Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fe3O4@SiO2 nanoparticle supported ionic liquid for green synthesis of antibacterially active 1-carbamoyl-1-phenylureas in water
Mahmoud Nasrollahzadeh *a,
Zahra Issaabadia and
S. Mohammad Sajadib
*a,
Zahra Issaabadia and
S. Mohammad Sajadib
aDepartment of Chemistry, Faculty of Science, University of Qom, Qom 3716146611, Iran. E-mail: mahmoudnasr81@gmail.com; Fax: +98 25 32103595; Tel: +98 25 32850953
bDepartment of Petroleum Geoscience, Faculty of Science, Soran University, PO Box 624, Soran, Kurdistan Regional Government, Iraq
First published on 2nd August 2018
Abstract
In the present work, we have designed a novel, heterogeneous and recyclable magnetic Brønsted acidic ionic liquid based on 5-phenyl-1H-tetrazole. The {Fe3O4@SiO2@(CH2)35-phenyl-1H-tetrazole-SO3H/Cl} ([FSTet-SO3H]Cl) was prepared via the immobilization of 5-phenyl-1H-tetrazole-bonded sulfonic acid onto the surface of silica-coated magnetic nanoparticles using 3-chloropropyltriethoxysilane as a linker. The catalyst was characterized by XRD, TEM, FESEM, EDS, TG-DTA, and FT-IR. The ability and high activity of this catalyst were demonstrated in the synthesis of 1-carbamoyl-1-phenylureas with good to excellent yields via a new, simple and one-pot procedure in aqueous media under reflux conditions. This procedure has advantages such as high yields, short reaction times, a simple methodology and work-up process, green reaction conditions, high stability, catalytic activity, and easy preparation, separation and reusability of the catalyst. The synthesis of these compounds was confirmed by FT-IR, 1H NMR, 13C NMR and CHN. In addition, we investigated the biological properties of the 1-carbamoyl-1-phenylureas as newly synthesized compounds. The described catalyst could be easily separated from the reaction mixture by additional magnetic force and reused several times without a remarkable loss of its catalytic activity and any considerable changes in the product yield and the reaction time.
Introduction
Ionic liquids (IL) are a class of liquids that contain only ions.1 In general, ionic liquids contain molten salts at a temperature above 800 °C, however today, ionic liquids are known as salts that are liquid at temperatures below 100 °C.2 Over the past few years, ionic liquids have attracted the attention of many scientists due to their capabilities as catalysts, reaction media, reagents and solvents.3 Ionic liquids have advantages such as a high thermal stability, being non-flammable, having a low vapor pressure, being recyclable and having excellent solvation properties.4 However, ionic liquids have limited applications due to their toxic nature.5 Recently, extensive studies have been conducted to eliminate the disadvantages associated with ionic liquids and replace them with safer and more productive ones. One of the best techniques is the combination of ionic liquids and magnetic nanoparticles (MNPs). MNPs act as a good support for the immobilization of the ionic liquids.6 Magnetic ionic liquids have certain specifications such as a large specific surface area, high stability, facile separation and recovery from the reaction mixture, good magnetic permeability and low toxicity and price.7Recently, solid acid catalysts have attracted a lot of attention in the field of organic reactions.8 In this regard, many Brønsted acids such as thiourea, TADDOL, amidinium and phosphate can be used as green and free-of-metal catalysts.9 If an alkane sulfonic acid group is covalently tethered to the IL cation, the IL is converted into a strong Brønsted acid.10 These SO3H-functionalized ionic liquids can act as good alternatives for homogenous and heterogeneous acidic catalysts due to their advantages such as being non-corrosive, nonvolatile and immiscible with many organic solvents.11
From the past to present, the synthesis of organic compounds has attracted the attention of many chemists due to their special importance in biological and medical studies. Among the organic compounds, 1-carbamoyl-1-phenylureas are an important class of compounds. However, there is no report on the synthesis of 1-carbamoyl-1-phenylureas in literature so far.
Over the past few years, the hydration of cyanamides has attracted a lot of attention as one of the most important ways of synthesizing N-monosubstituted ureas.12 However, the hydration of cyanamides suffers from several disadvantages including the use of corrosive bases or acids, low yields, the use of toxic organic solvents, long reaction times and tedious work-up.13 Therefore, the development of a new, easy and efficient method for the hydration of cyanamides is one of the most important challenges.
1-Carbamoyl-1-phenylureas are the parent compounds of a large and interesting class of organic substances. It is probable that the presence of three nitrogen atoms in their structure has led to greater biological properties, but still nobody has succeeded in synthesizing this important compound. In addition, they are important compounds which can be used as starting materials in coordination chemistry and organic synthesis in the future.
