Open Access Article
Open Access ArticleCatalytic conversion of CO2 into propylene carbonate in a continuous fixed bed reactor by immobilized ionic liquids
Liying Guo *,
Lili Deng,
Xianchao Jin,
Yirong Wang and
Haozhi Wang
*,
Lili Deng,
Xianchao Jin,
Yirong Wang and
Haozhi Wang
School of Petrochemical Engineering, Shenyang University of Technology, Liaoyang 111003, P. R. China. E-mail: lyguo1981@163.com
First published on 25th July 2018
Abstract
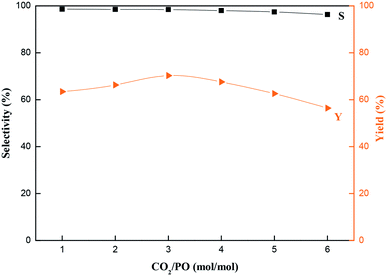
In this study, functionalized composite catalysts, namely, ILs (ILX/(ZnBr2)2), with hydroxyl, carboxyl and amino groups immobilized on a molecular sieve (MCM-22) support were synthesized with the help of a silane coupling agent, 3-chloropropyltriethoxysilane (CPTES). Cycloaddition of CO2 and propylene oxide (PO) was carried out in a fixed bed reactor. The results show that MCM-22-CPTES-[AeMIM][Zn2Br5] demonstrates the best catalytic properties for this reaction. A high selectivity and yield of propylene carbonate (PC) could be reached at 130 °C under a CO2 pressure of 2.0 MPa, with a LHSV of 0.75 h−1 and a molar ratio of CO2/PO of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The yield stabilized at 61.6% after 50 h in the fixed bed reactor.
1. The yield stabilized at 61.6% after 50 h in the fixed bed reactor.
1 Introduction
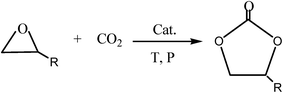
Carbon dioxide (CO2) is an attractive C1 source and has gained increasing worldwide attention as a raw material for sequestration and subsequent utilization.1–3 In view of reducing our carbon footprint, there has been increased research directed towards new methods of CO2 utilization.4,5 Numerous techniques have been developed for the preparation of these molecules, one of the most attractive synthetic pathways involves the cycloaddition of CO2 and epoxides to form cyclic carbonates.6–10 Scheme 1 shows the reaction used to synthesize cyclic carbonates. Propylene carbonate (PC) is a prominent example among these cyclic carbonates. PC can be employed as a plasticizer for novel mesoporous soft solid electrolytes,11 as an electrolytic component of electrolyte in lithium batteries12,13 and as an intermediate to produce polycarbonate14 on account of its high biodegradability and solvency, high flash and boiling points, low odor and evaporation rate.15–17 It also has applications in organic synthesis, such as the production of dimethyl carbonate (DMC) through transesterification with methanol.18Conventionally, homogeneous catalysts have been investigated for the synthesis of cyclic carbonates; the most common catalysts for this purpose are alkali metal,19,20 transition metal complexes21,22 and ILs.23–26 Generally, homogeneous catalysts are superior to heterogeneous catalysts in catalytic performances. However, homogeneous catalysts suffer from some drawbacks such as the large amount of catalyst required for the reaction, low stability, harsh reaction conditions and separation of catalyst from the products.27–29 Hence, the use of heterogeneous catalysts is the preferred choice for cycloaddition reactions. The development of an effective heterogeneous catalytic system that can overcome these problems and catalyze the cycloaddition of CO2 and epoxides remains an open challenge. Recently, immobilized IL catalysts have become the focus of many studies as they are easily separable after the reaction and can be reused in subsequent cycles.30,31 ILs have generally been immobilized on silica gel,32,33 polymers8,34,35 and molecular sieves36,37 because of their large specific surface area, high pore volume, and easy availability.
