Open Access Article
Open Access ArticleSKLB023 protects mice against acute liver injury by inhibiting proinflammatory cytokine production in both T cells and macrophages†
Jia Yua,
Lili Liub,
Huiming Zhanga,
Yating Wuc,
Heying Peid,
Liang Mad,
Anwen Xionge and
Caifeng Xie *cd
*cd
aDepartment of Pharmacy, The Third Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China
bDepartment of Pharmacy, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China
cSchool of Basic Medical Sciences, Nanchang University, 999 Xuefu Road, Honggutang New District, Nanchang, Jiangxi 330031, China. E-mail: xiecaifeng2013@163.com; Fax: +86-791-83827160; Tel: +86-791-83827160
dState Key Laboratory of Biotherapy, West China Hospital, West China Medical School, Sichuan University, Chengdu, China
eDepartment of Medical Oncology, Shanghai Pulmonary Hospital & Thoracic Cancer Institute, Tongji University School of Medicine, Shanghai, China
First published on 27th September 2018
Abstract
Acute liver failure is a severe clinical syndrome accompanied with excessive inflammatory response. Our previous study demonstrated that SKLB023, a novel thiazolidinedione derivative, showed potent anti-inflammatory activity in rheumatoid arthritis. The purpose of the present study is to evaluate the protective effect of SKLB023 on lipopolysaccharide (LPS)/D-GalN-induced liver failure and to explore the underlying molecular mechanisms. Our results showed that SKLB023 significantly improved mortality and liver injury as indicated by reduced serum levels of aminotransferases and alleviated pathological damage. Additionally, SKLB023 decreased the percentage of activated T cells and macrophages as well as the serum levels of cytokines in vivo. Furthermore, SKLB023 decreased levels of TNF-α and IL-6 secreted from liver macrophages (Kupffer cells) stimulated by LPS in vitro. Our results indicated that the protective effects of SKLB023 were associated with its significant impact on the inflammatory cytokines, which were produced by both T cells and macrophages.
1. Introduction
Acute liver failure, induced by bacteria, viral hepatitis, alcohol and other hepatotoxic drugs, is a severe clinical syndrome which is characterized by hepatic encephalopathy, severe coagulopathy, jaundice, and hydroperitoneum.1,2 Apart from liver transplantation, there are still lack of available therapies, with a high mortality (80–90%) observed in patients.3–5 Immune dysregulation plays a central role in the pathogenesis of ALF, in which massive leucocyte infiltration of the injured liver is contrasted by a depletion and dysfunction of immune cells in circulation.6,7 Additionally, autoimmune hepatitis in humans has been demonstrated to be initiated by activation of T cells and macrophages.8–10 These cells either directly attack liver parenchymal cells or induce tissue damage by the release of several proinflammatory cytokines, such as TNF-α and IFN-γ.Cytokines are categorized into Th1- and Th2-type cytokines, which are secreted from different helper T cell populations.11 Some studies suggested that Th1 cytokines behave as pro-inflammatory mediators involved in the pathogenesis of the liver toxicity, which has been demonstrated in immune-mediated liver injury induced by LPS and concanavalin A.12,13 Therefore, decreased of Th1-type cytokines and increased of Th2-type cytokines might be benefit to the treatment of ALF.
LPS/D-GalN-induced liver injury in mice is usually considered an experimental model for clinical endotoxic shock or septic shock.14 In this model, LPS exerts its effects by stimulating inflammatory cells and hepatic kupffer cells to produce various pro-inflammatory cytokines, including interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6).15,16 Injection of mice with GalN followed by LPS administration induces acute liver injury within 8 h after application.17,18 Some research papers indicated that depletion of monocytes/macrophages to be protective, reducing liver injury.19,20
In our previous work, (Z)-N-(3-chlorophenyl)-2-(4-((2,4-dioxothiazolidin-5-ylidene)methyl)phenoxy)acetamide (SKLB023, Fig. S1†) was synthesized and evaluated for its potently anti-inflammatory effect through inhibiting the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX-2).21 Furthermore, SKLB023 attenuated joint inflammation and cartilage destruction in arthritis models via suppression of NF-κB activation in macrophage.22 However, whether SKLB023 showed anti-inflammatory effect on LPS/D-GalN induced acute liver failure remains unknown until now. In the present study, we first investigated the protection of SKLB023 against LPS/D-GalN-induced acute liver injury in vivo, and then examined the inhibitory effect of SKLB023 on the activation of T cells and macrophages. Finally, we tested whether SKLB023 inhibited the production of proinflammatory cytokines secreted by T cells and macrophages. Our results demonstrated that SKLB023 protected mice from severe liver injury likely through inhibiting the production of cytokines and the activation of T cells and macrophages.
