Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The synthesis of imidazo[1,5-a]quinolines via a decarboxylative cyclization under metal-free conditions†
Zicong Yana,
Changfeng Wanb,
Yu Yanga,
Zhenggen Zha*a and
Zhiyong Wang *a
*a
aHefei National Laboratory for Physical Sciences at Microscale, CAS Key Laboratory of Soft Matter Chemistry & Center for Excellence in Molecular Synthesis of Chinese Academy of Sciences, Collaborative Innovation Center of Suzhou Nano Science and Technology & School of Chemistry and Materials Science, University of Science and Technology of China, 230026, Hefei, Anhui, P. R. China. E-mail: zwang3@ustc.edu.cn; Tel: +86-551-6360-3185
bCollege of Chemistry and Chemical Engineering, Jiangxi Normal University, 330022, Nanchang, P. R. China. E-mail: wanfeng@jxnu.edu.cn
First published on 25th June 2018
Abstract
An iodine-mediated decarboxylative cyclization was developed from α-amino acids and 2-methyl quinolines under metal-free conditions, affording a variety of imidazo[1,5-a]quinolines with moderate to good yields.
Introduction
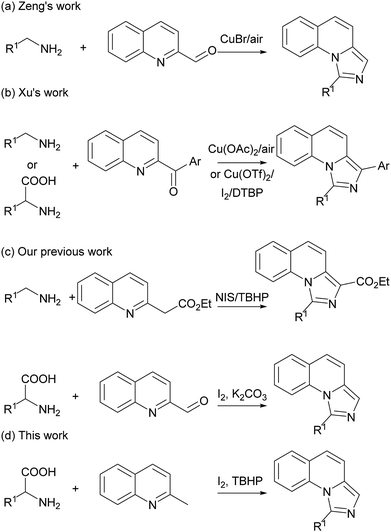
The imidazo[1,5-a]quinoline moiety is an important class of fused N-heterocyclic compounds such as skeletons of NK1 receptor ligands,1 antitumor drug C-1311,2 potential antimicrobial natural product cribrostatin 6,3 inhibitor targeting phosphodiesterase 10A4 and highly efficient ligands of central benzodiazepine receptors.5 As the structure for the precursors of N-heterocyclic carbene6 and other transformations,7 much effort has been done for the synthesis of imidazo[1,5-a]quinolines. The previous strategies mainly focused in Vilsmeier-type cyclizations using N-2-pyridylmethylamides as starting materials.8 Subsequently, various new protocols have also been developed to synthesize these compounds.9 Recently, Zeng and Xu groups independently reported copper-catalyzed imidazole synthesis via the oxidative C–H amination reactions (Scheme 1a and b).10 These elegant protocols led to new reactivity pathways and made great progress in imidazole synthesis. However, these reported methods still suffered from the usage of transition-metal catalysts. Our group also developed previously some oxidative C–H amination reactions for imidazole synthesis under metal-free conditions.11 The main drawbacks are the narrow scope of the substrates and the difficulty of the available starting materials. Therefore, a more environmental-friendly methodology with readily available starting materials for the preparation of imidazo[1,5-a]quinolines is also highly desirable.On the other hand, decarboxylative reactions were widely used in organic synthesis, especially in pericyclic reactions for heterocycles.12 Recently, a series of cascade decarboxylative reactions involving azomethine ylides have been developed to construct C–C and C–N bonds.13 As a facile, stable and cheap starting materials, amino acids has long been neglected in organic synthesis.14 Our group also developed some decarboxylative cyclization reactions from amino acids.15 As our continuing interest in the decarboxylative reaction for the synthesis of N-heterocycles, herein we report a new decarboxylative cascade reaction for the synthesis of imidazo[1,5-a]quinolines starting from readily available materials under metal-free condition (Scheme 1c and d).
Results and discussion
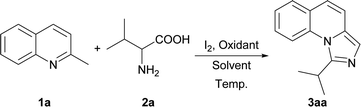
Initially, we employed 2-methylquinoline (1a) and valine (2a) as model substrates. The results are summarized in Table 1. Firstly, the reaction of 1 equiv. of 1a and 1.5 equiv. of 2a was carried out in the presence of 1 equiv. of I2 in N,N-dimethylformamide (DMF) at 80 °C for 3 h to give rise to a 20% yield of the expected product 1-isopropylimidazo[1,5-a]quinoline (3aa) (Entry 1, Table 1). Then the influence of oxidants on this reaction was investigated (Entries 2–6, Table 1). It was found that the yield could be improved to 84% by using 3 equiv. of tert-butyl hydroperoxide (TBHP) while the other oxidants, such as di-tert-butyl peroxide (DTBP), dioxygen and peroxysulfates had little influence on the reaction or deteriorated the reaction.| Entry | Oxidant | Solvent | Temp. (°C) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (1.0 equiv., 0.2 mmol), 2a (1.5 equiv., 0.3 mmol), I2 (1.0 equiv.), oxidant (3 equiv.) in solvent (0.5 mL).b Isolated yield.c The reaction was carried out without I2.d The reaction was carried out with 20% of I2. | ||||
| 1 | — | DMF | 80 | 20 |
| 2 | TBHP | DMF | 80 | 84 |
| 3 | O2 | DMF | 80 | 84 |
| 4 | DTBP | DMF | 80 | 18 |
| 5 | K2S2O8 | DMF | 80 | 0 |
| 6 | (NH4)2S2O8 | DMF | 80 | Trace |
| 7 | TBHP | DMA | 80 | 81 |
| 8 | TBHP | DMSO | 80 | 57 |
| 9 | TBHP | H2O | 80 | n. d. |
| 10 | TBHP | Tol. | 80 | n. d. |
| 11 | TBHP | MeCN | 80 | Trace |
| 12 | TBHP | MeOH | 80 | n. d. |
| 13 | TBHP | THF | 80 | n. d. |
| 14 | TBHP | 1,4-Dioxane | 80 | n. d. |
| 15 | TBHP | DMF/H2O | 80 | 37 |
| 16 | TBHP | DMF/MeOH | 80 | 49 |
| 17 | TBHP | DMF | 25 | 25 |
| 18 | TBHP | DMF | 50 | 43 |
| 19 | TBHP | DMF | 100 | 83 |
| 20 | TBHP | DMF | 120 | 83 |
| 21c | TBHP | DMF | 80 | 0 |
| 22d | TBHP | DMF | 80 | 17 |
Afterwards several solvents were also examined (Entries 7–16, Table 1). N,N-Dimethylacetamide (DMA) had a little negative influence on the reaction (Entry 7, Table 1) while dimethyl sulfoxide (DMSO) reduced the reaction yield remarkably. Other solvents, such as ethyl nitrile, toluene, tetrahydrofuran (THF), methanol, 1,4-dioxane and water would result in the failure of the reaction (Entries 9–14, Table 1). The mixed solvents deteriorated the reaction either (Entries 15–16, Table 1). Subsequently the different temperature was examined for this reaction. When the reaction temperature was increased to 100 °C, the corresponding product can be obtained with a yield of 83%. (Entry 19, Table 1). Further increase of the temperature could not improve the yield (Entry 20, Table 1). The product 3aa was obtained with the yields of 25% and 43% when the reactions were carried out at 25 °C and 50 °C, respectively (Entry 17–18, Table 1). This indicated that reducing the reaction temperature would destroy this reaction. The reaction could not proceed without I2 and the yield of the product decreased to 17% while only 20% of the I2 was added (Entries 21–22, Table 1). Finally, the optimized reaction conditions were obtained as described in entry 2 of Table 1: 1.0 equiv. of 2-methyl quinoline 1a and 1.5 equiv. of α-amino acid 2a as the reaction substrates, 1.0 equiv. of iodine and 3.0 equiv. of TBHP as the oxidants, the reaction being carried out in 0.5 mL of DMF at 80 °C for 3 h.