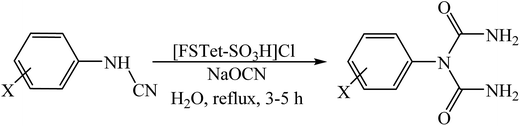
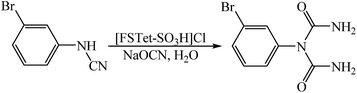
In this research, we have designed a heterogeneous and recyclable magnetic Brønsted acidic ionic liquid catalyst by using 5-phenyl-1H-tetrazole. Although many ionic liquid based imidazoles are known, only very few ionic liquid based tetrazoles have been described.14 However, it is noteworthy that there is no report on the synthesis of magnetic ionic liquid based tetrazoles. Therefore, this report could create a new approach for the production of magnetic ionic liquid based tetrazoles. Next, we investigated the catalytic activity of [FSTet-SO3H]Cl in the synthesis of 1-carbamoyl-1-phenylureas via a one-pot procedure in aqueous media under reflux conditions (Scheme 1). The results show that the catalyst has excellent catalytic activity in this reaction. The products were prepared in good to excellent yields and characterized by FT-IR, 1H NMR, 13C NMR, CHN and melting point determination.
Experimental
Reagents and methods
All materials of commercial reagent grade were purchased from the Merck and Aldrich companies and used without further purification. FT-IR spectra were recorded on a Nicolet 370 FT/IR spectrometer (Thermo Nicolet, USA) using pressed KBr pellets. X-ray diffraction (XRD) measurements were carried out with a Philips powder diffractometer type PW 1373 goniometer. It was equipped with a graphite monochromator crystal. The X-ray wavelength was 1.5405 Å and the diffraction patterns were recorded in the 2θ range (10–80) with a scanning speed of 2° min−1. TEM images were taken using a Philips EM208 transmission electron microscope with an accelerating voltage of 90 kV. Scanning electron microscopy (SEM) of the {Fe3O4@SiO2@(CH2)35-phenyl-1H-tetrazole-SO3H/HCl} was performed on a Cam scan MV2300. The chemical compositions of the synthesized catalyst were determined by EDS (energy dispersive X-ray spectroscopy) performed in SEM. Thermal analysis (TG-DTG) was carried out using an STA 1500 Rheometric Scientific (England). The flow rate of air was 120 mL min−1 and the ramping rate of the sample was 2 °C min−1. VSM measurements were recorded using a SQUID magnetometer at 298 K (Quantum Design MPMS XL). Melting points were taken in open capillaries using BUCHI 510 melting point apparatus and are uncorrected.General protocol for the synthesis of the {Fe3O4@SiO2@(CH2)35-phenyl-1H-tetrazole-SO3H/Cl}
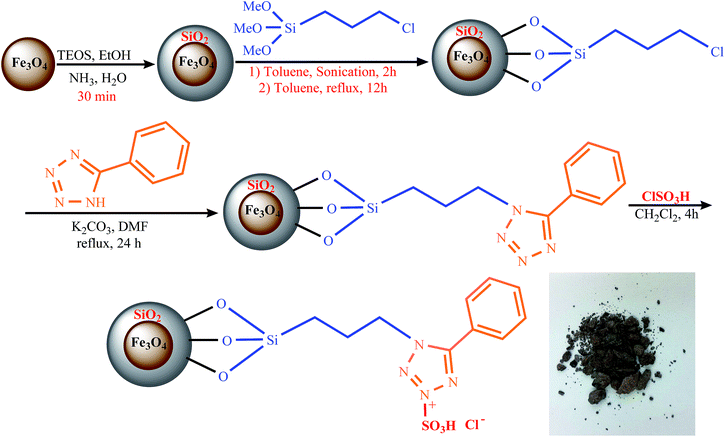
Firstly, in a 250 mL flask, 1.0 g of Fe3O4, 20.0 mL of H2O, 3.0 mL of NH3, 80.0 mL of EtOH and 3.0 mL of tetraethylorthosilicate (TEOS) were mixed and refluxed to afford silica-coated Fe3O4 (Fe3O4@SiO2). In the next step, 3.0 g of Fe3O4@SiO2, 10.0 mmol of (3-chloropropyl)trimethoxysilane and 80.0 mL of dry toluene were refluxed under nitrogen for 12 h. The as-prepared Fe3O4@SiO2@(CH2)3Cl was separated with an external magnet, washed with dry toluene and anhydrous diethyl ether, and dried at 80 °C for 6 h under vacuum. Then 5-phenyl-1H-tetrazole (0.73 g, 5.0 mmol) and K2CO3 (0.69 g, 5.0 mmol) in 50.0 mL of DMF were added to the Fe3O4@SiO2@(CH2)3Cl and the mixture was refluxed for 24 h. The resulting solid was filtered, washed with ethanol and distilled water and dried at 80 °C for 6 h under vacuum. Finally, chlorosulfonic acid (1.165 g, 10.0 mmol) was added dropwise to the Fe3O4@SiO2@(CH2)35-phenyl-1H-tetrazole in dry dichloromethane and the mixture was stirred for 3 h. Then with similar steps of filtering, washing and drying, {Fe3O4@SiO2@(CH2)35-phenyl-1H-tetrazole-SO3H/HCl} as a magnetically recoverable catalyst was obtained.General protocol for the one-pot synthesis of 1-carbamoyl-1-phenylureas