2. Results
2.1. Effect of SKLB023 on survival rate after LPS/GalN administration
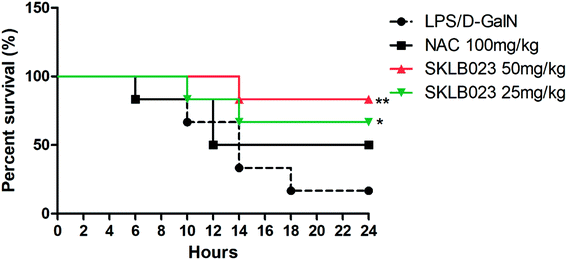
To investigate the effects of SKLB023 on LPS/GalN-induced lethality, the survival rate was observed within 24 h after LPS/GalN treatment (Fig. 1). The results showed that the survival rate of the LPS/GalN treated group was 16%. 25 and 50 mg kg−1 SKLB023 significantly increased the survival rate (66% and 83%), which higher than that of 100 mg kg−1 N-acetyl-L-cysteine (50%). N-Acetyl-L-cysteine (NAC) is commonly used in the treatment of ALF as a thiol antioxidant.23 In the present study, NAC was employed as positive control.2.2. Effect of SKLB023 on LPS/D-GalN-induced acute hepatic injury
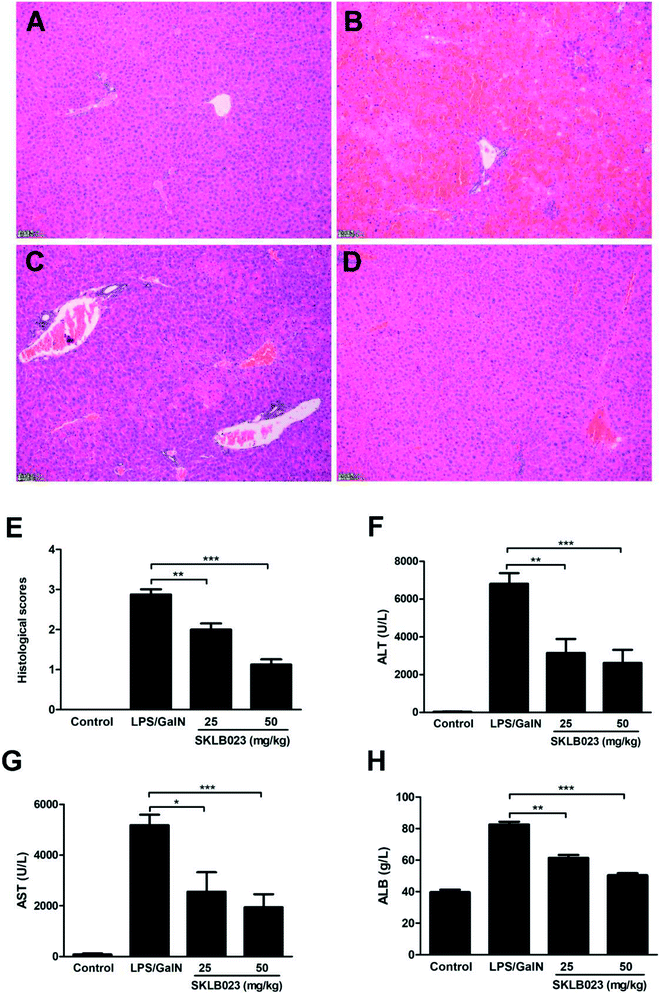
LPS/D-GalN-induced acute hepatic injury mice model was performed and SKLB023 was orally administrated. Histological examination of liver tissue sections showed no pathological changes in normal mice (Fig. 2A). However, congestion, hepatocyte necrosis, destruction of hepatic architecture, and massive immigration of inflammatory cells into the sinusoids were observed at 8 h after LPS/D-GalN administration (Fig. 2B). The treatment with 25 and 50 mg kg−1 of SKLB023 only showed patchy necrosis of hepatocytes and a slight immigration of inflammatory cells (Fig. 2C and D). In addition, the histological scores of mice significantly decreased by 25 mg kg−1 (**P < 0.01) and 50 mg kg−1 (***P < 0.001) of SKLB023, which compared with the LPS injected mice (Fig. 2E). Furthermore, Mice treated with SKLB023 showed a significant reduction of plasma AST, ALT and ALB (Fig. 2F–H), as compared with that of LPS/D-GalN group. SKLB023 also slightly decreased the serum level of ALP (Fig. S2†).2.3. Effect of SKLB023 on the accumulation of T cells and macrophages in mice livers with LPS/D-GalN injection
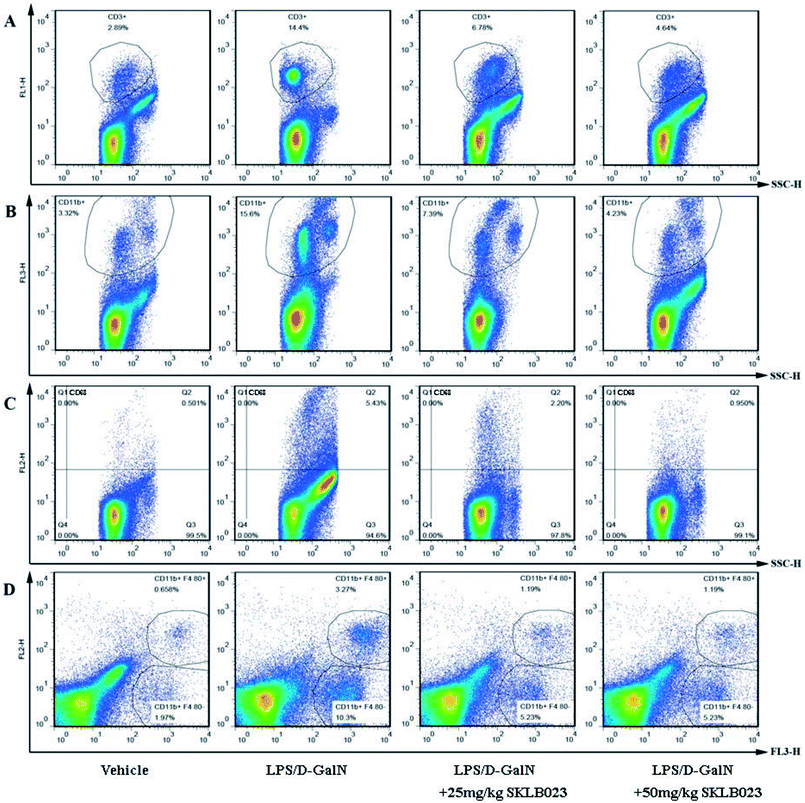
The T cells and macrophages in mice liver with LPS/D-GalN injection were isolated and analyzed by FACS. The percentage of CD3 positive T cells in liver of vehicle mice was 2.89%, but the percentage was increased significantly to 14.4% after treated with LPS/D-GalN. The administration of SKLB023 at the doses of 25 and 50 mg kg−1 decreased the percentage to 6.78% and 4.64% (Fig. 3A). CD11b, CD68 and F4/80 positive cells staining showed that SKLB023 possessed great impact on the phenotype of macrophages at 25 and 50 mg kg−1 in LPS/D-GalN (Fig. 3B–D). 