Although immobilized IL catalysts show excellent catalytic performance in cycloaddition reactions, they are primarily applied in a batch reactor and must be disassembled after each reaction.32–37 Hence, it is crucial to devise heterogeneously immobilized IL catalysts that can be used for continuous catalysis in fixed bed reactor because there are many attractive features in fixed bed reactors, particularly the realization of large scale production under continuous operating conditions. To the best of our knowledge, Takahashi et al.33 first reported the continuous operation of immobilized IL catalysts in a fixed bed reactor. The conversion of the epoxide was 80%, but the reaction pressure was as high as about 10 MPa. Xiong et al.38 prepared ILs immobilized on coconut shell activated carbon (CSAC) as catalysts. These catalysts were used in the cycloaddition of CO2 to epichlorohydrin (ECH) in a fixed bed reactor and showed excellent catalytic performances; the conversion of ECH was high for these immobilized IL catalysts after 50 h. Fang et al.39 prepared an efficient catalyst comprising PS-MimCl, ZnBr2 and phenol formaldehyde resin, and applied it for PC synthesis from CO2 and PO in a fixed bed reactor. The as-prepared catalyst was applied for a 72 h reaction and demonstrated good selectivity and yield of PC.
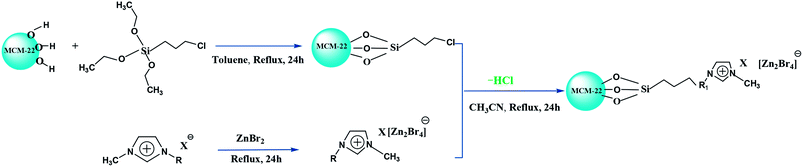
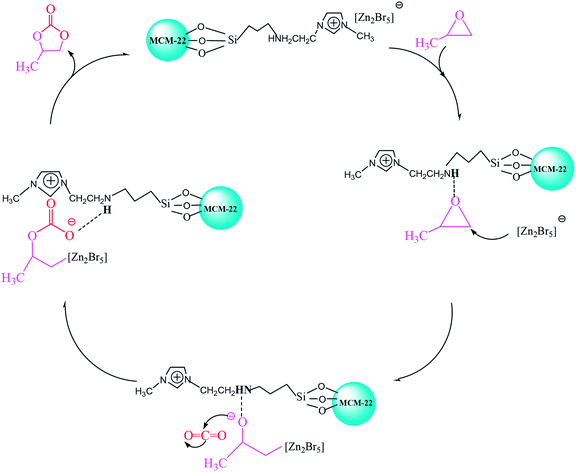
In this study, we report three composite ILs, namely, [HeMIM]Cl/(ZnBr2)2, [CeMIM]Cl/(ZnBr2)2 and [AeMIM][Zn2Br5],37 covalently immobilized on a molecular sieve (MCM-22) support with the help of a silane coupling agent, 3-chloropropyltriethoxysilane (CPTES). These composites exhibited good chemical and thermal stability, and were applied as novel heterogeneous catalysts in the preparation of PC through the cycloaddition of CO2 and PO. In addition, flexible automation, process reliability and low mechanical consumption were easily achieved in continuous industrial operation.
2 Experimental
2.1 Reagents
N-Methyl imidazole (99.0%), chloroethanol (99.0%), chloroacetic acid (99.0%), 2-bromoethylamine hydrogen bromide salt, zinc bromide (98.0%), toluene (99.5%), acetonitrile (99.0%), and propylene oxide (99.5%), used in this study, were of analytic grade and purchased from Sinopharm Chemical Reagent Co., Ltd. (China). 3-Chloropropyltriethoxysilane (CPTES, 95%) was purchased from Aladdin Chemical Co. CO2 gas (99.95%) was offered by Petrochina Liaoyang Yifang Petrochemical Company (China). MCM-22 was provided by Petrochina Fushun Petrochemical Company (China). All materials were used without further purification.2.2 Characterization of MCM-22-CPTES-ILX/(ZnBr2)2