In a typical procedure, to a mixture of arylcyanamide (1.0 mmol) and sodium cyanate (0.065 g, 1.0 mmol) 0.2 g of the {Fe3O4@SiO2@(CH2)35-phenyl-1H-tetrazole-SO3H/Cl} catalyst in 10.0 mL of H2O was added and stirred under reflux conditions for an appropriate amount of time. The reaction progress was monitored using TLC. After completion of the reaction, the catalyst was recovered magnetically and the mixture was cooled for the product to precipitate. All of the products were purified via recrystallization from EtOAC–n-hexane. The products were obtained in high yields and characterized by FT-IR, 1H NMR, 13C NMR, CHN and melting points.Results and discussion
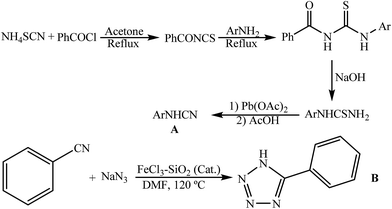
Our recent studies have shown that arylcyanamides and tetrazoles could be used as highly active compounds in organic synthesis.8,15 However, a lack of convenient methods for the preparation of the arylcyanamides and ionic liquid based tetrazoles strongly restricts their potential application in organic synthesis. In this work, the arylcyanamides and 5-phenyl-1H-tetrazole were prepared according to our recent work on aryl amines15c and benzonitrile15d (Scheme 2).Due to our interest in green protocols, in the present work, for the first time we have reported the synthesis of 1-carbamoyl-1-phenylureas using [FSTet-SO3H]Cl as a magnetic Brønsted acidic ionic liquid catalyst in water as a green solvent.
In this work, by combining the advantages of a Brønsted acidic ionic liquid as a H+ source and silica-coated magnetic nanoparticles (Fe3O4@SiO2) with high surface area, excellent thermal stability, low toxicity, and easy synthesis and separation from the reaction mixture by an external magnet, a [FSTet-SO3H]Cl nanocomposite has been produced using a simple method. To the best of our knowledge, this is the first report wherein an ionic liquid based tetrazole has been immobilized on the Fe3O4@SiO2 surface as a powerful catalytic support.
Characterization of the [FSTet-SO3H]Cl
As shown in Scheme 3, the [FSTet-SO3H]Cl was prepared in four steps: (1) coating the Fe3O4 with the silica (Fe3O4@SiO2), (2) linking the Fe3O4@SiO2 with the 3-chloropropyltriethoxysilane, (3) grafting the Fe3O4@SiO2@(CH2)3Cl with 5-phenyl-1H-tetrazole and (4) converting the Fe3O4@SiO2@(CH2)35-phenyl-1H-tetrazole to an ionic liquid by chlorosulfonic acid. The synthesized catalyst was characterized using FT-IR, XRD, TEM, FE-SEM, EDS, VSM, TGA and DTG.The chemical composition of Fe3O4@SiO2, Fe3O4@SiO2@(CH2)3Cl, Fe3O4@SiO2@(CH2)35-phenyl-1H-tetrazole, and [FSTet-SO3H]Cl was analyzed by Energy Dispersive X-ray Spectroscopy (EDS). The EDS spectra of these materials indicate the presence of the corresponding elements in their structure. The EDS spectrum of [FSTet-SO3H]Cl confirmed that it was composed of Fe, Si, N, C, O and S (Fig. 1). Fig. 1 confirmed that Fe, Si, N, C, and O were the main components present in both Fe3O4@SiO2@(CH2)35-phenyl-1H-tetrazole and [FSTet-SO3H]Cl along with the S element which is present only in [FSTet-SO3H]Cl further demonstrating the formation of an ionic liquid in the case of [FSTet-SO3H]Cl.