3.32% CD11b positive cells were present in vehicle mice liver, but the injection of LPS increased the percentage of CD11b positive cells to 15.6%. The CD11b positive cells were decreased by SKLB023 treatment at 25 and 50 mg kg−1 to 7.39% and 4.23% (Fig. 3B). SKLB023 suppressed the accumulation of CD68 positive cells in the liver of mice injected with LPS/D-GalN (Fig. 3C). In addition, Fig. 3D showed the percentage of CD11b and F4/80 co-positive cells in the liver of mice in each group. 0.658% CD11b and F4/80 co-positive cells were present in vehicle mice liver, but the percentage was increased to 3.27% in LPS/D-GalN treated mice. However, the treatment of SKLB023 at 25 and 50 mg kg−1 inhibited the accumulation of CD11b and F4/80 co-positive cells in the liver.2.4. Effect of SKLB023 on the levels of serum inflammatory cytokines in LPS/D-GalN stimulated mice
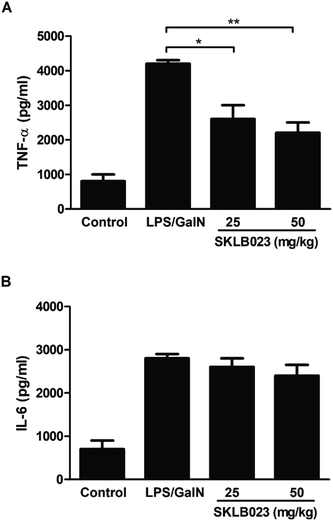
The levels of serum TNF-α and IL-6 were determined by ELISA, and the results were showed in Fig. 4. LPS/D-GalN administration significantly increased the levels of TNF-α and IL-6 in the serum of mice, however, SKLB023 significantly inhibited the production of TNF-α in the serum (Fig. 4A). SKLB023 also suppressed the expression of IL-6 (Fig. 4B), although the difference was not statistically significant.2.5. Effect of SKLB023 on proinflammatory cytokines production in T cells in vitro
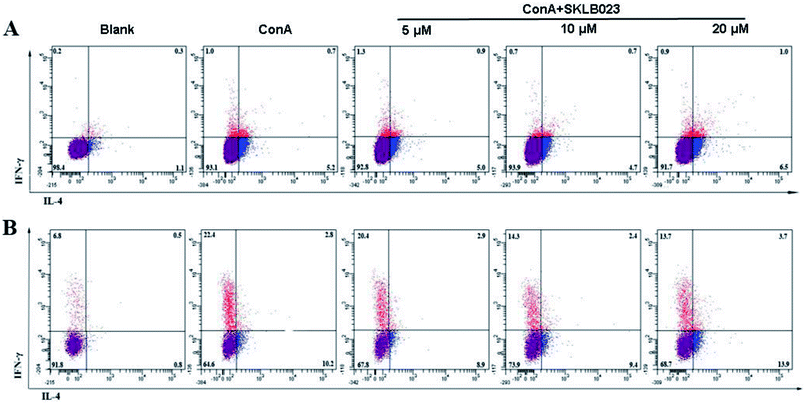
The effect of SKLB023 on the activation of T cells was investigated by flow cytometry analysis. As illustrated in Fig. S3A,† SKLB023 possessed little impact on the proliferation of CD3 positive cells. However, moderate decrease of the percentage of CD4 positive T cells (CD4+) as well as moderate elevation of CD8 positive T cells (CD8+) at different concentrations was showed in Fig. S3B† (31.0, 29.4, and 28.0% for CD4+; 2.7, 3.9, and 3.5% for CD8+ at 5, 10, and 20 μM of SKLB023, respectively).The production of IFN-γ and IL-4 in T cells and culture supernatants of the spleen cells isolated from mice were measured by FACS (Fig. 5). The expression levels of intracellular cytokines IFN-γ and IL-4, produced by CD4+ or CD8+ T cells were determined by Flow cytometry (Fig. 5). The production of IFN-γ was raised after stimulated by ConA in both CD4+ T cells (Fig. 5A, from 0.2% to 1.0%) and CD8+ T cells (Fig. 5B, from 6.8% to 22.4%). However, SKLB023 suppressed the production of IFN-γ at different concentrations (0.7% and 0.9% for CD4+ T cells at 10 and 20 μM, and 20.4, 14.3, and 13.7% for CD8+ 123 at 5, 10, and 20 μM of SKLB023, respectively). The production of IL-4 was also raised after stimulated by ConA in both CD4+ T cells (from 1.1% to 5.2%) and CD8+ T cells (from 0.8% to 10.2%). However, as illustrated in Fig. 5, SKLB023 possessed little impact on the production of IL-4 in CD4+ and CD8+ cells at different concentrations.