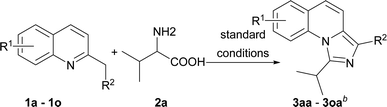
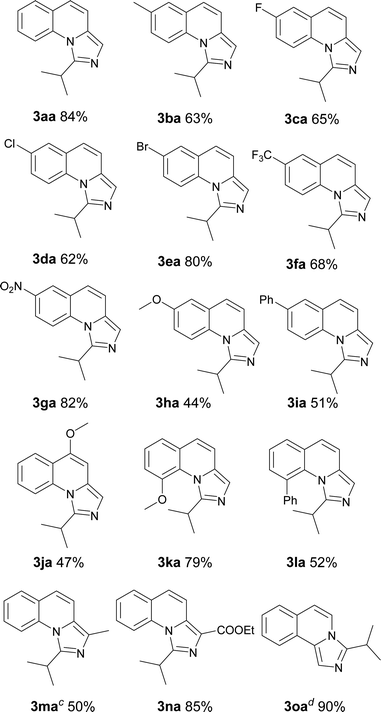
With the optimized conditions in hand, we explored the scope of the reaction substrates. Firstly, different substrates with groups on R1 and R2 were examined, and the results were listed in Table 2. Generally, 6-substituted 2-methylquinolines could be converted to the corresponding products in moderate to good yields (3ba–3ia). The substrates with electron-withdrawing groups presented more efficient than that with the electron-donating groups in this reaction. For example, 2-methyl-6-methoxylquinoline only afforded the product (3ha) with 44% yield while 2-methyl-6-nitroquinoline gave the product with a high yield of 81% (3ga). Subsequently, the substitution position of methoxyl group was investigated. The yields between 4-methoxyl and 6-methoxyl product were almost the same. However, the 8-methoxyl product was obtained with an abnormal yield of 79% (3ha, 3ja and 3ka). This implied that steric hindrance had little influence on the reaction when 2-methyl-8-phenylquinoline was employed (3ia vs. 3la). The 2-ethylquinoline showed lower reactivity. We could not obtain the desired product under the standard conditions but only the corresponding ketone was obtained. The desired imidazo[1,5-a]quinoline can be obtained with a yield of 50% when the reaction temperature was increased to 120 °C (3ma). Agreeing with former reports,11b an electron-withdrawing substituent on the carbon of methyl at 2-position favoured the reaction (3na). 1-Methylisoquinoline could also converted to the desired product well under standard conditions (3oa).
| a Reaction conditions: 1a–1o (1.0 equiv., 0.2 mmol), 2a (1.5 equiv., 0.3 mmol), I2 (1.0 equiv.), oxidant (3 equiv.) in solvent (0.5 mL).b Isolated yield.c This reaction was carried out at 120 °C.d This product was obtained with the starting material of 1-methylisoquinoline so the structure was different from 3 as shown. |
|---|
 |
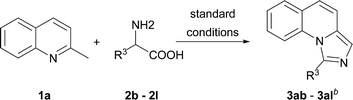
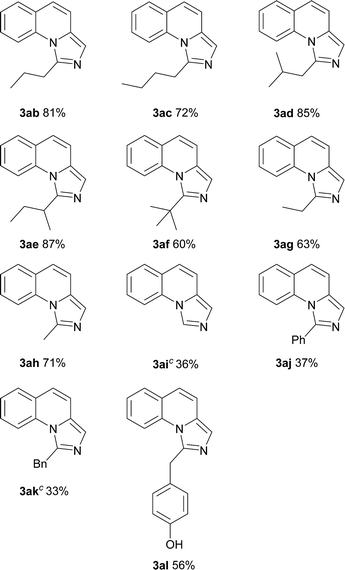
On the other hand, the scope of the amino acids were investigated (Table 3). Alkyl amino acids worked well in this reaction to afford the desired products with moderate to good yields (3ab–3ah), while the aromatic amino acids, such as phenylglycine and phenylalanine, can also be employed as the substrate to afford the corresponding products with lower yields (3aj–3ak). This may be due to the fact that under these conditions, aromatic amino acids are more active and some side reactions can occur. For instance, part of the amino acids might be decarboxylated first, which could not convert to desired products. As for tyrosine, the phenolic hydroxyl group could tolerate the reaction conditions to give the desired product with good yield (3al). To our delight, non-substituted imidazo[1,5-a]quinoline (3ai) can be obtained at 120 °C by virtue of this method, which was challenging from other methods.9a,11a
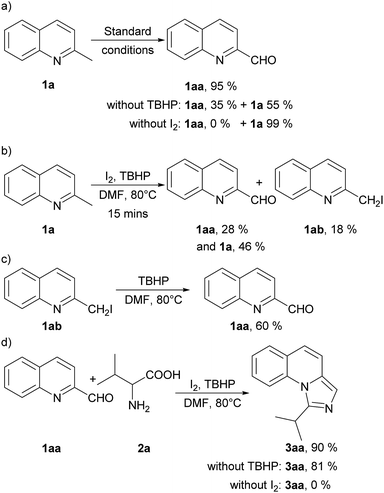
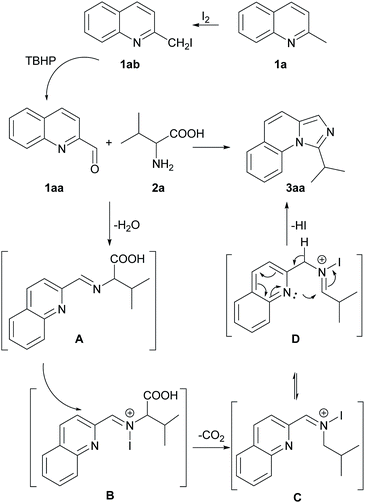
To get an insight into the mechanism of this process, we conducted several control experiments (Scheme 2). Firstly, only 2-methylquinoline (1a) was employed under the standard conditions and quinoline-2-carbaldehyde (1aa) was obtained with a yield of 95%. The yield decreased when TBHP was absent with 55% of 1a recovered, and no corresponding aldehyde was found without iodine (Scheme 2a). Besides, the aldehyde (1aa) and 2-(iodomethyl) quinoline (1ab) could be detected at 15 minutes of the model reaction (Scheme 2b). Moreover, 2-(iodomethyl) quinoline (1ab) was employed under the standard conditions without I2 and 1aa was obtained with a yield of 60% (Scheme 2c). When quinoline-2-carbaldehyde (1aa) and valine (2a) were used as the reaction substrates under standard conditions, 90% of 3aa could be afforded. It should be noted that the yield decreased slightly when TBHP was absent, but no corresponding product 3aa was detected without iodine (Scheme 2d). These experiments indicated that 1aa might be an intermediate of this reaction under the promotion of iodine and TBHP. In the subsequent transformation, TBHP might not be important but I2 is needed.
Based on the experimental results above and the previous reports,10c,11a a possible reaction pathway was proposed as shown in Scheme 3. Initially, 2-methylquinoline (1a) is quickly substituted with iodine to afford 2-(iodomethyl) quinoline (1ab) and subsequently oxidized to quinoline-2-carbaldehyde (1aa). Thereafter, 1aa produces imine A with the amino acid 2a. The imine A goes through N-iodination process, generating intermediate B. Afterward, the intermediate B undergoes a decarboxylative pathway to generate intermediate C at high temperature. Finally, C transforms to D and then cyclization happens easily through an intramolecular nucleophilic attack to give the final product 3aa.
Conclusions
In summary, we developed a facile decarboxylative cyclization to construct imidazo[1,5-a]quinolines with readily available starting material under metal-free condition. Compared to previous reports, the substrate scope of the primary α-amino acid was largely extended. In particular, the synthesis avoids the metal residues in the products, which provides a useful method for the pharmaceutical synthesis.Experimental
Materials and methods
Products were purified by flash chromatography on 200–300 mesh silica gels using petroleum ether/ethyl acetate as eluent. NMR spectra were recorded on 400 MHz (101 MHz for 13C NMR) Bruker NMR spectrometer with CDCl3 or DMSO-d6 as the solvent and tetramethylsilane (TMS) as the internal standard. High resolution mass spectra (ESI) were recorded on a Waters™ Q-TOF Premier. Melting points were determined on a melting point apparatus and are uncorrected.General procedure for the synthesis of imidazo[1,5-a]quinolines (3)
2-Methyl quinoline (1a, 28 mg, 0.2 mmol), valine (2a, 35 mg, 0.3 mmol) and iodine (51 mg, 0.2 mmol) were added to 0.5 mL of N,N-dimethylformamide. Then 60 μL of tert-butyl hydroperoxide was added and the solution was kept at 80 °C for 3 h. After the reaction was finished, the reaction mixture was washed and extracted by CH2Cl2. The organic phase was dried by Na2SO4 and then evaporated under reduced pressure. The resulting residue was purified by silica gel column chromatography (PE/EtOAc) to afford the desired product 3.1-Isopropyl imidazo[1,5-a]quinoline (3aa)
The title compound was prepared according to the general working procedure and purified by column chromatography to give the product as a yellow oil. Silica gel TLC Rf = 0.6 (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc = 3
EtOAc = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 8.07–8.05 (d, J = 8.5 Hz, 1H), 7.52–7.50 (d, J = 7.7 Hz, 1H), 7.41–7.37 (t, J = 7.9 Hz, 1H), 7.27–7.24 (m, 2H), 7.15–7.12 (d, J = 9.6 Hz, 1H), 6.81–6.79 (d, J = 9.3 Hz, 1H), 3.77–3.67 (sept, J = 6.6 Hz, 1H), 1.47–1.45 (d, J = 6.6 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 149.3, 133.1, 130.2, 128.7, 127.7, 126.0, 124.8, 120.6, 117.5, 116.9, 30.0, 21.6; HRMS (ESI) m/z calcd for C14H15N2 [M + H]+ 211.1235, found 211.1236.
1); 1H NMR (400 MHz, CDCl3) δ 8.07–8.05 (d, J = 8.5 Hz, 1H), 7.52–7.50 (d, J = 7.7 Hz, 1H), 7.41–7.37 (t, J = 7.9 Hz, 1H), 7.27–7.24 (m, 2H), 7.15–7.12 (d, J = 9.6 Hz, 1H), 6.81–6.79 (d, J = 9.3 Hz, 1H), 3.77–3.67 (sept, J = 6.6 Hz, 1H), 1.47–1.45 (d, J = 6.6 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 149.3, 133.1, 130.2, 128.7, 127.7, 126.0, 124.8, 120.6, 117.5, 116.9, 30.0, 21.6; HRMS (ESI) m/z calcd for C14H15N2 [M + H]+ 211.1235, found 211.1236.