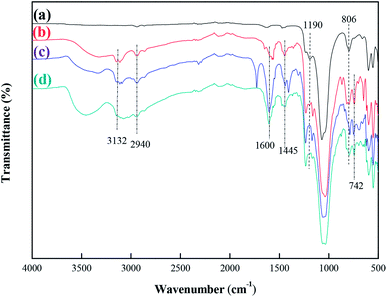
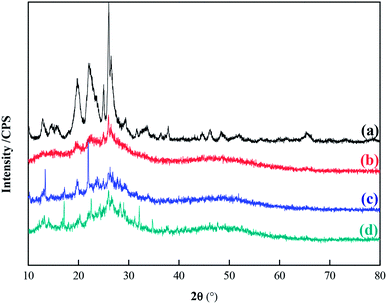
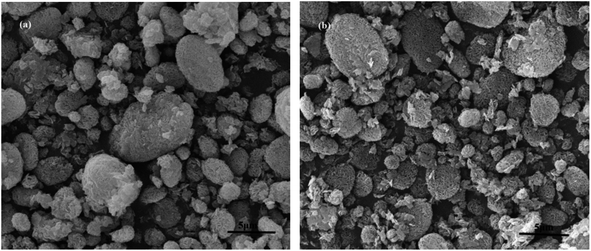
Fourier transform infrared (FT-IR) spectra of MCM-22-CPTES-ILX/(ZnBr2)2 catalysts were recorded on a MAGNA-IR750 from Thermo Nicolet Corporation. The powder X-ray diffraction (XRD) patterns were obtained using Rigaku Miniflex patterns from Rigaku Corporation. A scan speed of 5° min−1 was used for the X-ray diffractometer over a scan range of 10–80°. Thermogravimetric analysis (TGA) was conducted using a PerkinElmer TGA4000 instrument. The morphology and surface structure of the molecular sieve was obtained with a Quanta 450 Sigma field emission scanning electron microscope (FE-SEM). The loading mass of ILs and Zn were investigated by elemental analysis and ICP. Then, the purity of PC was determined by 1790F gas chromatography from Agilent Technologies. The catalytic experimental fixed bed reactor (Tianjin Pengxiang Technology Co., Ltd, China) was used for the catalytic process.2.3 Preparation of MCM-22-CPTES-ILX/(ZnBr2)2
2.4 Typical procedure for the synthesis of PC from PO and CO2
The cycloaddition reaction of CO2 and PO was carried out in a fixed bed reactor. The catalyst was placed in the middle of the packed column and both ends were filled with quartz granules. Then, the fixed bed reactor was sealed and placed under nitrogen (N2) atmosphere. CO2 was fed into the fixed bed reactor from a high pressure cylinder, while propylene oxide was fed through a pump. PO was heated in the preheater and into the packed column to react with CO2; no additional solvent was added during the reaction. The reaction temperature and the pressure were investigated for optimization. The products were analyzed on a gas chromatograph, equipped with a FID and SE-54 column, to calculate selectivity and yield.3 Results and discussion
3.1 Characterization of MCM-22-CPTES-ILX/(ZnBr2)2
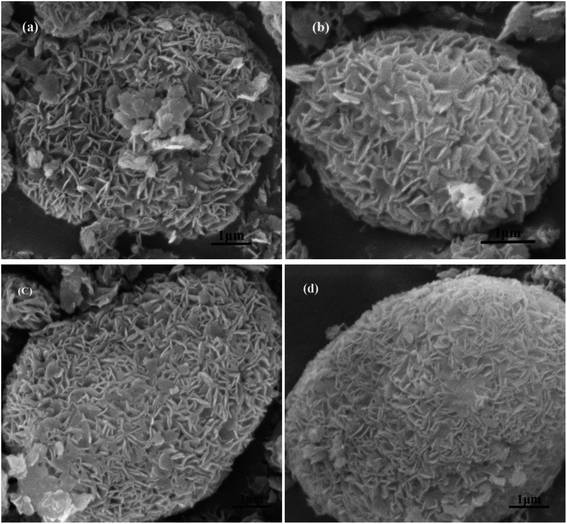 | ||
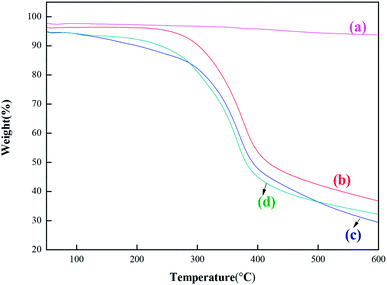
| Fig. 4 SEM of MCM-22 and MCM-22-CPTES-ILX/(ZnBr2)2. (The morphology characterizations of (a) MCM-22 and (b–d) MCM-22-CPTES-ILX/(ZnBr2)2). | ||
| Catalyst | N (wt%) | C (wt%) | H (wt%) | IL grafted [mmol g−1] | Znc [mmol g−1] |
|---|---|---|---|---|---|