2.6. Effect of SKLB023 on proinflammatory cytokines production in liver macrophages
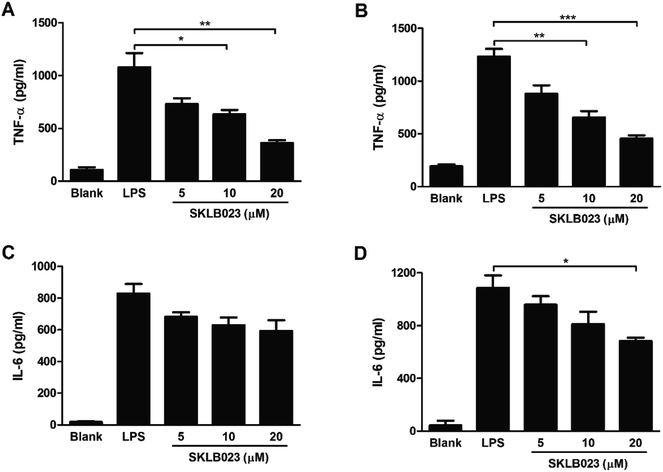
Our previous studies indicated that SKLB023 shown potential anti-inflammatory activity in RAW264.7 cells.22 In present investigation, we analyzed the effect of SKLB023 on the cytokines production of liver macrophages (Kupffer cells) stimulated by LPS in vitro. SKLB023 significantly decreased the cytokines secreted by these macrophages. The expression levels of TNF-α and IL-6 were decreased by SKLB023 in a dose-dependent manner at 48 and 72 hours (Fig. 6). The level of TNF-α increased to 1079.2 pg ml−1 at 48 h and 1232.1 pg ml−1 at 72 h after LPS stimulated, however, different concentrations of SKLB023 significantly decreased the level of TNF-α to 729.5, 633.1 (*P < 0.05), and 370.2 (**P < 0.01) pg ml−1 at 48 h (Fig. 6A), and 874.6, 649.7 (**P < 0.01), and 455.3 (***P < 0.001) pg ml−1 at 72 h (Fig. 6B). Additionally, SKLB023 decreased the level of IL-6 at 5, 10, and 20 μM within 48 h (Fig. 6C) and 72 h (Fig. 6D).3. Discussion
LPS/D-GalN-induced liver injury is a well-established experimental model that closely resembles human fulminant hepatitis in both morphological and functional features. When sensitized by the specific hepatotoxic D-GalN, a low dose of LPS could induce severe hepatic damage.24,25 In the present study, a novel synthetic small-molecule compound (SKLB023) ameliorated liver damage in LPS/D-GalN-induced liver injury model with a reduction in serum TNF-α and IL-6. SKLB023 significantly elevated the survival rate of LPS/D-GalN-induced ALF, and also decreased the activation T cells and macrophages in the liver of mice stimulated by LPS. Furthermore, SKLB023 significantly suppressed the production of inflammatory cytokines in both T cells and macrophages in vitro.In LPS/D-GalN-induced liver injury model, we found the number of CD3 positive T cells and the percentage of CD68, CD11b and F4/80 positive cells were decreased by SKLB023, which means that both T cells and macrophages participated in the LPS-induced liver injury, and SKLB023 would prevented mice from attack by decreasing the activation of T cells and macrophages.