1-Isopropyl-7-methyl-imidazo[1,5-a]quinoline (3ba)
The title compound was prepared according to the general working procedure and purified by column chromatography to give the product as a brown oil. Silica gel TLC Rf = 0.45 (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc = 3
EtOAc = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 8.06–8.04 (d, J = 8.6 Hz, 1H), 7.40 (s, 1H), 7.35 (s, 1H), 7.31–7.29 (d, J = 8.6 Hz, 1H), 7.21 (d, J = 8.1 Hz, 1H), 6.86–6.84 (d, J = 9.4 Hz, 1H), 3.85–3.75 (sept, J = 6.7 Hz, 1H), 2.44 (s, 3H), 1.56–1.54 (d, J = 6.7 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 149.0, 134.4, 131.0, 130.0, 128.7, 128.7, 126.0, 120.5, 117.4, 116.8, 29.8, 21.5, 20.7; HRMS (ESI) m/z calcd for C15H17N2 [M + H]+ 225.1392, found 225.1390.
1); 1H NMR (400 MHz, CDCl3) δ 8.06–8.04 (d, J = 8.6 Hz, 1H), 7.40 (s, 1H), 7.35 (s, 1H), 7.31–7.29 (d, J = 8.6 Hz, 1H), 7.21 (d, J = 8.1 Hz, 1H), 6.86–6.84 (d, J = 9.4 Hz, 1H), 3.85–3.75 (sept, J = 6.7 Hz, 1H), 2.44 (s, 3H), 1.56–1.54 (d, J = 6.7 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 149.0, 134.4, 131.0, 130.0, 128.7, 128.7, 126.0, 120.5, 117.4, 116.8, 29.8, 21.5, 20.7; HRMS (ESI) m/z calcd for C15H17N2 [M + H]+ 225.1392, found 225.1390.
7-Flouro-1-isopropyl-imidazo[1,5-a]quinoline (3ca)
The title compound was prepared according to the general working procedure and purified by column chromatography to give the product as a yellow solid. Silica gel TLC Rf = 0.6 (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc = 3
EtOAc = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); mp = 74–76 °C; 1H NMR (400 MHz, CDCl3) δ 8.16–8.12 (dd, J = 9.1, 4.4 Hz, 1H), 7.40 (s, 1H), 7.31–7.27 (m, 2H), 7.25–7.20 (m, 1H), 6.87–6.84 (d, J = 9.3 Hz, 1H), 3.81–3.71 (sept, J = 6.7 Hz, 1H), 1.56 (d, J = 6.7 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 160.4, 157.9 (d, 1JCF = 251.5 Hz), 149.2, 129.9, 129.5, 129.5 (d, 4JCF = 2.2 Hz), 127.9, 127.8 (d, 3JCF = 8.3 Hz), 121.2, 119.7, 119.7 (d, 4JCF = 2.7 Hz), 118.8, 118.6, 118.5 (d, 3JCF = 8.5 Hz), 115.0, 114.8 (d, 2JCF = 23.5 Hz), 114.0, 113.7 (d, 2JCF = 22.3 Hz), 29.9, 21.4; HRMS (ESI) m/z calcd for C14H14N2F [M + H]+ 229.1141, found 229.1138.
1); mp = 74–76 °C; 1H NMR (400 MHz, CDCl3) δ 8.16–8.12 (dd, J = 9.1, 4.4 Hz, 1H), 7.40 (s, 1H), 7.31–7.27 (m, 2H), 7.25–7.20 (m, 1H), 6.87–6.84 (d, J = 9.3 Hz, 1H), 3.81–3.71 (sept, J = 6.7 Hz, 1H), 1.56 (d, J = 6.7 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 160.4, 157.9 (d, 1JCF = 251.5 Hz), 149.2, 129.9, 129.5, 129.5 (d, 4JCF = 2.2 Hz), 127.9, 127.8 (d, 3JCF = 8.3 Hz), 121.2, 119.7, 119.7 (d, 4JCF = 2.7 Hz), 118.8, 118.6, 118.5 (d, 3JCF = 8.5 Hz), 115.0, 114.8 (d, 2JCF = 23.5 Hz), 114.0, 113.7 (d, 2JCF = 22.3 Hz), 29.9, 21.4; HRMS (ESI) m/z calcd for C14H14N2F [M + H]+ 229.1141, found 229.1138.
7-Chloro-1-isopropyl-imidazo[1,5-a]quinoline (3da)
The title compound was prepared according to the general working procedure and purified by column chromatography to give the product as a yellow oil. Silica gel TLC Rf = 0.6 (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc = 2
EtOAc = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1H NMR (400 MHz, CDCl3) δ 8.10–8.08 (d, J = 9.0 Hz, 1H), 7.59 (s, 1H), 7.46–7.44 (d, J = 9.0 Hz, 1H), 7.39 (s, 1H), 7.30–7.27 (d, J = 9.6 Hz, 1H), 6.84–6.82 (d, J = 9.4 Hz, 1H), 3.80–3.70 (sept, J = 6.7 Hz, 1H), 1.57–1.55 (d, J = 6.7 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 149.5, 131.5, 130.17, 130.0, 127.8, 127.5, 127.5, 121.3, 119.4, 118.8, 118.3, 30.0, 21.4; HRMS (ESI) m/z calcd for C14H14N2Cl [M + H]+ 245.0846, found 245.0844.
1) 1H NMR (400 MHz, CDCl3) δ 8.10–8.08 (d, J = 9.0 Hz, 1H), 7.59 (s, 1H), 7.46–7.44 (d, J = 9.0 Hz, 1H), 7.39 (s, 1H), 7.30–7.27 (d, J = 9.6 Hz, 1H), 6.84–6.82 (d, J = 9.4 Hz, 1H), 3.80–3.70 (sept, J = 6.7 Hz, 1H), 1.57–1.55 (d, J = 6.7 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 149.5, 131.5, 130.17, 130.0, 127.8, 127.5, 127.5, 121.3, 119.4, 118.8, 118.3, 30.0, 21.4; HRMS (ESI) m/z calcd for C14H14N2Cl [M + H]+ 245.0846, found 245.0844.
7-Bromo-1-isopropyl-imidazo[1,5-a]quinoline (3ea)
The title compound was prepared according to the general working procedure and purified by column chromatography to give the product as a yellow oil. Silica gel TLC Rf = 0.15 (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc = 10
EtOAc = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 8.03 (m, 1H), 7.75–7.74 (d, J = 2.3 Hz, 1H), 7.60–7.57 (m, 1H), 7.39 (s, 1H), 7.29–7.27 (m, 1H), 6.83–6.80 (m, 1H), 3.80–3.69 (sept, J = 6.6 Hz, 1H), 1.56–1.54 (d, J = 6.6 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 149.5, 131.9, 130.9, 130.3, 130.0, 127.9, 121.3, 119.3, 118.8, 118.5, 117.8, 30.0, 21.3; HRMS (ESI) m/z calcd for C14H14N2Br [M + H]+ 289.0340, found 289.0340.
1); 1H NMR (400 MHz, CDCl3) δ 8.03 (m, 1H), 7.75–7.74 (d, J = 2.3 Hz, 1H), 7.60–7.57 (m, 1H), 7.39 (s, 1H), 7.29–7.27 (m, 1H), 6.83–6.80 (m, 1H), 3.80–3.69 (sept, J = 6.6 Hz, 1H), 1.56–1.54 (d, J = 6.6 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 149.5, 131.9, 130.9, 130.3, 130.0, 127.9, 121.3, 119.3, 118.8, 118.5, 117.8, 30.0, 21.3; HRMS (ESI) m/z calcd for C14H14N2Br [M + H]+ 289.0340, found 289.0340.
1-Isopropyl-7-(trifluoromethyl)-imidazo[1,5-a]quinoline (3fa)
The title compound was prepared according to the general working procedure and purified by column chromatography to give the product as a yellow needle solid. Silica gel TLC Rf = 0.5 (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc = 3
EtOAc = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); mp = 60–62 °C; 1H NMR (400 MHz, CDCl3) δ 8.27–8.24 (d, J = 8.9 Hz, 1H), 7.88 (s, 1H), 7.74–7.72 (d, J = 8.9 Hz, 1H), 7.41 (s, 1H), 7.34–7.32 (d, J = 9.3 Hz, 1H), 6.95–6.92 (d, J = 9.3 Hz, 1H), 3.84–3.74 (sept, J = 6.6 Hz, 1H), 1.57 (d, J = 6.6 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 150.0, 135.0, 130.2, 127.8, 125.1, 122.4, 119.7 (q, 1JCF = 272.0 Hz), 127.4, 127.0, 126.7, 126.4 (q, 2JCF = 33.0 Hz), 126.1, 125.8, 125.7, 125.7, 125.7 (q, JCF = 3.9 Hz), 124.2, 124.2, 124.1, 124.1 (q, JCF = 3.4 Hz), 121.5, 119.9, 119.1, 117.4, 30.1, 21.4; HRMS (ESI) m/z calcd for C15H14N2F3 [M + H]+ 279.1109, found 279.1107.