| a Fresh catalyst.b Reaction after 50 h.c Zn loading obtained from ICP analysis. | |||||
| MCM-22 | — | — | — | — | — |
| MCM-22-CPTES-[HeMIM]Cl/(ZnBr2)2 | 3.98 | 13.64 | 3.55 | 1.42 | 2.79 |
| MCM-22-CPTES-[CeMIM]Cl/(ZnBr2)2 | 3.75 | 13.01 | 3.29 | 1.34 | 2.61 |
| MCM-22-CPTES-[AeMIM][Zn2Br5]a | 4.87 | 12.26 | 3.27 | 1.16 | 2.29 |
| MCM-22-CPTES-[AeMIM][Zn2Br5]b | 3.91 | 11.73 | 2.26 | 0.93 | 1.64 |
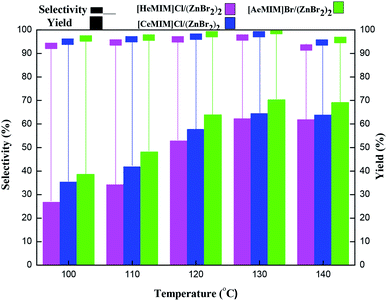
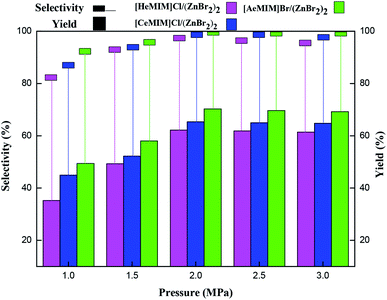
3.2 Catalytic activity of immobilized ILX/(ZnBr2)2
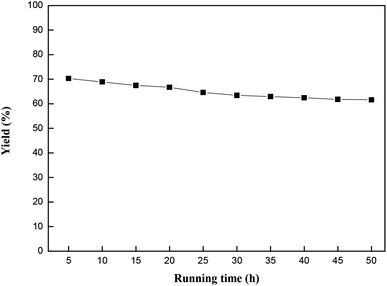 | ||
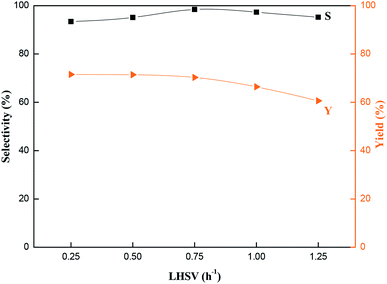
Fig. 9 Effects of reaction time on the catalytic performance of MCM-22-CPTES-[AeMIM][Zn2Br5]. Reaction conditions: temperature, 130 °C; pressure, 2.0 MPa; LHSV, 0.75 h−1; CO2/PO (mol mol−1), 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
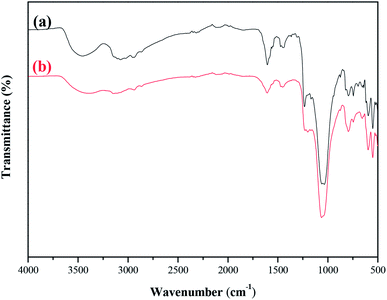 | ||
| Fig. 10 FT-IR spectra comparison of the fresh and 50 h-reaction-used MCM-22-CPTES-[AeMIM][Zn2Br5]. (a) Fresh catalyst (b) after 50 h of reaction. | ||
4 Conclusions
In conclusion, MCM-22 immobilized composite ILs (ILX/(ZnBr2)2) with CPTES catalysts were prepared successfully and used in the cycloaddition reaction of CO2 and PO. Among the three investigated catalysts, MCM-22-CPTES-[AeMIM][Zn2Br5] exhibited excellent catalytic performance in the fixed bed reactor. Probably, hydroxyl, carboxyl and amino groups of functionalized ILX/(ZnBr2)2 would remove a hydrogen atom in the immobilization process. Hence, only the amino hydrogen can form a hydrogen bond with the O atom of PO, which greatly affects the reaction. The amino, bromide ion and Lewis acid zinc bromide of ILX/(ZnBr2)2 in the catalyst played essential roles in promoting the catalytic activity and were main reasons for the excellent selectivity and yield of PC using MCM-22-CPTES-[AeMIM][Zn2Br5] as a catalyst. We demonstrate a truly heterogeneous and environmentally friendly catalyst that is capable of realizing the continuous catalysis of PC from PO and CO2 in a solvent-free reaction, which can contribute to a green and environmental protection technology.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by National Natural Science Foundation of China (21706163) and Liaoning province Department of Education Foundation (LQGD2017020).References
- W. M. Ren, Z. W. Liu, Y. Q. Wen, R. Zhang and X. B. Lu, J. Am. Chem. Soc., 2009, 131, 11509–11518 CrossRef PubMed.