The cytokine secretion pattern of CD4+ T cells defines two main subsets of helper cells. Th1 cells produce IL-2, IFN-γ, and TNF-α, whereas Th2 cells secrete IL-4, IL-10, and IL-13. The importance of CD4+ T cell dichotomy is underlined by the growing body of evidence that the outcome of numerous diseases critically depends on the Th1/Th2 balance in the accompanying immune responses.11,26 Our results showed that SKLB023 decreased the expression of IFN-γ and increased the production of IL-4 at the same time, which indicated SKLB023 ameliorated LPS/D-GalN-induced acute liver injury by regulating the polarization of Th1/Th2 balance.
Macrophages play an important role in innate immunity and participate as effector cells in adaptive immune responses. They secrete multiple inflammatory mediators (e.g., IL-1β, IL-6, and TNF-α) when induced in Th1-dominated immune responses and are therefore termed inflammatory macrophages.27 Our results showed that SKLB023 decreased the production of TNF-α and IL-6 secreted by macrophages. It is reported that IL-6 produced by APCs can modulate the differentiation of CD4+ T cells into effectors Th1 or Th2 and it shifted the Th1/Th2 balance toward the Th2 direction.28 It seems that SKLB023 may suppress the expression of IL-6 and then modulated the differentiation of CD4+ T cells. However, we need some additional experiments to identify this hypothesis.
In our previous study, pharmacokinetic profiles of SKLB023 in mice were determined by Ultra Performance Liquid Chromatography (UPLC) assay, which suggested that the oral bioavailability of SKLB023 was 18.10%, with the half-time of 2.16 h.21 Furthermore, the acute toxicity of SKLB023 on mouse was evaluated and the results showed that the LD50 of SKLB023 was higher than 2 g kg−1 (p.o.) (unpublished data). The long-term toxicity of SKLB023 is conducting in our present project, and we here show a representative H&E staining result for histopathological evaluation of the liver, kidneys, heart, lungs, and spleen organs of mice treated with SKLB023 (50 mg kg−1, p.o.) for one month (Fig. S4†). Both acute toxicity and H&E staining results indicated that SKLB023 is a potent anti-inflammatory compound with good safety and pharmacokinetic profiles.
In conclusion, our results indicated that SKLB023 protected mice from LPS/D-GalN-induced liver failure. These beneficial effects of SKLB023 seem to be associated with the inhibition of pro-inflammatory cytokines secreted by T cells and macrophages, which finally protects mice from liver injury. Therefore, SKLB023 offers a novel effective therapeutic strategy for the treatment of acute liver failure.
4. Materials and methods
4.1. Animal ethics and statement
BALB/c mice (6–8 weeks old; weight range 20–22 g) were obtained from Huafukang Biotechnology Company. Animals were maintained under constant conditions (temperature: 22 ± 2 °C, humidity: 40–60%, 12 h light/12 h dark cycle). Standard lab chow and tap water were available ad libitum. All animal studies were carried out in accordance with the NIH guidelines for the care and use of laboratory animals (NIH Publication no. 85–23 Rev. 1985) and was approved by the Animal Care and Use Committee of Sichuan University in China (IACUC number: 20100318).4.2. Reagents
SKLB023 was prepared as previously described.21 Yield 92.8%; analytical HPLC purity = 98.53%; white solid. N-Acetyl-L-cysteine (NAC), LPS, D-GalN and other analytical grade chemicals were purchased from Sigma. Antibodies were obtained from eBioscience.4.3. LPS/D-GalN-induced acute hepatic inflammation
BALB/c mice were treated with SKLB023 (25 mg kg−1 and 50 mg kg−1, dissolved in 0.25% CMC, p.o.) or equal volume of vehicle immediately after the injection of LPS (0.35 μg kg−1, Salmonella abortus equi) and D-GalN (750 mg kg−1). Mice were sacrificed at 8 h after LPS/D-GalN challenge.18 Plasma enzyme activities of ALT, AST, ALP and ALB were measured by using an automated procedure, and the levels of serum TNF-α and IL-6 were analyzed by ELISA (R&D system, USA) according to manufacturer's instructions. In addition, parts of liver tissue in each mouse were quickly put into cold PBS for flow cytometry analysis, and other parts were fixed in formalin for H&E analysis.To calculate the survival rate, mice were challenged with 750 mg kg−1 D-GalN and 1.5 μg kg−1 LPS, which was reported as a lethal dose.29 100 mg kg−1 NAC, 25 and 50 mg kg−1 SKLB023 were respectively pre-administrated 1 h before GalN/LPS challenge. The number of dead mice was counted every 2 h and the survival rates of mice were monitored for 24 h after GalN/LPS injection.