1); mp = 60–62 °C; 1H NMR (400 MHz, CDCl3) δ 8.27–8.24 (d, J = 8.9 Hz, 1H), 7.88 (s, 1H), 7.74–7.72 (d, J = 8.9 Hz, 1H), 7.41 (s, 1H), 7.34–7.32 (d, J = 9.3 Hz, 1H), 6.95–6.92 (d, J = 9.3 Hz, 1H), 3.84–3.74 (sept, J = 6.6 Hz, 1H), 1.57 (d, J = 6.6 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 150.0, 135.0, 130.2, 127.8, 125.1, 122.4, 119.7 (q, 1JCF = 272.0 Hz), 127.4, 127.0, 126.7, 126.4 (q, 2JCF = 33.0 Hz), 126.1, 125.8, 125.7, 125.7, 125.7 (q, JCF = 3.9 Hz), 124.2, 124.2, 124.1, 124.1 (q, JCF = 3.4 Hz), 121.5, 119.9, 119.1, 117.4, 30.1, 21.4; HRMS (ESI) m/z calcd for C15H14N2F3 [M + H]+ 279.1109, found 279.1107.
1-Isopropyl-7-nitro-imidazo[1,5-a]quinoline (3ga)
The title compound was prepared according to the general working procedure and purified by column chromatography to give the product as an orange needle solid. Silica gel TLC Rf = 0.45 (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc = 3
EtOAc = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); mp = 190–192 °C; 1H NMR (400 MHz, CDCl3) δ 8.47–8.24 (m, 3H), 7.44–7.43 (d, J = 4.8 Hz, 1H), 7.40–7.36 (dd, J = 9.3, 5.5 Hz, 1H), 7.00–6.96 (m, 1H), 3.79–3.74 (sept, J = 6.4 Hz, 1H), 1.57 (d, J = 6.5, 6H); 13C NMR (101 MHz, CDCl3) δ 150.5, 143.9, 136.7, 130.2, 126.5, 123.8, 122.3, 122.1, 120.0, 119.8, 117.6, 30.2, 21.4; HRMS (ESI) m/z calcd for C14H14N3O2 [M + H]+ 256.1086, found 256.1083.
1); mp = 190–192 °C; 1H NMR (400 MHz, CDCl3) δ 8.47–8.24 (m, 3H), 7.44–7.43 (d, J = 4.8 Hz, 1H), 7.40–7.36 (dd, J = 9.3, 5.5 Hz, 1H), 7.00–6.96 (m, 1H), 3.79–3.74 (sept, J = 6.4 Hz, 1H), 1.57 (d, J = 6.5, 6H); 13C NMR (101 MHz, CDCl3) δ 150.5, 143.9, 136.7, 130.2, 126.5, 123.8, 122.3, 122.1, 120.0, 119.8, 117.6, 30.2, 21.4; HRMS (ESI) m/z calcd for C14H14N3O2 [M + H]+ 256.1086, found 256.1083.
1-Isopropyl-7-methoxy-imidazo[1,5-a]quinoline (3ha)
The title compound was prepared according to the general working procedure and purified by column chromatography to give the product as a brown oil. Silica gel TLC Rf = 0.30 (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc = 3
EtOAc = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 8.11–8.08 (d, J = 8.8 Hz, 1H), 7.37 (s, 1H), 7.26–7.24 (d, J = 9.6 Hz, 1H), 7.10–7.08 (m, 2H), 6.88–6.86 (d, J = 9.3 Hz, 1H), 3.90 (s, 3H), 3.84–3.74 (sept, J = 6.7 Hz, 1H), 1.56 (d, J = 6.7 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 156.3, 148.7, 129.9, 127.4, 127.3, 120.6, 120.4, 118.2, 118.0, 115.1, 111.2, 55.5, 29.8, 21.5; HRMS (ESI) m/z calcd for C15H17N2O [M + H]+ 241.1341, found 241.1342.
1); 1H NMR (400 MHz, CDCl3) δ 8.11–8.08 (d, J = 8.8 Hz, 1H), 7.37 (s, 1H), 7.26–7.24 (d, J = 9.6 Hz, 1H), 7.10–7.08 (m, 2H), 6.88–6.86 (d, J = 9.3 Hz, 1H), 3.90 (s, 3H), 3.84–3.74 (sept, J = 6.7 Hz, 1H), 1.56 (d, J = 6.7 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 156.3, 148.7, 129.9, 127.4, 127.3, 120.6, 120.4, 118.2, 118.0, 115.1, 111.2, 55.5, 29.8, 21.5; HRMS (ESI) m/z calcd for C15H17N2O [M + H]+ 241.1341, found 241.1342.
1-Isopropyl-7-phenyl-imidazo[1,5-a]quinoline (3ia)
The title compound was prepared according to the general working procedure and purified by column chromatography to give the product as a yellow oil. Silica gel TLC Rf = 0.15 (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc = 10
EtOAc = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 8.24–8.22 (d, J = 8.8 Hz, 1H), 7.82 (s, 1H), 7.75–7.73 (d, J = 8.8 Hz, 1H), 7.67–7.65 (d, J = 7.5 Hz, 4H), 7.50–7.46 (t, J = 7.5 Hz, 2H), 7.40–7.37 (m, 2H), 7.28–7.26 (t, J = 6.8 Hz, 1H), 6.98–6.96 (d, J = 9.3 Hz, 2H), 3.90–3.80 (sept, J = 6.6 Hz, 1H), 1.59 (d, J = 6.6 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 149.4, 139.7, 137.6, 130.2, 128.9, 127.6, 127.0, 126.8, 126.5, 126.4, 120.8, 120.7, 117.9, 117.3, 30.0, 21.5. HRMS (ESI) m/z calcd for C20H19N2 [M + H]+ 287.1548, found 287.1550.
1); 1H NMR (400 MHz, CDCl3) δ 8.24–8.22 (d, J = 8.8 Hz, 1H), 7.82 (s, 1H), 7.75–7.73 (d, J = 8.8 Hz, 1H), 7.67–7.65 (d, J = 7.5 Hz, 4H), 7.50–7.46 (t, J = 7.5 Hz, 2H), 7.40–7.37 (m, 2H), 7.28–7.26 (t, J = 6.8 Hz, 1H), 6.98–6.96 (d, J = 9.3 Hz, 2H), 3.90–3.80 (sept, J = 6.6 Hz, 1H), 1.59 (d, J = 6.6 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 149.4, 139.7, 137.6, 130.2, 128.9, 127.6, 127.0, 126.8, 126.5, 126.4, 120.8, 120.7, 117.9, 117.3, 30.0, 21.5. HRMS (ESI) m/z calcd for C20H19N2 [M + H]+ 287.1548, found 287.1550.
1-Isopropyl-5-methoxy-imidazo[1,5-a]quinoline (3ja)
The title compound was prepared according to the general working procedure and purified by column chromatography to give the product as a green oil. Silica gel TLC Rf = 0.50 (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc = 3
EtOAc = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 8.15–8.12 (d, J = 8.7 Hz, 1H), 8.08–8.06 (d, J = 8.1 Hz, 1H), 7.57–7.53 (m, 1H), 7.42–7.38 (m, 1H), 7.17 (s, 1H), 6.50 (s, 1H), 3.94 (m, 3H), 3.80–3.71 (sept, J = 6.5 Hz, 1H), 1.54 (d, J = 6.6 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 148.5, 147.9, 133.4, 130.1, 128.5, 124.4, 123.4, 121.3, 117.9, 116.7, 91.9, 55.3, 30.0, 21.5; HRMS (ESI) m/z calcd for C15H17N2O [M + H]+ 241.1341, found 241.1338.
1); 1H NMR (400 MHz, CDCl3) δ 8.15–8.12 (d, J = 8.7 Hz, 1H), 8.08–8.06 (d, J = 8.1 Hz, 1H), 7.57–7.53 (m, 1H), 7.42–7.38 (m, 1H), 7.17 (s, 1H), 6.50 (s, 1H), 3.94 (m, 3H), 3.80–3.71 (sept, J = 6.5 Hz, 1H), 1.54 (d, J = 6.6 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 148.5, 147.9, 133.4, 130.1, 128.5, 124.4, 123.4, 121.3, 117.9, 116.7, 91.9, 55.3, 30.0, 21.5; HRMS (ESI) m/z calcd for C15H17N2O [M + H]+ 241.1341, found 241.1338.