- M. Y. He, Y. H. Sun and B. X. Han, Angew. Chem., Int. Ed., 2013, 52, 9620–9633 CrossRef PubMed.
- Q. Su, X. Q. Yao, W. G. Cheng and S. J. Zhang, Green Chem., 2017, 19, 2957–2965 RSC.
- W. Z. Zhang, M. W. Yang and X. B. Lu, Green Chem., 2016, 18, 4181–4184 RSC.
- C. Kohrt and T. Werner, ChemSusChem, 2015, 8, 2031–2034 CrossRef PubMed.
- M. North, R. Pasquale and C. Young, Green Chem., 2010, 12, 1514–1539 RSC.
- Y. Leng, D. Lu, P. P. Jiang, C. J. Zhang, J. W. Zhao and W. J. Zhang, Catal. Commun., 2016, 74, 99–103 CrossRef.
- A. H. Jadhav, G. M. Thorat, K. Lee, A. C. Lim, H. Kang and J. G. Seo, Catal. Today, 2016, 265, 56–67 CrossRef.
- F. Adam, J. N. Appaturi and E. P. Ng, J. Mol. Catal. A: Chem., 2014, 386, 42–48 CrossRef.
- S. M. Sadeghzadeh, Green Chem., 2015, 17, 3059–3066 RSC.
- C. Chotsuwan, S. Boonrungsiman, T. Chokanarojwong and S. Dongbang, J. Solid State Electrochem., 2017, 21, 3011–3019 CrossRef.
- L. F. Zhang, S. Q. Du, Q. L. Song, Y. Liu and S. W. Guo, Chem. Res. Chin. Univ., 2017, 33, 779–784 CrossRef.
- J. H. Clements, Ind. Eng. Chem. Res., 2003, 42, 663–674 CrossRef.
- P. T. Anastas and R. L. Lankey, Green Chem., 2000, 2, 289–295 RSC.
- S. Y. Huang, S. G. Liu, J. P. Li, N. Zhao, W. Wei and Y. H. Sun, Catal. Lett., 2006, 112, 187–191 CrossRef.
- G. L. Yu, X. R. Chen and C. L. Chen, React. Kinet. Catal. Lett., 2009, 97, 69–75 CrossRef.
- T. Sakakura and K. Kohno, Chem. Commun., 2009, 45, 1312–1330 RSC.
- H. Wang, M. H. Wang, N. Zhao, W. Wei and Y. H. Sun, Catal. Lett., 2005, 105, 253–257 CrossRef.
- M. A. Fuchs, C. Altesleben, S. C. Staudt, O. Walter, T. A. Zevaco and E. Dinjus, Catal. Sci. Technol., 2014, 4, 1658–1673 RSC.
- J. Song, Z. Zhang, B. Han, S. Hu, W. Li and Y. Xie, Green Chem., 2008, 10, 1337–1341 RSC.
- A. Decortes, A. M. Castilla and A. W. Kleij, Angew. Chem., Int. Ed., 2010, 49, 9822–9837 CrossRef PubMed.
- T. Ema, Y. Miyazaki, J. Shimonishi, C. Maeda and J. Y. Hasegawa, J. Am. Chem. Soc., 2014, 136, 15270–15279 CrossRef PubMed.
- J. J. Peng and Y. Q. Deng, Chin. J. Catal., 2001, 22, 598–600 Search PubMed.
- Y. G. Zhang and J. Y. G. Chan, Energy Environ. Sci., 2010, 3, 408–417 RSC.
- J. Q. Wang, J. Y. Leong and Y. G. Zhang, Green Chem., 2014, 16, 4515–4519 RSC.
- F. W. Li, L. F. Xiao, C. G. Xia and B. Hu, Tetrahedron Lett., 2004, 45, 8307–8310 CrossRef.
- M. I. Kim, D. K. Kim, K. V. Bineesh, D. W. Kim, M. Selvaraj and D. W. Park, Catal. Today, 2013, 200, 24–29 CrossRef.
