.6% lower than the Cd concentration in the CK. The statistically lower concentration of DTPA-extractable Cd observed in amendment-treated soils might be due to the fact that several retention processes of the amendments, such as precipitation, diffusion, surface sorption, and ion exchange, contributed towards stabilization of Cd in the soil. In addition, DTPA–Cd concentration increased significantly with the decrease of size of soil aggregates (from 0.20–2.0 mm to <0.002 mm) for all treatments with amendments (Table 3). The substantially high concentration of Cd measured in the fine aggregate fraction could be explained by the fact that the small soil particles mainly consisted of silt and clay which have large specific surface areas and a high proportion of reactive substrates that tend to attract more Cd. Moreover, this metal can act as a binding agent in the formation of clay-polyvalent metal–organic matter complexes.33 Similar to the mass loading of total Cd in the different fractions of soil aggregates, the mass loading value of available Cd was the highest in the size fractions of 0.2–2 mm and 0.02–0.2 mm (Fig. 2B). Furthermore, a positive correlation between the distribution of the mass loading of total Cd and available Cd in soil aggregates was observed.
| Treatment | Bulk soil | 0.2–2 mm | 0.02–0.2 mm | 0.002–0.02 mm | <0.002 mm |
|---|---|---|---|---|---|
| Total Cd (mg kg−1) | |||||
| CK | 1.305 ± 0.034a | 1.519 ± 0.013b | 1.132 ± 0.013a | 1.442 ± 0.093a | 1.909 ± 0.069a |
| CM | 1.298 ± 0.011a | 1.567 ± 0.042ab | 1.064 ± 0.045a | 1.336 ± 0.020ab | 1.780 ± 0.084ab |
| RM | 1.302 ± 0.026a | 1.616 ± 0.063ab | 1.056 ± 0.104a | 1.321 ± 0.100ab | 1.741 ± 0.150b |
| HS | 1.311 ± 0.021a | 1.659 ± 0.019a | 1.010 ± 0.080a | 1.218 ± 0.070b | 1.598 ± 0.060b |
| BC | 1.307 ± 0.024a | 1.632 ± 0.019ab | 1.039 ± 0.022a | 1.275 ± 0.087ab | 1.663 ± 0.016b |
| Fe-BC | 1.304 ± 0.013a | 1.641 ± 0.059ab | 1.026 ± 0.035a | 1.258 ± 0.074ab | 1.627 ± 0.028b |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| DTPA-Cd (mg kg−1) | |||||
| CK | 0.423 ± 0.002a | 0.359 ± 0.051a | 0.396 ± 0.061a | 0.555 ± 0.033a | 0.630 ± 0.062a |
| CM | 0.305 ± 0.002b | 0.243 ± 0.041b | 0.295 ± 0.048b | 0.452 ± 0.038b | 0.441 ± 0.006b |
| RM | 0.283 ± 0.006b | 0.226 ± 0.025bc | 0.269 ± 0.054bc | 0.434 ± 0.022b | 0.434 ± 0.062b |
| HS | 0.188 ± 0.002d | 0.178 ± 0.016d | 0.190 ± 0.021c | 0.247 ± 0.014c | 0.142 ± 0.005c |
| BC | 0.218 ± 0.011c | 0.214 ± 0.023c | 0.216 ± 0.005c | 0.279 ± 0.018c | 0.161 ± 0.009c |
| Fe-BC | 0.209 ± 0.007c | 0.206 ± 0.039c | 0.206 ± 0.051c | 0.268 ± 0.037c | 0.152 ± 0.004c |
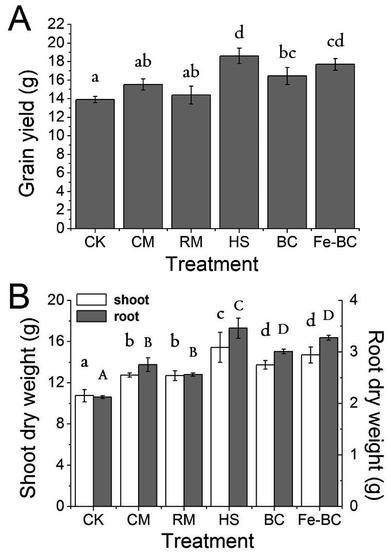
3.2 The effect of amendments on alleviation of Cd phytotoxicity to wheat
In this study, the concentration of Cd in the grain of wheat grown in naturally polluted soil (CK) exceeded the maximum permissible concentration (MPC) (0.2 mg kg−1) set by the Chinese National Food Quality Standard for Cd.36 This result suggests that there is a potential health risk in consuming wheat grown in local unamended soils. However, our results as a whole indicate that the application of amendments, particularly HS, BC or Fe-BC, lowered Cd concentrations in wheat grain to a safe level for human consumption. Furthermore, results indicate that remediation of polluted agricultural soil is possible with the simple application of soil amendments.