4.4. T cells and macrophages analysis in the liver of mice injected with LPS/D-GalN
Liver samples were collected at 8 h and then perfused in situ with 10 ml cold PBS to remove circulating peripheral blood lymphocytes. Then, liver fragments were pressed through a sterile stainless steel screen in 50 ml cold PBS. The hepatocytes were collected at the bottom by low-speed centrifugation for analysis of macrophages, and the supernatant was collected for analysis of T cells. Macrophages and T cells were prepared as described previously30,31 with slight modifications. Macrophages were further separated from hepatocytes by gradient centrifugation, and the method of selective adherence to plastic was used to purify the obtained cell population. The supernatant obtained from the low-speed centrifugation was collected and centrifuged, and the pellet was resuspended. The cell suspension was then layered on top of a density cushion of 40%/70% discontinuous Percoll (Pharmacia Biotech, Uppsala, Sweden) and centrifuged to obtain the lymphocyte fraction at the interface. Cells were collected, lysed with RBC lysing buffer and washed with cold PBS. T cells and macrophages were stained with anti-mouse CD3, CD11b, CD68 and F4/80 antibodies (eBioscience, San Diego, CA) and subjected to FACS analysis.4.5. Histological analysis
Livers were removed (n = 8 per group) from animals under pentobarbital anesthesia (100 mg kg−1; intraperitoneal injection). Livers were sectioned, fixed in 10% formalin, and embedded in paraffin. The sections were then stained with hematoxylin and eosin. Extent of liver injury was evaluated according to Heijnen's technique.32,33 Briefly, liver injury was assessed as the sum of individual scores ranging from 0 to 3 (0, no injury; 1, mild; 2, moderate; 3, severe) for the following parameters: cytoplasmic color fading, vacuolization, nuclear condensation, nuclear fragmentation, nuclear fading, and erythrocyte stasis.4.6. T cells isolation and pro-inflammatory cytokines analysis
Splenocytes were collected from BALB/c mice, treated with RBC lysis buffer (Sigma, St. Louis, MO), and T cells were incubated in 6-well Costar plates in the presence of Con A (5 μg ml−1) and SKLB023 at the concentration of 5, 10, 20 μM for 72 h. Cytokine contents (IL-4 and IFN-γ) were measured in 72 hour supernatants of the spleen cell cultures using a specific enzyme-linked immunosorbent assay (ELISA) for mice (MiniKit; Endogen), according to manufacturer's instructions. Cells were then stained for the surface expression of CD3, CD4, and CD8 (Biolegend, CA, USA). Samples were then washed and examined by flow cytometry with CellQuest software (BD FACS Calibur).4.7. Preparation of liver macrophages (Kupffer cells) and cytokines analysis
BALB/c mice were anaesthetized by diethyl ether inhalation and liver samples were collected after perfusion. The liver tissues were dispersed by 0.1% type IV collagenase digestion and filtered through a 74 μm stainless steel wire mesh to remove undigested tissue. Macrophages was prepared as described in Materials and methods (4.4). Macrophages were cultured with DMEM medium alone or activated with 2 ng ml−1 IFN-γ and 100 ng ml−1 LPS, and treated in absence or presence of SKLB023 (5, 10, 20 μM).34 Supernatant of activated macrophages cultured for 48 h and 72 h was collected, and the levels of TNF-α and IL-6 in supernatant were determined by ELISA (R&D system, USA) according to manufacturer's instructions.4.8. Statistical analysis
Data were expressed as mean ± SD and differences between group means were calculated by Student's t test or by ANOVA where appropriate. P values less than or equal to 0.05 were considered significant.5. Author contributions
J. Y. and C. F. X. designed the study, analyzed the results and wrote the manuscript; J. Y., L. L. Y., and Y. T. W. conducted the research and analyzed the results; H. M. Z. and A. W. X. participated in the study design and wrote the manuscript; H. Y. P. and L. M. conducted the animal experiment and analyzed the results.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
This work is supported by grant to J.-Yu from the Science and Research Fund of Jiangxi Health and Family Planning Commission (No. 20187011), grant to C.-F. Xie from Natural Science Foundation of Jiangxi Province (20161BAB215201), and grant to A.-W. Xiong from National Natural Science Foundation of China (81502292).References
- S. Krawitz, V. Lingiah and N. T. Pyrsopoulos, Acute Liver Failure: Mechanisms of Disease and Multisystemic Involvement, Clin. Liver Dis., 2018, 22, 243–256 CrossRef PubMed.