1-Isopropyl-9-methoxy-imidazo[1,5-a]quinoline (3ka)
The title compound was prepared according to the general working procedure and purified by column chromatography to give the product as a green oil. Silica gel TLC Rf = 0.55 (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc = 3
EtOAc = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1H NMR (400 MHz, CDCl3) δ 7.41 (s, 1H), 7.33–7.29 (m, 1H), 7.18–7.16 (d, J = 8.3 Hz, 2H), 7.00–6.98 (d, J = 8.1 Hz, 1H), 6.79–6.77 (d, J = 9.2 Hz, 1H), 3.92 (m, 3H), 3.75–3.65 (sept, J = 6.7 Hz, 1H), 1.36 (d, J = 6.7 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 154.6, 149.7, 130.5, 129.1, 125.7, 122.7, 121.6, 119.7, 118.0, 109.9, 55.3, 30.2, 22.9; HRMS (ESI) m/z calcd for C15H17N2O [M + H]+ 241.1341, found 241.1345.
1) 1H NMR (400 MHz, CDCl3) δ 7.41 (s, 1H), 7.33–7.29 (m, 1H), 7.18–7.16 (d, J = 8.3 Hz, 2H), 7.00–6.98 (d, J = 8.1 Hz, 1H), 6.79–6.77 (d, J = 9.2 Hz, 1H), 3.92 (m, 3H), 3.75–3.65 (sept, J = 6.7 Hz, 1H), 1.36 (d, J = 6.7 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 154.6, 149.7, 130.5, 129.1, 125.7, 122.7, 121.6, 119.7, 118.0, 109.9, 55.3, 30.2, 22.9; HRMS (ESI) m/z calcd for C15H17N2O [M + H]+ 241.1341, found 241.1345.
1-Isopropyl-9-phenyl-imidazo[1,5-a]quinoline (3la)
The title compound was prepared according to the general working procedure and purified by column chromatography to give the product as a yellow oil. Silica gel TLC Rf = 0.35 (PE: EtOAc = 10: 1); 1H NMR (400 MHz, CDCl3) δ 7.56–7.53 (m, 1H), 7.45–7.43 (d, J = 9.2 Hz, 3H), 7.40–7.36 (t, J = 7.4 Hz, 2H), 7.32–7.28 (t, J = 7.4 Hz, 1H), 7.26–7.24 (d, J = 9.4 Hz, 1H), 7.20–7.18 (d, J = 6.9 Hz, 2H), 6.92–6.90 (d, J = 9.2 Hz, 1H), 2.83–2.73 (sept, J = 6.7 Hz, 1H), 0.69–0.67 (d, J = 6.7 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 154.5, 141.0, 133.0, 131.0, 130.9, 129.5, 129.2, 129.0, 127.60, 127.6, 126.5, 125.6, 122.1, 120.3, 117.7, 29.8, 21.3; HRMS (ESI) m/z calcd for C20H19N2 [M + H]+ 287.1548, found 287.1552.1-Isopropyl-3-methyl-imidazo[1,5-a]quinoline (3ma)
The title compound was prepared according to the general working procedure except the temperature was 120 °C and purified by column chromatography to give the product as a yellow solid. Silica gel TLC Rf = 0.25 (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc = 5
EtOAc = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); mp = 110–112 °C; 1H NMR (400 MHz, CDCl3) δ 8.13–8.11 (d, J = 8.5 Hz, 1H), 7.60–7.58 (d, J = 7.6 Hz, 1H), 7.49–7.45 (t, J = 7.4 Hz, 1H), 7.36–7.33 (t, J = 7.4 Hz, 1H), 7.20–7.17 (d, J = 9.4 Hz, 1H), 6.84–6.81 (d, J = 9.4 Hz, 1H), 3.86–3.76 (sept, J = 6.7 Hz, 1H), 2.49 (s, 3H), 1.57–1.55 (d, J = 6.7 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 148.0, 133.3, 128.6, 128.5, 127.5, 126.2, 126.1, 124.6, 118.8, 117.1, 116.9, 29.7, 21.7, 12.5; HRMS (ESI) m/z calcd for C15H17N2 [M + H]+ 225.1392, found 225.1390.
1); mp = 110–112 °C; 1H NMR (400 MHz, CDCl3) δ 8.13–8.11 (d, J = 8.5 Hz, 1H), 7.60–7.58 (d, J = 7.6 Hz, 1H), 7.49–7.45 (t, J = 7.4 Hz, 1H), 7.36–7.33 (t, J = 7.4 Hz, 1H), 7.20–7.17 (d, J = 9.4 Hz, 1H), 6.84–6.81 (d, J = 9.4 Hz, 1H), 3.86–3.76 (sept, J = 6.7 Hz, 1H), 2.49 (s, 3H), 1.57–1.55 (d, J = 6.7 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 148.0, 133.3, 128.6, 128.5, 127.5, 126.2, 126.1, 124.6, 118.8, 117.1, 116.9, 29.7, 21.7, 12.5; HRMS (ESI) m/z calcd for C15H17N2 [M + H]+ 225.1392, found 225.1390.
Ethyl 1-isopropyl-imidazo[1,5-a]quinoline-3-carboxylate (3na)
The title compound was prepared according to the general working procedure and purified by column chromatography to give the product as a pale yellow solid. Silica gel TLC Rf = 0.2 (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DCM = 2
DCM = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); mp = 118–120 °C; 1H NMR (400 MHz, CDCl3) δ 8.30–8.28 (d, J = 8.5 Hz, 1H), 8.15–8.13 (d, J = 9.3 Hz, 1H), 7.77–7.75 (d, J = 7.5 Hz, 1H), 7.65–7.61 (t, J = 7.6 Hz, 1H), 7.57–7.41 (t, J = 7.4 Hz, 1H), 7.33–7.31 (d, J = 9.5 Hz, 1H), 4.51–4.46 (q, J = 7.0 Hz, 2H), 3.93–3.83 (sept, J = 6.3 Hz, 1H), 1.64–1.62 (d, J = 6.4 Hz, 6H), 1.48–1.45 (t, J = 7.0 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 163.8, 150.0, 134.6, 132.8, 129.2, 128.9, 125.8, 125.7, 125.6, 121.9, 118.1, 117.3, 60.6, 30.3, 21.5, 14.6; HRMS (ESI) m/z calcd for C17H18N2O2Na [M + Na]+ 305.1266, found 305.1265.
1); mp = 118–120 °C; 1H NMR (400 MHz, CDCl3) δ 8.30–8.28 (d, J = 8.5 Hz, 1H), 8.15–8.13 (d, J = 9.3 Hz, 1H), 7.77–7.75 (d, J = 7.5 Hz, 1H), 7.65–7.61 (t, J = 7.6 Hz, 1H), 7.57–7.41 (t, J = 7.4 Hz, 1H), 7.33–7.31 (d, J = 9.5 Hz, 1H), 4.51–4.46 (q, J = 7.0 Hz, 2H), 3.93–3.83 (sept, J = 6.3 Hz, 1H), 1.64–1.62 (d, J = 6.4 Hz, 6H), 1.48–1.45 (t, J = 7.0 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 163.8, 150.0, 134.6, 132.8, 129.2, 128.9, 125.8, 125.7, 125.6, 121.9, 118.1, 117.3, 60.6, 30.3, 21.5, 14.6; HRMS (ESI) m/z calcd for C17H18N2O2Na [M + Na]+ 305.1266, found 305.1265.
3-Isopropyl-imidazo[5,1-a]isoquinoline (3oa)
The title compound was prepared according to the general working procedure and purified by column chromatography to give the product as a pale yellow solid, mp = 73–75 °C, silica gel TLC Rf = 0.3 (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc = 3
EtOAc = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 8.00–7.98 (d, J = 7.9 Hz, 1H), 7.77 (s, 1H), 7.65–7.63 (d, J = 7.5 Hz, 1H), 7.57–7.55 (d, J = 7.7 Hz, 1H), 7.52–7.48 (t, J = 7.5 Hz, 1H), 7.42–7.38 (t, J = 7.4 Hz, 1H), 6.82–6.80 (d, J = 7.4 Hz, 1H), 3.41–3.31 (sept, J = 6.8 Hz, 1H), 1.48 (d, J = 6.8 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 146.0, 128.4, 128.1, 127.0, 127.7, 126.6, 125.1, 122.3, 119.5, 118.2, 113.5, 26.1, 20.8; HRMS (ESI) m/z calcd for C14H15N2 [M + H]+ 211.1235, found 211.1235.
1); 1H NMR (400 MHz, CDCl3) δ 8.00–7.98 (d, J = 7.9 Hz, 1H), 7.77 (s, 1H), 7.65–7.63 (d, J = 7.5 Hz, 1H), 7.57–7.55 (d, J = 7.7 Hz, 1H), 7.52–7.48 (t, J = 7.5 Hz, 1H), 7.42–7.38 (t, J = 7.4 Hz, 1H), 6.82–6.80 (d, J = 7.4 Hz, 1H), 3.41–3.31 (sept, J = 6.8 Hz, 1H), 1.48 (d, J = 6.8 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 146.0, 128.4, 128.1, 127.0, 127.7, 126.6, 125.1, 122.3, 119.5, 118.2, 113.5, 26.1, 20.8; HRMS (ESI) m/z calcd for C14H15N2 [M + H]+ 211.1235, found 211.1235.