3.3 Correlations between Cd analysis and wheat growth
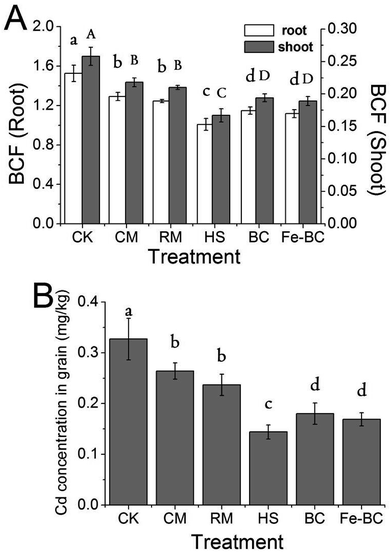
A strong correlation was found between soil DTPA–Cd and its accumulation in wheat tissues (shoot, root and grain) (Table 4), indicating that DTPA–Cd in soil is the likely source of Cd that transfers into plant tissues. The negative correlation between DTPA–Cd and wheat biomass accumulation (dry weight of tissues) suggests that the available Cd in soil may induce phytotoxicity and significantly inhibit plant growth. Moreover, Table 4 shows that the correlation of Cd mass loading (total Cd or DTPA–Cd) in soil aggregates with wheat growth or wheat Cd accumulation strongly depended on the size of aggregate; the mass loading of Cd in the fraction of 0.2–2 mm negatively correlated with DTPA–Cd concentration in the bulk soil and Cd accumulation in wheat tissues, and positively correlated with wheat dry weight. Conversely, the size fraction distribution of Cd in small aggregates, especially the <0.002 mm fraction, displayed strongly positive correlations with DTPA–Cd concentration in the bulk soil and Cd accumulation in wheat tissues. These results also confirmed that soil aggregates can significantly influence retention of Cd in soil. In situ addition of amendments, especially HS and (Fe)-BC, altered the distribution of Cd among soil aggregates and translocated Cd from small aggregates into large ones (Fig. 2). Therefore, the re-distribution of Cd among soil aggregates is likely the determining factor that controlled the efficacy of soil amendments in alleviating the phytotoxic effects of Cd on wheat.| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | (11) | (12) | (13) | (14) | (15) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a *Correlation is significant at the 0.05 level; italics and **correlation is significant at the 0.01 level (8–14) refer to Cd mass loading in different fractions. | |||||||||||||||
| Shoot dry weight (1) | 1.000 | ||||||||||||||
| Root dry weight (2) | 0.972** | 1.000 | |||||||||||||
| Grain yield (3) | 0.870** | 0.912** | 1.000 | ||||||||||||
| DTPA–Cd concentration in bulk soil (4) | −0.814 ** | −0.737** | −0.745** | 1.000 | |||||||||||
| Cd concentration in grain (5) | −0.892** | −0.853** | −0.817** | 0.942** | 1.000 | ||||||||||
| BCF (shoot) (6) | −0.908** | −0.881** | −0.788** | 0.856** | 0.966** | 1.000 | |||||||||
| BCF (root) (7) | −0.823** | −0.811** | −0.729** | 0.797** | 0.944** | 0.962** | 1.000 | ||||||||
| Total Cd in 0.2–2 mm (8) | −0.947** | 0.919** | 0.798** | −0.844** | −0.940** | −0.962** | −0.902** | 1.000 | |||||||
| Total Cd in 0.02–0.2 mm (9) | −0.907** | −0.873** | −0.783** | 0.863** | 0.963** | 0.969** | 0.940** | −0.984** | 1.000 | ||||||
| Total Cd in 0.002–0.02 mm (10) | −0.936** | −0.905** | −0.742** | 0.782** | 0.886** | 0.930** | 0.855** | −0.986** | 0.949** | 1.000 | |||||
| Total Cd in <0.002 mm (11) | −0.964** | −0.948** | −0.844** | 0.852** | 0.932** | 0.947** | 0.868** | −0.988** | 0.960** | 0.968** | 1.000 | ||||
| DTPA–Cd in 0.2–2 mm (12) | 0.932** | 0.897** | 0.813** | −0.868** | −0.992** | −0.909** | −0.834** | 0.980** | −0.963** | −0.959** | −0.981** | 1.000 | |||
| DTPA–Cd in 0.02–0.2 mm (13) | −0.558* | −0.509* | −0.423 | 0.545* | 0.706** | 0.720** | 0.814** | −0.699** | 0.789** | 0.640** | 0.623** | −0.651** | 1.000 | ||