- W. Bernal, G. Auzinger, A. Dhawan and J. Wendon, Acute liver failure, Lancet, 2010, 376, 190–201 CrossRef.
- P. Patel, N. Okoronkwo and N. T. Pyrsopoulos, Future Approaches and Therapeutic Modalities for Acute Liver Failure, Clin. Liver Dis., 2018, 22, 419–427 CrossRef PubMed.
- D. H. Van Thiel, J. Brems, A. Nadir, R. Idilman, A. Colantoni, D. Holt and S. Edelstein, Liver transplantation for fulminant hepatic failure, J. Gastroenterol., 2002, 37, 78–81 CrossRef PubMed.
- S. H. Kim, Y. S. Kim, S. S. Kang, K. Bae, T. M. Hung and S. M. Lee, Anti-apoptotic and hepato protective effects of gomisin A on fulminant hepatic failure induced by D-galactosamine and lipopolysaccharide in mice, J. Pharmacol. Sci., 2008, 106, 225–233 CrossRef CAS.
- C. G. Antoniades, P. A. Berry, J. A. Wendon and D. Vergani, The importance of immune dysfunction in determining outcome in acute liver failure, J. Hepatol., 2008, 49, 845–861 CrossRef CAS PubMed.
- Z. Wu, M. Han, T. Chen, W. Yan and Q. Ning, Acute liver failure: mechanisms of immune-mediated liver injury, Liver Int., 2010, 30, 782–794 CrossRef CAS PubMed.
- C. G. Antoniades, A. Quaglia, L. S. Taams, R. R. Mitry, M. Hussain, R. Abeles, L. A. Possamai, M. Bruce, M. McPhail, C. Starling, B. Wagner, A. Barnardo, S. Pomplun, G. Auzinger, W. Bernal, N. Heaton, D. Vergani, M. R. Thursz and J. Wendon, Source and characterization of hepatic macrophages in acetaminophen-induced acute liver failure in humans, Hepatology, 2012, 56, 735–746 CrossRef CAS PubMed.
- Q. Yang, Y. Shi, J. He and Z. Chen, The evolving story of macrophages in acute liver failure, Immunol. Lett., 2012, 147, 1–9 CrossRef CAS PubMed.
- C. Tuncer, Y. H. Oo, N. Murphy, D. H. Adams and P. F. Lalor, The regulation of T-cell recruitment to the human liver during acute liver failure, Liver Int., 2013, 33, 852–863 CrossRef CAS PubMed.
- T. R. Mosmann and S. Sad, The expanding universe of T-cell subsets: Th1, Th2 and more, Immunol. Today, 1996, 17, 138–146 CrossRef CAS PubMed.
- T. R. Mosmann, H. Cherwinski, M. W. Bond, M. A. Giedlin and R. L. Coffman, Two types of murine helper T cell clone. I. definition according to profiles of lymphokine activities and secreted proteins, J. Immunol., 1986, 136, 2348–2357 CAS.
- Y. Tanaka, A. Takahashi, K. Watanabe, K. Takayama, T. Yahata, S. Habu and T. Nishimura, A pivotal role of IL-12 in Th1-dependent mouse liver injury, Int. Immunol., 1996, 8, 569–576 CrossRef CAS PubMed.
- Z. Dong, H. Wei, R. Sun and Z. Tian, The roles of innate immune cells in liver injury and regeneration, Cell. Mol. Immunol., 2007, 4, 241–252 CAS.
- H. Tsutsui and S. Nishiguchi, Importance of Kupffer cells in the development of acute liver injuries in mice, Int. J. Mol. Sci., 2014, 15, 7711–7730 CrossRef PubMed.
- S. B. Yee, P. E. Ganey and R. A. Roth, The role of Kupffer cells and TNF-α in monocrotaline and bacterial lipopolysaccharide-induced liver injury, Toxicol. Sci., 2003, 71, 124–132 CrossRef CAS PubMed.
- C. Galanos, M. A. Freudenberg and W. Reutter, Galactosamine induced sensitization to the lethal effects of endotoxin, Proc. Natl. Acad. Sci. U. S. A., 1979, 76, 5939–5943 CrossRef CAS.
- A. M. Wolf, D. Wolf, H. Rumpold, S. Ludwiczek, B. Enrich, G. Gastl, G. Weiss and H. Tilg, The kinase inhibitor imatinib mesylate inhibits TNF-{alpha} production in vitro and prevents TNF-dependent acute hepatic inflammation, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 13622–13627 CrossRef CAS PubMed.