1-Propyl-imidazo[1,5-a]quinoline (3ab)
The title compound was prepared according to the general working procedure and purified by column chromatography to give the product as a yellow oil. Silica gel TLC Rf = 0.40 (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc = 2
EtOAc = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 8.15–8.13 (d, J = 8.1 Hz, 1H), 7.65–7.63 (d, J = 7.5 Hz, 1H), 7.54–7.50 (m, 1H), 7.41–7.36 (m, 2H), 7.27–7.25 (m, 1H), 6.94–6.92 (d, J = 9.5 Hz, 1H), 3.38–3.34 (t, J = 7.4 Hz, 2H), 2.07–1.99 (m, 2H), 1.17–1.13 (t, J = 7.3 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 144.1, 133.2, 130.3, 128.7, 127.7, 125.9, 124.8, 120.6, 117.4, 116.6, 34.3, 20.6, 14.0; HRMS (ESI) m/z calcd for C14H15N2 [M + H]+ 211.1235, found 211.1230.
1); 1H NMR (400 MHz, CDCl3) δ 8.15–8.13 (d, J = 8.1 Hz, 1H), 7.65–7.63 (d, J = 7.5 Hz, 1H), 7.54–7.50 (m, 1H), 7.41–7.36 (m, 2H), 7.27–7.25 (m, 1H), 6.94–6.92 (d, J = 9.5 Hz, 1H), 3.38–3.34 (t, J = 7.4 Hz, 2H), 2.07–1.99 (m, 2H), 1.17–1.13 (t, J = 7.3 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 144.1, 133.2, 130.3, 128.7, 127.7, 125.9, 124.8, 120.6, 117.4, 116.6, 34.3, 20.6, 14.0; HRMS (ESI) m/z calcd for C14H15N2 [M + H]+ 211.1235, found 211.1230.
1-Butyl-imidazo[1,5-a]quinoline (3ac)
The title compound was prepared according to the general working procedure and purified by column chromatography to give the product as an orange oil. Silica gel TLC Rf = 0.65 (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc = 6
EtOAc = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 8.14–8.12 (d, J = 8.5 Hz, 1H), 7.63–7.61 (d, J = 7.7 Hz, 1H), 7.53–7.49 (t, J = 7.8 Hz, 1H), 7.40–7.35 (m, 2H), 7.25–7.23 (m, 1H), 6.92–6.90 (d, J = 9.4 Hz, 1H), 3.38–3.35 (t, J = 7.7 Hz, 2H), 2.02–1.95 (m, 2H), 1.61–1.54 (m, 2H), 1.05–1.00 (t, J = 7.3 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 144.2, 133.2, 130.3, 128.7, 127.7, 125.9, 124.8, 120.6, 120.6, 117.5, 116.6, 32.1, 29.3, 22.6, 13.9; HRMS (ESI) m/z calcd for C15H17N2 [M + H]+ 225.1392, found 225.1391.
1); 1H NMR (400 MHz, CDCl3) δ 8.14–8.12 (d, J = 8.5 Hz, 1H), 7.63–7.61 (d, J = 7.7 Hz, 1H), 7.53–7.49 (t, J = 7.8 Hz, 1H), 7.40–7.35 (m, 2H), 7.25–7.23 (m, 1H), 6.92–6.90 (d, J = 9.4 Hz, 1H), 3.38–3.35 (t, J = 7.7 Hz, 2H), 2.02–1.95 (m, 2H), 1.61–1.54 (m, 2H), 1.05–1.00 (t, J = 7.3 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 144.2, 133.2, 130.3, 128.7, 127.7, 125.9, 124.8, 120.6, 120.6, 117.5, 116.6, 32.1, 29.3, 22.6, 13.9; HRMS (ESI) m/z calcd for C15H17N2 [M + H]+ 225.1392, found 225.1391.
1-Isobutyl-imidazo[1,5-a]quinoline (3ad)
The title compound was prepared according to the general working procedure and purified by column chromatography to give the product as a yellow oil. Silica gel TLC Rf = 0.25 (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc = 5
EtOAc = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 8.13–8.10 (d, J = 8.5 Hz, 1H), 7.65–7.63 (d, J = 7.7 Hz, 1H), 7.54–7.50 (t, J = 7.8 Hz, 1H), 7.41–7.37 (m, 2H), 7.27–7.25 (m, 1H), 6.95–6.92 (d, J = 9.4 Hz, 1H), 3.28–3.26 (d, J = 7.0 Hz, 2H), 2.47–2.35 (m, 1H), 1.10–1.09 (d, J = 6.6 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 143.5, 133.1, 130.3, 128.7, 127.7, 125.9, 124.9, 120.6, 120.6, 117.5, 116.6, 41.1, 26.6, 22.6; HRMS (ESI) m/z calcd for C15H17N2 [M + H]+ 225.1392, found 225.1393.
1); 1H NMR (400 MHz, CDCl3) δ 8.13–8.10 (d, J = 8.5 Hz, 1H), 7.65–7.63 (d, J = 7.7 Hz, 1H), 7.54–7.50 (t, J = 7.8 Hz, 1H), 7.41–7.37 (m, 2H), 7.27–7.25 (m, 1H), 6.95–6.92 (d, J = 9.4 Hz, 1H), 3.28–3.26 (d, J = 7.0 Hz, 2H), 2.47–2.35 (m, 1H), 1.10–1.09 (d, J = 6.6 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ 143.5, 133.1, 130.3, 128.7, 127.7, 125.9, 124.9, 120.6, 120.6, 117.5, 116.6, 41.1, 26.6, 22.6; HRMS (ESI) m/z calcd for C15H17N2 [M + H]+ 225.1392, found 225.1393.
1-(sec-Butyl)-imidazo[1,5-a]quinoline (3ae)
The title compound was prepared according to the general working procedure and purified by column chromatography to give the product as a brown oil. Silica gel TLC Rf = 0.70 (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc = 3
EtOAc = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 8.15–8.13 (d, J = 8.6 Hz, 1H), 7.63–7.61 (d, J = 7.7 Hz, 1H), 7.52–7.49 (t, J = 7.9 Hz, 1H), 7.38–7.35 (m, 2H), 7.27–7.23 (m, 1H), 6.92–6.89 (d, J = 9.3 Hz, 1H), 3.64–3.56 (m, 1H), 2.24–2.14 (m, 1H), 1.86–1.75 (m, 1H), 1.55–1.53 (d, J = 6.7 Hz, 3H), 1.05–1.01 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 148.7, 133.2, 130.1, 128.7, 127.6, 126.1, 124.7, 120.7, 120.5, 117.5, 116.9, 36.7, 28.5, 18.9, 11.9; HRMS (ESI) m/z calcd for C15H17N2 [M + H]+ 225.1392, found 225.1396.
1); 1H NMR (400 MHz, CDCl3) δ 8.15–8.13 (d, J = 8.6 Hz, 1H), 7.63–7.61 (d, J = 7.7 Hz, 1H), 7.52–7.49 (t, J = 7.9 Hz, 1H), 7.38–7.35 (m, 2H), 7.27–7.23 (m, 1H), 6.92–6.89 (d, J = 9.3 Hz, 1H), 3.64–3.56 (m, 1H), 2.24–2.14 (m, 1H), 1.86–1.75 (m, 1H), 1.55–1.53 (d, J = 6.7 Hz, 3H), 1.05–1.01 (t, J = 7.4 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 148.7, 133.2, 130.1, 128.7, 127.6, 126.1, 124.7, 120.7, 120.5, 117.5, 116.9, 36.7, 28.5, 18.9, 11.9; HRMS (ESI) m/z calcd for C15H17N2 [M + H]+ 225.1392, found 225.1396.
1-(tert-Butyl)-imidazo[1,5-a]quinoline (3af)
The title compound was prepared according to the general working procedure and purified by column chromatography to give the product as a yellow oil. Silica gel TLC Rf = 0.55 (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc = 3
EtOAc = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 8.42–8.40 (d, J = 8.7 Hz, 1H), 7.63–7.61 (d, J = 7.7 Hz, 1H), 7.54–7.50 (t, J = 7.9 Hz, 1H), 7.40–7.37 (m, 2H), 7.28–7.26 (d, J = 9.1 Hz, 1H), 6.95–6.92 (d, J = 9.3 Hz, 1H), 1.75 (s, 9H); 13C NMR (101 MHz, CDCl3) δ 151.5, 133.2, 131.8, 128.7, 126.7, 126.5, 124.8, 120.8, 120.4, 120.3, 117.9, 34.8, 30.5; HRMS (ESI) m/z calcd for C15H17N2 [M + H]+ 225.1392, found 225.1391.
1); 1H NMR (400 MHz, CDCl3) δ 8.42–8.40 (d, J = 8.7 Hz, 1H), 7.63–7.61 (d, J = 7.7 Hz, 1H), 7.54–7.50 (t, J = 7.9 Hz, 1H), 7.40–7.37 (m, 2H), 7.28–7.26 (d, J = 9.1 Hz, 1H), 6.95–6.92 (d, J = 9.3 Hz, 1H), 1.75 (s, 9H); 13C NMR (101 MHz, CDCl3) δ 151.5, 133.2, 131.8, 128.7, 126.7, 126.5, 124.8, 120.8, 120.4, 120.3, 117.9, 34.8, 30.5; HRMS (ESI) m/z calcd for C15H17N2 [M + H]+ 225.1392, found 225.1391.