| DTPA–Cd in 0.002–0.02 mm (14) | −0.827** | −0.772** | −0.690** | 0.805** | 0.769** | 0.717** | 0.601** | −0.853** | 0.800** | 0.866** | 0.863** | −0.918** | 0.385 | 1.000 | |
| DTPA–Cd in <0.002 mm (15) | −0.940** | −0.944** | −0.889** | 0.820** | 0.891** | 0.907** | 0.802** | −0.941** | 0.904** | 0.913** | 0.975** | −0.946** | 0.505* | 0.830** | 1.000 |
The primary purpose of this study was to investigate whether application of amendments into Cd-polluted soil could reduce the toxic levels of Cd in the edible parts of crops as well as determine the potential mechanisms that may control translocation of Cd into grains. Table 4 provides the correlations between measured variables. Grain–Cd was significantly and positively correlated with DPTA-Cd in the bulk soil (r = 0.942, p < 0.01) but negatively correlated with Cd mass loading in macroaggregates (r = 0.940 and 0.992 for total Cd and DTPA–Cd respectively, p < 0.01). Moreover, a significant positive correlation between grain–Cd and BCF (shoot and root) was observed (Table 4). After soil Cd is taken up by roots and transported to actively transpiring parts of the plant, Cd is then remobilized via the phloem or xylem to the grains.37
4. Conclusions
Results of this study indicate that soil aggregates significantly influenced retention and bioavailability of Cd in soil. The addition of amendments was responsible for immobilizing Cd in the soil and alleviating Cd phytotoxicity in wheat that was grown in the polluted soil by re-distributing more Cd ions from the fraction of smaller soil aggregates to the fraction of larger soil aggregates. The addition of soil amendments was effective in promoting the formation of large aggregates (0.2–2 mm and 0.02–0.2 mm) with greater mass loading of Cd (total Cd or DTPA extractable Cd). Active substances in the amendments likely attached to soil Cd and formed clay-poluvalent metal–organic matter complexes. Additional results obtained in this study were the significant negative correlations between the greater mass loading of Cd in macroaggregates (0.2–2 mm) and lower Cd accumulation in wheat tissues. In addition, the mitigation of Cd phytotoxicity (as indicated by the correlations between level of reduction in plant growth and Cd accumulation in plant tissues) was dependent upon the type of amendment applied. The addition of humic substances was most effective; it resulted in a 56.9% decrease of Cd concentration in wheat grain compared to that of the CK. This reduction likely corresponded with the 138.1% increase in total Cd accumulation in the soil fraction of 0.2–2 mm. This study fully demonstrates that addition of soil amendments alters soil Cd distribution among different sizes of soil aggregates and this mechanism of Cd immobilization can be easily applied by land managers to control or reduce Cd contamination in agricultural lands.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by the National Key Research and Development Program of China (2016YFD0800707), the National Key Technology R&D Program of China (2015BAD05B03), Natural Science Foundation of China (41271490 & 21706278). We are grateful to two anonymous reviewers for their constructive comments and suggestions on this manuscript.Notes and references
- H. Cui, K. Ma, Y. Fan, X. Peng, J. Mao, D. Zhou, Z. Zhang and J. Zhou, Environ. Sci. Pollut. Res., 2016, 23(11), 1–10 CrossRef PubMed.