- N. Van Rooijen and A. Sanders, Kupffer cell depletion by liposome-delivered drugs: comparative activity of intracellular clodronate, propamidine and ethylenediaminetetraacetic acid (EDTA), Hepatology, 1996, 23, 1239–1243 CrossRef CAS PubMed.
- H. Tsutsui, M. Imamura, J. Fujimoto and K. Nakanishi, The TLR4/TRIF-mediated activation of NLRP3 inflammasome underlies endotoxin-induced liver injury in mice, Gastroenterol. Res. Pract., 2010, 2010, 641865 Search PubMed.
- L. Ma, C. Xie, Y. Ma, J. Liu, M. Xiang, X. Ye, H. Zheng, Z. Chen, Q. Xu, T. Chen, J. Chen, J. Yang, N. Qiu, G. Wang, X. Liang, A. Peng, S. Yang, Y. Wei and L. Chen, Synthesis and biological evaluation of novel 5-benzylidenethiazolidine-2,4-dione derivatives for the treatment of inflammatory diseases, J. Med. Chem., 2011, 54, 2060–2068 CrossRef CAS PubMed.
- C. Xie, L. Ma, J. Liu, X. Li, H. Pei, M. Xiang and L. Chen, SKLB023 blocks joint inflammation and cartilage destruction in arthritis models via suppression of nuclear factor-kappa B activation in macrophage, PLoS One, 2013, 8, e56349 CrossRef CAS PubMed.
- S. M. Riordan and R. Williams, Liver: management of non-acetaminophen-induced ALF, Nat. Rev. Gastroenterol. Hepatol., 2010, 7, 75–77 CrossRef CAS PubMed.
- R. Silverstein, D-Galactosamine lethality model: scope and limitations, J. Endotoxin Res., 2004, 10, 147–162 CAS.
- E. A. Wilhelm, C. R. Jesse, S. S. Roman, C. W. Nogueira and L. Savegnago, Hepatoprotective effect of 3-alkynyl selenophene on acute liver injury induced by D-galactosamine and lipopolysaccharide, Exp. Mol. Pathol., 2009, 87, 20–26 CrossRef CAS PubMed.
- P. Kidd, Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease, Altern. Med. Rev., 2003, 8, 223–246 Search PubMed.
- H. L. Twigg 3rd, Macrophages in innate and acquired immunity, Semin. Respir. Crit. Care Med., 2004, 25, 21–31 CrossRef PubMed.
- S. Diehl and M. Rincón, The two faces of IL-6 on Th1/Th2 differentiation, Mol. Immunol., 2002, 39, 531–536 CrossRef CAS PubMed.
- G. Tiegs, J. Barsig, B. Matiba, S. Uhlig and A. Wendel, Potentiation by granulocyte macrophage colony-stimulating factor of lipopolysaccharide toxicity in mice, J. Clin. Invest., 1994, 93, 2616–2622 CrossRef CAS PubMed.
- P. Z. Li, J. Z. Li, M. Li, J. P. Gong and K. He, An efficient method to isolate and culture mouse Kupffer cells, Immunol. Lett., 2014, 158, 52–56 CrossRef CAS PubMed.
- X. Shen, Y. Wang, F. Gao, F. Ren, R. W. Busuttil, J. W. Kupiec-Weglinski and Y. Zhai, CD4 T cells promote tissue inflammation via CD40 signaling without de novo activation in a murine model of liver ischemia/reperfusion injury, Hepatology, 2009, 50, 1537–1546 CrossRef CAS PubMed.
- B. H. Heijnen, I. H. Straatsburg, D. J. Gouma and T. M. van Gulik, Decrease in core liver temperature with 10 degrees C by in situ hypothermic perfusion under total hepatic vascular exclusion reduces liver ischemia and reperfusion injury during partial hepatectomy in pigs, Surgery, 2003, 134, 806–817 CrossRef PubMed.
- S. J. Johnson, J. E. Hines and A. D. Burt, Phenotypic modulation of perisinusoidal cells following acute liver injury: a quantitative analysis, Int. J. Exp. Pathol., 1992, 73, 765–772 CAS.
- Y. W. Kim, R. J. Zhao, S. J. Park, J. R. Lee, I. J. Cho, C. H. Yang, S. G. Kim and S. C. Kim, Anti-inflammatory effects of liquiritigenin as a consequence of the inhibition of NF-kappaB-dependent iNOS and proinflammatory cytokines production, Br. J. Pharmacol., 2008, 154, 165–173 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The following are available online. Fig. S1: chemical structure of SKLB023; Fig. S2: the effect of SKLB023 on the serum level of ALP in LPS/D-GalN-induced ALF model. Fig. S3: the effect of SKLB023 on the proliferation of T cells. Fig. S4: histopathological evaluation of organs isolated from mice treated with SKLB023 for one month. See DOI: 10.1039/c8ra03720e |
| This journal is © The Royal Society of Chemistry 2018 |