1-Ethyl-imidazo[1,5-a]quinoline (3ag)
The title compound was prepared according to the general working procedure and purified by column chromatography to give the product as a brown oil. Silica gel TLC Rf = 0.35 (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc = 10
EtOAc = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 8.18–8.16 (d, J = 8.5 Hz, 1H), 7.64–7.62 (d, J = 7.9 Hz, 1H), 7.53–7.50 (t, J = 7.8 Hz, 1H), 7.41–7.37 (m, 2H), 7.27–7.25 (m, 1H), 6.95–6.92 (d, J = 9.1 Hz, 1H), 3.44–3.39 (q, J = 6.9 Hz, 2H), 1.60–1.56 (t, J = 7.3 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 145.1, 133.2, 130.4, 128.7, 127.8, 125.9, 124.9, 120.7, 120.5, 117.4, 116.6, 25.8, 11.8; HRMS (ESI) m/z calcd for C13H13N2 [M + H]+ 197.1079, found 197.1079.
1); 1H NMR (400 MHz, CDCl3) δ 8.18–8.16 (d, J = 8.5 Hz, 1H), 7.64–7.62 (d, J = 7.9 Hz, 1H), 7.53–7.50 (t, J = 7.8 Hz, 1H), 7.41–7.37 (m, 2H), 7.27–7.25 (m, 1H), 6.95–6.92 (d, J = 9.1 Hz, 1H), 3.44–3.39 (q, J = 6.9 Hz, 2H), 1.60–1.56 (t, J = 7.3 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ 145.1, 133.2, 130.4, 128.7, 127.8, 125.9, 124.9, 120.7, 120.5, 117.4, 116.6, 25.8, 11.8; HRMS (ESI) m/z calcd for C13H13N2 [M + H]+ 197.1079, found 197.1079.
1-Methyl-imidazo[1,5-a]quinoline (3ah)
The title compound was prepared according to the general working procedure and purified by column chromatography to give the product as a yellow oil. Silica gel TLC Rf = 0.30 (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc = 6
EtOAc = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 8.23–8.21 (d, J = 8.5 Hz, 1H), 7.65–7.63 (dd, J = 7.7, 1.4 Hz, 1H), 7.54–7.49 (m, 1H), 7.41–7.37 (m, 1H), 7.33 (s, 1H), 7.26–7.24 (m, 1H), 6.95–6.92 (d, J = 9.4 Hz, 1H), 3.09 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 140.0, 133.3, 130.3, 128.6, 127.7, 125.7, 124.9, 120.6, 120.5, 117.3, 116.2, 19.6; HRMS (ESI) m/z calcd for C12H11N2 [M + H]+ 183.0922, found 183.0923.
1); 1H NMR (400 MHz, CDCl3) δ 8.23–8.21 (d, J = 8.5 Hz, 1H), 7.65–7.63 (dd, J = 7.7, 1.4 Hz, 1H), 7.54–7.49 (m, 1H), 7.41–7.37 (m, 1H), 7.33 (s, 1H), 7.26–7.24 (m, 1H), 6.95–6.92 (d, J = 9.4 Hz, 1H), 3.09 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 140.0, 133.3, 130.3, 128.6, 127.7, 125.7, 124.9, 120.6, 120.5, 117.3, 116.2, 19.6; HRMS (ESI) m/z calcd for C12H11N2 [M + H]+ 183.0922, found 183.0923.
Imidazo[1,5-a]quinoline (3ai)
The title compound was prepared according to the general working procedure except the temperature was 120 °C and purified by column chromatography to give the product as a brown oil. Silica gel TLC Rf = 0.15 (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc = 3
EtOAc = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, CDCl3) δ 8.65 (s, 1H), 7.98–7.96 (d, J = 8.3 Hz, 1H), 7.68–7.66 (d, J = 7.8 Hz, 1H), 7.58–7.54 (m, 1H), 7.48–7.41 (m, 2H), 7.34–7.32 (d, J = 9.5 Hz, 1H), 7.05–7.03 (d, J = 9.5 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ 130.9, 128.8, 128.6, 127.8, 125.6, 124.2, 122.4, 121.3, 116.8, 114.6; HRMS (ESI) m/z calcd for C11H9N2 [M + H]+ 169.0766, found 169.0765.
1); 1H NMR (400 MHz, CDCl3) δ 8.65 (s, 1H), 7.98–7.96 (d, J = 8.3 Hz, 1H), 7.68–7.66 (d, J = 7.8 Hz, 1H), 7.58–7.54 (m, 1H), 7.48–7.41 (m, 2H), 7.34–7.32 (d, J = 9.5 Hz, 1H), 7.05–7.03 (d, J = 9.5 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ 130.9, 128.8, 128.6, 127.8, 125.6, 124.2, 122.4, 121.3, 116.8, 114.6; HRMS (ESI) m/z calcd for C11H9N2 [M + H]+ 169.0766, found 169.0765.
1-Phenyl-imidazo[1,5-a]quinoline (3aj)
The title compound was prepared according to the general working procedure and purified by column chromatography to give the product as a pale yellow solid. Silica gel TLC Rf = 0.30 (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc = 6
EtOAc = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); mp = 113–115 °C; 1H NMR (400 MHz, CDCl3) δ 7.66–7.60 (m, 3H), 7.54–7.50 (m, 5H), 7.34–7.26 (m, 2H), 7.19–7.14 (m, 1H), 7.03–7.00 (d, J = 9.4 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ 142.3, 133.7, 132.3, 130.5, 129.5, 129.2, 128.7, 128.6, 127.3, 125.5, 125.1, 122.3, 121.4, 117.4, 117.1; HRMS (ESI) m/z calcd for C17H13N2 [M + H]+ 245.1079, found 245.1082.
1); mp = 113–115 °C; 1H NMR (400 MHz, CDCl3) δ 7.66–7.60 (m, 3H), 7.54–7.50 (m, 5H), 7.34–7.26 (m, 2H), 7.19–7.14 (m, 1H), 7.03–7.00 (d, J = 9.4 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ 142.3, 133.7, 132.3, 130.5, 129.5, 129.2, 128.7, 128.6, 127.3, 125.5, 125.1, 122.3, 121.4, 117.4, 117.1; HRMS (ESI) m/z calcd for C17H13N2 [M + H]+ 245.1079, found 245.1082.
1-Benzyl-imidazo[1,5-a]quinoline (3ak)
The title compound was prepared according to the general working procedure except the temperature was 120 °C and purified by column chromatography to give the product as a pale yellow solid. Silica gel TLC Rf = 0.40 (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc = 3
EtOAc = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); mp = 95–97 °C; 1H NMR (400 MHz, CDCl3) δ 8.00–7.98 (d, J = 7.7 Hz, 1H), 7.61–7.59 (m, 1H), 7.47 (s, 1H), 7.35–7.25 (m, 5H), 7.23–7.15 (m, 3H), 6.98–6.96 (d, J = 9.4 Hz, 1H), 4.83 (s, 2H); 13C NMR (101 MHz, CDCl3) δ 141.4, 136.9, 132.6, 130.7, 128.9, 128.5, 128.2, 127.8, 126.7, 125.7, 125.0, 121.2, 121.0, 117.3, 116.9, 37.8; HRMS (ESI) m/z calcd for C18H15N2 [M + H]+ 259.1235, found 259.1230.
1); mp = 95–97 °C; 1H NMR (400 MHz, CDCl3) δ 8.00–7.98 (d, J = 7.7 Hz, 1H), 7.61–7.59 (m, 1H), 7.47 (s, 1H), 7.35–7.25 (m, 5H), 7.23–7.15 (m, 3H), 6.98–6.96 (d, J = 9.4 Hz, 1H), 4.83 (s, 2H); 13C NMR (101 MHz, CDCl3) δ 141.4, 136.9, 132.6, 130.7, 128.9, 128.5, 128.2, 127.8, 126.7, 125.7, 125.0, 121.2, 121.0, 117.3, 116.9, 37.8; HRMS (ESI) m/z calcd for C18H15N2 [M + H]+ 259.1235, found 259.1230.
4-(Imidazo[1,5-a]quinolin-1-ylmethyl)-phenol (3al)
The title compound was prepared according to the general working procedure and purified by column chromatography to give the product as a brown solid, mp = 224–225 °C. Silica gel TLC Rf = 0.55 (DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = 10
MeOH = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); 1H NMR (400 MHz, DMSO) δ 9.26 (s, 1H), 8.09–8.07 (d, J = 8.2 Hz, 1H), 7.75–7.74 (d, J = 7.4 Hz, 1H), 7.47–7.38 (m, 4H), 7.12–7.10 (d, J = 9.1 Hz, 1H), 6.91–6.89 (d, J = 8.1 Hz, 2H), 6.66–6.64 (d, J = 8.0 Hz, 2H), 4.68 (s, 2H); 13C NMR (101 MHz, DMSO) δ 155.9, 141.9, 131.8, 130.2, 129.0, 128.5, 128.0, 127.1, 125.1, 125.08, 121.0, 120.6, 117.4, 117.0, 115.5, 36.1; HRMS (ESI) m/z calcd for C18H15N2O [M + H]+ 275.1184, found 275.1185.