- K. Ilg, W. Wilcke, G. Safronov, F. Lang, A. Fokin and M. Kaupenjohann, Geoderma, 2004, 123(1–2), 153–162 CrossRef CAS.
- Y. Gong, Y. Liu, Z. Xiong and D. Zhao, Environ. Sci. Technol., 2014, 48(7), 3986–3994 CrossRef CAS PubMed.
- M. Voltolini, N. Taş, S. Wang, E. L. Brodie and J. B. Ajo-Franklin, Geoderma, 2017, 305, 382–393 CrossRef CAS.
- H. Zhao and X. Li, Environ. Pollut., 2013, 174(5), 297 CrossRef CAS PubMed.
- B. Huang, Z. Li, J. Huang, L. Guo, X. Nie, Y. Wang, Y. Zhang and G. Zeng, J. Hazard. Mater., 2014, 264(2), 176 CrossRef CAS PubMed.
- G. Shi, S. Xia, J. Ye, Y. Huang, C. Liu and Z. Zhang, Environ. Exp. Bot., 2015, 111, 127–134 CrossRef CAS.
- M. Rizwan, S. Ali, M. Adrees, M. Ibrahim, T. Dcw, M. Zia-Ur-Rehman, Z. A. Zahir, J. Rinklebe, F. M. G. Tack and Y. S. Ok, Chemosphere, 2017, 182, 90–105 CrossRef CAS PubMed.
- L. Z. Li, C. Tu, W. J. G. M. Peijnenburg and Y. M. Luo, Environ. Pollut., 2017, 221, 351–358 CrossRef CAS PubMed.
- Z. Y. Hseu, S. W. Su, H. Y. Lai, H. Y. Guo, T. C. Chen and Z. S. Chen, Soil Sci. Plant Nutr., 2010, 56(1), 31–52 CrossRef CAS.
- F. Guo, C. Ding, Z. Zhou, G. Huang and X. Wang, Ecotoxicol. Environ. Saf., 2017, 148, 303 CrossRef PubMed.
- P. Janoš, J. Vávrová, L. Herzogová and V. Pilařová, Geoderma, 2010, 159(3–4), 335–341 CrossRef.
- C. C. Gilmour, G. S. Riedel, G. Riedel, S. Kwon, R. Landis, S. S. Brown, C. A. Menzie and U. Ghosh, Environ. Sci. Technol., 2013, 47(22), 13001–13010 CrossRef CAS PubMed.
- H. He, T. Nfy, A. Yao, R. Qiu, W. C. Li and Z. Ye, Chemosphere, 2017, 189, 247 CrossRef CAS PubMed.
- R. Van Poucke, J. Ainsworth, M. Maeseele, Y. S. Ok, E. Meers and F. M. G. Tack, Appl. Geochem., 2018, 88, 122–130 CrossRef CAS.
- N. Bolan, A. Kunhikrishnan, R. Thangarajan, J. Kumpiene, J. Park, T. Makino, M. B. Kirkham and K. Scheckel, J. Hazard. Mater., 2013, 266(4), 141–166 Search PubMed.