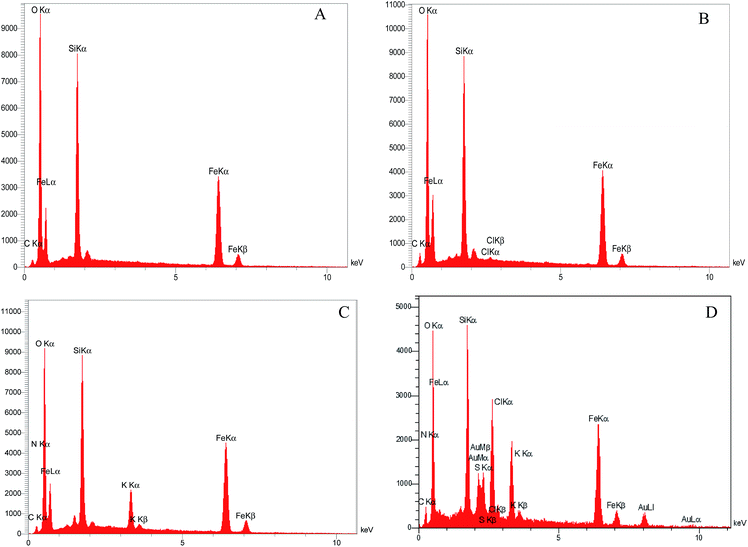 | ||
| Fig. 1 The EDS spectra of Fe3O4@SiO2 (A), Fe3O4@SiO2@(CH2)3-Cl (B), Fe3O4@SiO2@(CH2)35-phenyl-1H-tetrazole (C) and [FSTet-SO3H]Cl (D). | ||
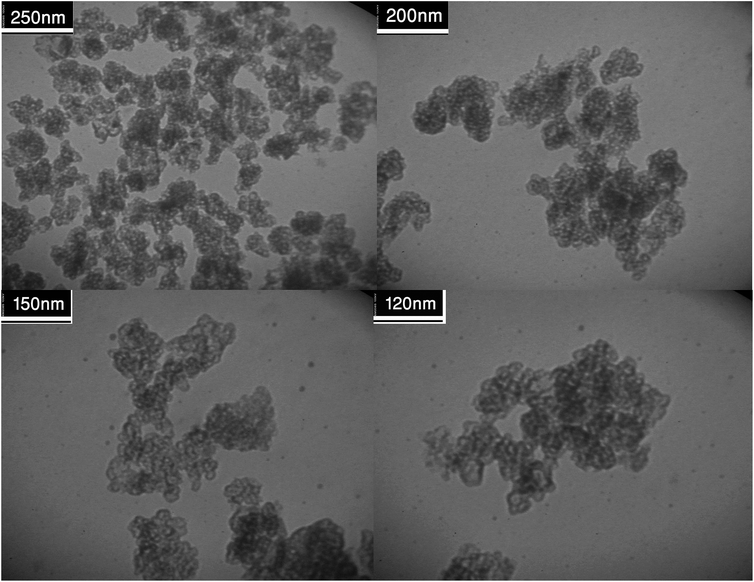
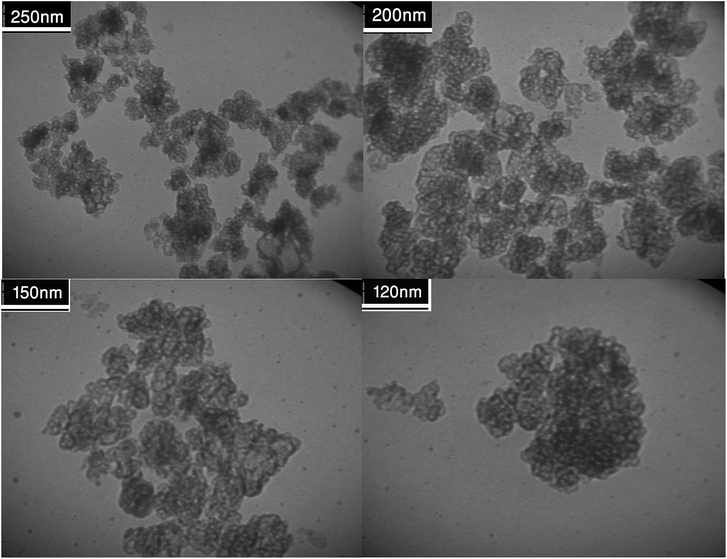
Fig. 2 shows the FESEM images of Fe3O4@SiO2, Fe3O4@SiO2@(CH2)3Cl, Fe3O4@SiO2@(CH2)35-phenyl-1H-tetrazole and [FSTet-SO3H]Cl. These materials show a spherical morphology with an average particle size in the range 23–39 nm. The morphology of [FSTet-SO3H]Cl was also studied from TEM images at different magnifications (Fig. 3). The morphology calculated from the FESEM images was in agreement with TEM images in the case of [FSTet-SO3H]Cl. However, the average particle size was found to be smaller in the TEM images.