1); 1H NMR (400 MHz, DMSO) δ 9.26 (s, 1H), 8.09–8.07 (d, J = 8.2 Hz, 1H), 7.75–7.74 (d, J = 7.4 Hz, 1H), 7.47–7.38 (m, 4H), 7.12–7.10 (d, J = 9.1 Hz, 1H), 6.91–6.89 (d, J = 8.1 Hz, 2H), 6.66–6.64 (d, J = 8.0 Hz, 2H), 4.68 (s, 2H); 13C NMR (101 MHz, DMSO) δ 155.9, 141.9, 131.8, 130.2, 129.0, 128.5, 128.0, 127.1, 125.1, 125.08, 121.0, 120.6, 117.4, 117.0, 115.5, 36.1; HRMS (ESI) m/z calcd for C18H15N2O [M + H]+ 275.1184, found 275.1185.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the financial support from the National Natural Science Foundation of China (21432009, 21672200, 21472177 and 21772185), and the assistance of the product characterization from the Chemistry Experiment Teaching Center of University of Science and Technology of China. This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences, Grant No. XDB20000000.Notes and references
- A. Cappelli, G. Giuliani, M. Anzini, D. Riitano, G. Giorgi and S. Vomero, Bioorg. Med. Chem., 2008, 16, 6850 CrossRef PubMed.
- A. Wiśniewska, A. Chrapkowska, A. Kot-Wasik, J. Konopa and Z. Mazerska, Acta Biochim. Pol., 2007, 54, 831 Search PubMed.
- (a) G. R. Pettit, J. C. Collins, J. C. Knight, D. L. Herald, R. A. Nieman, M. D. Williams and R. K. Pettit, J. Nat. Prod., 2003, 66, 544 CrossRef PubMed; (b) M. D. Markey and T. R. Kelly, J. Org. Chem., 2008, 73, 7441 CrossRef PubMed; (c) D. Knueppel and S. F. Martin, Angew. Chem., Int. Ed., 2009, 48, 2569 CrossRef PubMed; (d) M. Mohamed, T. P. Gonçalves, R. J. Whitby, H. F. Sneddon and D. C. Harrowven, Chem.–Eur. J., 2011, 17, 13698 CrossRef PubMed.
- M. S. Malamas, Y. Ni, J. Erdei, H. Stange, R. Schindler, H.-J. Lankau, C. Grunwald, K. Y. Fan, K. Parris, B. Langen, U. Egerland, T. Hage, K. L. Marquis, S. Grauer, J. Brennan, R. Navarra, R. Graf, B. L. Harrison, A. Robichaud, T. Kronbach, M. N. Pangalos, N. Hoefgen and N. J. Brandon, J. Med. Chem., 2011, 54, 7621 CrossRef PubMed.
- A. Cappelli, M. Anzini, F. Castriconi, G. Grisci, M. Paolino, C. Braile, S. Valenti, G. Giuliani, S. Vomero, A. D. Capua, L. Betti, G. Giannaccini, A. Lucacchini, C. Ghelardini, L. D. C. Mannelli, M. Frosini, L. Ricci, G. Giorgi, M. P. Mascia and G. Biggio, J. Med. Chem., 2016, 59, 3353 CrossRef PubMed.
- (a) M. Alcarazo, S. J. Roseblade, A. R. Cowley, R. Fernández, J. M. Brown and J. M. Lassaletta, J. Am. Chem. Soc., 2005, 127, 3290 CrossRef PubMed; (b) F. E. Hahn, Angew. Chem., Int. Ed., 2006, 45, 1348 CrossRef PubMed; (c) K. G. Kishore, O. Ghashghaei, C. Estarellas, M. M. Mestre, C. Monturiol, N. Kielland, J. M. Kelly, A. F. Francisco, S. Jayawardhana, D. Muñoz-Torrero, B. Pérez, F. J. Luque, R. Gámez-Montaño and R. Lavilla, Angew. Chem., Int. Ed., 2016, 55, 8994 CrossRef PubMed; (d) H. Erguven, D. C. Leitch, E. N. Keyzer and B. A. Arndtsen, Angew. Chem., Int. Ed., 2017, 56, 6078 CrossRef PubMed.
- (a) D. S. Roman, V. Poiret, G. Pelletier and A. B. Charette, Eur. J. Org. Chem., 2015, 67 CrossRef; (b) Z. Tan, H. Zhao, C. Zhou, H. Jiang and M. Zhang, J. Org. Chem., 2016, 81, 9939 CrossRef PubMed; (c) S.-S. Wu, C.-T. Feng, D. Hu, Y.-K. Huang, Z. Li, Z.-G. Luo and S.-T. Ma, Org. Biomol. Chem., 2017, 15, 1680 RSC.
- (a) J. D. Bower and G. R. Ramage, J. Chem. Soc., 1955, 2834 RSC; (b) K. Winterfeld and H. Franzke, Angew. Chem., 1963, 75, 1101 CrossRef.
- (a) D. C. Mohan, S. N. Rao, C. Ravi and S. Adimurthy, Org. Biomol. Chem., 2015, 13, 5602 RSC; (b) Z. Li, S.-S. Wu, Z.-G. Luo, W.-K. Liu, C.-T. Feng and S.-T. Ma, J. Org. Chem., 2016, 81, 4386 CrossRef PubMed.
- (a) M. Li, Y. Xie, Y. Ye, Y. Zou, H. Jiang and W. Zeng, Org. Lett., 2014, 16, 6232 CrossRef PubMed; (b) H. Wang, W. Xu, Z. Wang, L. Yu and K. Xu, J. Org. Chem., 2015, 80, 2431 CrossRef PubMed; (c) H. Wang, W. Xu, L. Xin, W. Liu, Z. Wang and K. Xu, J. Org. Chem., 2016, 81, 3681 CrossRef PubMed.
- (a) Q. Wang, S. Zhang, F. Guo, B. Zhang, P. Hu and Z. Wang, J. Org. Chem., 2012, 77, 11161 CrossRef PubMed; (b) Y. Yan, Y. Zhang, Z. Zha and Z. Wang, Org. Lett., 2013, 15, 2274 CrossRef PubMed.
- (a) N. Rodríguez and L. J. Goossen, Chem. Soc. Rev., 2011, 40, 5030 RSC; (b) Y. Wei, P. Hu, M. Zhang and W. Su, Chem. Rev., 2017, 117, 8864 CrossRef PubMed.
- (a) D. Seidel, Acc. Chem. Res., 2015, 48, 317 CrossRef PubMed; (b) L. Zheng, F. Yang, Q. Dang and X. Bai, Org. Lett., 2008, 10, 889 CrossRef PubMed; (c) H.-P. Bi, L. Zhao, Y.-M. Liang and C.-J. Li, Angew. Chem., Int. Ed., 2009, 48, 792 CrossRef PubMed; (d) C. Zhang and D. Seidel, J. Am. Chem. Soc., 2010, 132, 1798 CrossRef PubMed; (e) H.-P. Bi, Q. Teng, M. Guan, W.-W. Chen, Y.-M. Liang, X. Yao and C.-J. Li, J. Org. Chem., 2010, 75, 783 CrossRef PubMed; (f) Y. Kang and D. Seidel, Org. Lett., 2016, 18, 4277 CrossRef PubMed; (g) D. Singh, V. Kumar, N. Devi, C. C. Malakar, R. Shankar and V. Singh, Adv. Synth. Catal., 2017, 359, 1213 CrossRef.
- (a) D. Zhao, T. Wang, Q. Shen and J.-X. Li, Chem. Commun., 2014, 50, 4302 RSC; (b) K. Xu, Z. Wang, J. Zhang, L. Yu and J. Tan, Org. Lett., 2015, 17, 4476 CrossRef PubMed; (c) A. Joshi, D. C. Mohan and S. Adimurthy, Org. Lett., 2016, 18, 464 CrossRef PubMed; (d) P. Olsén, M. Oschmann, E. V. Johnston and B. Åkermark, Green Chem., 2018, 20, 469 RSC; (e) P. Peng, J. Xiong, B. Li, G. Mo, R. Chen and Z. Wang, Chin. J. Org. Chem., 2013, 33, 1891 CrossRef.
- (a) Z. Zhang, J. Zhang, J. Tan and Z. Wang, J. Org. Chem., 2008, 73, 5180 CrossRef PubMed; (b) Q. Wang, C. Wan, Y. Gu, J. Zhang, L. Gao and Z. Wang, Green Chem., 2011, 13, 578 RSC; (c) Y. Yan and Z. Wang, Chem. Commun., 2011, 47, 9513 RSC; (d) Y. Li, F. Guo, Z. Zha and Z. Wang, Sustainable Chem. Processes, 2013, 1, 8 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra03786h |
| This journal is © The Royal Society of Chemistry 2018 |