- W. L. Lindsay and W. A. Norvell, Soil Sci. Soc. Am. J., 1978, 42, 1–8 CrossRef.
- Z. X. Rao, D. Y. Huang, J. S. Wu, Q. H. Zhu, H. H. Zhu, C. Xu, J. Xiong, H. Wang and M. M. Duan, Environ. Pollut., 2018, 239, 198–204 CrossRef CAS PubMed.
- M. C. Manna, A. Swarup, R. H. Wanjari, B. Mishra and D. K. Shahi, Soil Tillage Res., 2007, 94(2), 397–409 CrossRef.
- Y. G. Wang, C. Combe and M. M. Clark, J. Membr. Sci., 2001, 183, 49–60 CrossRef CAS.
- F. M. D. Fonseca, M. M. D. Oliveira and L. N. H. Arakaki, J. Hazard. Mater., 2006, 137(1), 288–292 CrossRef PubMed.
- J. Wang, Z. Meng, X. Li, F. X. Liu and J. Xu, Environ. Pollut., 2017, 227, 175–182 CrossRef PubMed.
- M. Y. Chang and R. S. Juang, J. Colloid Interface Sci., 2004, 278(1), 18–25 CrossRef CAS PubMed.
- L. C. C. D. Silva, L. B. O. D. Santos, G. Abate, I. C. Cosentino, M. C. A. Fantini, J. C. Masini and J. R. Matos, Microporous Mesoporous Mater., 2008, 110(2), 250–259 CrossRef.
- G. Guibaud, F. Bordas, A. Saaid, P. D'Abzac and E. V. Hullebusch, Colloids Surf., B, 2008, 63(1), 48–54 CrossRef CAS PubMed.
- K. Quenea, I. Lamy, P. Winterton, A. Bermond and C. Dumat, Geoderma, 2009, 149(3), 217–223 CrossRef CAS.
- R. A. Sutherland, Environ. Pollut., 2003, 121(2), 229–237 CrossRef CAS PubMed.
- D. Varrica, G. Dongarrà, G. Sabatino and F. Monna, Environ. Geol., 2003, 44(2), 222–230 CAS.
- G. E. Brown Jr, A. L. Foster and J. D. Ostergren, Proc. Natl. Acad. Sci. U. S. A., 1999, 96(7), 3388–3395 CrossRef CAS.
- B. Yousaf, G. Liu, R. Wang, M. Zia-ur-Rehman, M. S. Rizwan, M. Imtiaz, G. Murtaza and A. Shakoor, Environ. Earth Sci., 2016, 75, 374–384 CrossRef.
- B. Yousaf, G. Liu, Q. Abbas, R. Wang, M. Imtiaz and M. Zia-ur-Rehman, Land Degrad Dev., 2017, 28(8), 1–12 Search PubMed.
- G. Guo, Y. Zhang, C. Zhang, S. Wang, Z. Yan and F. Li, Geoderma, 2013, 200–201(2), 108–113 CrossRef CAS.
- M. I. Umer and S. M. Rajab, Eurasian Journal of Silence, 2012, 1(1), 45–50 Search PubMed.
- P. Xu, C. X. Sun, X. Z. Ye, W. D. Xiao, Q. Zhang and Q. Wang, Ecotoxicol. Environ. Saf., 2016, 132, 94 CrossRef CAS PubMed.
- M. K. Uddin, Chem. Eng. J., 2016, 308, 438–462 CrossRef.
- Chinese Ministry of Health, Maximum levels of contaminants in foods, GB 2762-2005, 2005, http://jckspaqj.aqsiq.gov.cn/zwyxspjyjy/gnxgbz/200610/t20061027_16395.htm.
- M. S. Rodda, G. Li and R. J. Reid, Plant Soil, 2011, 347(1–2), 105–114 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2018 |