- S. Liang, H. Liu, T. Jiang, J. Song, G. Yang and B. Han, Chem. Commun., 2011, 47, 2131–2133 RSC.
- D. Bai, S. Duan, L. Hai and H. Jing, ChemCatChem, 2012, 4, 1752–1758 CrossRef.
- Z. Z. Yang, Y. N. Zhao and L. N. He, RSC Adv., 2011, 1, 545–567 RSC.
- J. Sun, W. G. Cheng, W. Fan, Y. H. Wang, Z. Y. Meng and S. J. Zhang, Catal. Today, 2009, 148, 361–367 CrossRef.
- M. I. Kim, S. J. Choi, D. W. Kim and D. W. Park, J. Ind. Eng. Chem., 2014, 20, 3102–3107 CrossRef.
- T. Takahashi, T. Watahiki, S. Kitazume, H. Yasuda and T. Sakakura, Chem. Commun., 2006, 1664–1666 RSC.
- J. Sun, J. Q. Wang, W. G. Cheng, J. X. Zhang, X. H. Li, S. J. Zhang and Y. B. She, Green Chem., 2012, 14, 654–660 RSC.
- J. Sun, W. G. Cheng, W. Fan, Y. H. Wang, Z. Y. Meng and S. J. Zhang, Catal. Today, 2009, 148, 361–367 CrossRef.
- W. G. Cheng, X. Chen, J. Sun, J. Q. Wang and S. J. Zhang, Catal. Today, 2013, 200, 117–124 CrossRef.
- L. Y. Guo, L. L. Deng, X. C. Jin, H. Wu and L. Z. Yin, Catal. Lett., 2017, 147, 2290–2297 CrossRef.
- Y. L. Zhang, Z. T. Tan, B. L. Liu, D. S. Mao and C. R. Xiong, Catal. Commun., 2015, 68, 73–76 CrossRef.
- Z. Z. Zhang, X. L. Sun, X. W. Zhang and X. C. Fang, Catal. Lett., 2016, 146, 2098–2104 CrossRef.
- L. Y. Guo, B. Zhang, Z. M. Wang, X. Y. Ma and P. C. Huang, Acta Polym. Sin., 2015, 5, 556–563 Search PubMed.
- E. H. Lee, J. Y. Ahn, M. M. Dharman, D. W. Park, S. W. Park and I. Kim, Catal. Today, 2008, 131, 130–134 CrossRef.
- Y. Xie, K. L. Ding, Z. M. Liu, J. J. Li, G. M. An, R. T. Tao, Z. Y. Sun and Z. Z. Yang, Chem.–Eur. J., 2010, 16, 6687–6692 CrossRef PubMed.
- F. Adam, S. Balakrishnan and P. L. Wong, J. Phys. Sci., 2006, 17, 1–13 Search PubMed.
- F. Adam, J. N. Appaturi, R. Thankappan and M. A. M. Nawi, Appl. Surf. Sci., 2010, 257, 811–816 CrossRef.
- J. N. Appaturi and F. Adam, Appl. Catal., B, 2013, 136, 150–159 CrossRef.
- M. H. Valkenberg, C. deCastro and W. F. Hölderich, Green Chem., 2002, 4, 88–93 RSC.
- W. Hammond, E. Prouzet and S. D. Mahanti, Microporous Mesoporous Mater., 1999, 27, 19–25 CrossRef.
- M. B. Yue, L. B. Sun and Y. Cao, Microporous Mesoporous Mater., 2008, 114, 74–81 CrossRef.
- V. B. Saptal and B. M. Bhanage, ChemCatChem, 2016, 8, 244–250 CrossRef.
- W. H. Zhang, P. P. He, S. Wu, J. Xu, Y. X. Li, G. Zhang and X. Y. Wei, Appl. Catal., A, 2016, 509, 111–117 CrossRef.
- W. L. Dai, L. Chen, S. F. Yin, S. L. Luo and C. T. Au, Catal. Lett., 2010, 135, 295–340 CrossRef.
- J. Yue, G. W. Chen, Q. Yuan, L. A. Luo and Y. Gonthier, Chem. Eng. Sci., 2007, 62, 2096–2108 CrossRef.
- Y. C. Zhao, C. Q. Yao, G. W. Chen and Q. Yuan, Green Chem., 2013, 15, 446–452 RSC.
| This journal is © The Royal Society of Chemistry 2018 |