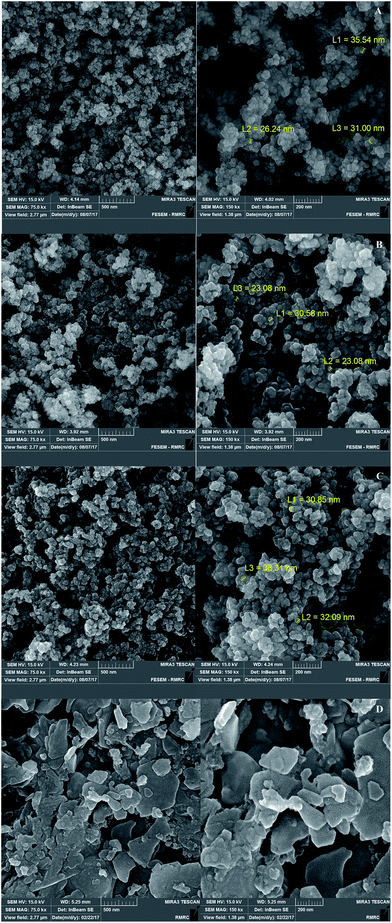 | ||
| Fig. 2 FESEM images of Fe3O4@SiO2 (A), Fe3O4@SiO2@(CH2)3Cl (B), Fe3O4@SiO2@(CH2)35-phenyl-1H-tetrazole (C) and [FSTet-SO3H]Cl (D). | ||
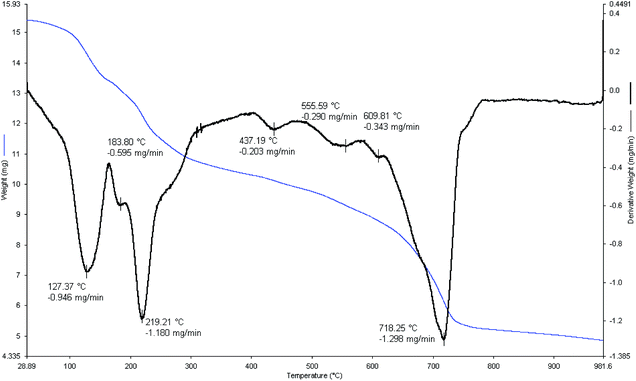
The thermal stability of [FSTet-SO3H]Cl was also analyzed by TGA (thermogravimetric analysis) and DTA (differential thermal analysis) experiments in air flow (120 mL min−1) at a heating rate of 2 °C min−1 on an autonomic TG-DTA. As shown in Fig. 4, three weight loss stages were observed in air flow for [FSTet-SO3H]Cl. The first weight-loss step mainly happened at 120–190 °C and is associated with desorbed water or other organic solvents which were employed during the preparation steps of the catalyst. In the second stage at 218–300 °C, a weight loss is observed which can be attributed to the thermal decomposition of 5-phenyl-1H-tetrazole functionalized with chlorosulfonic acid on the surface of the silica coating. Finally, the weight loss observed at 650–750 °C is due to the decomposition of the catalyst.
FT-IR analysis was carried out to confirm the structure and the formation of [FSTet-SO3H]Cl. Fig. 5 shows the FT-IR spectra of 5-phenyl-1H-tetrazole (A), Fe3O4@SiO2 (B), Fe3O4@SiO2@(CH2)3Cl (C), Fe3O4@SiO2@(CH2)35-phenyl-1H-tetrazole (D) and [FSTet-SO3H]Cl (E). The structure of 5-phenyl-1H-tetrazole was in agreement with the FT-IR spectra data. The disappearance of one strong and sharp absorption band (CN stretching band) in benzonitrile, and the appearance of an NH stretching band in the FT-IR spectroscopy, was evidence for the formation of 5-phenyl-1H-tetrazole (Fig. 5A). In Fig. 5B–E, the peak at 3600–3100 cm−1 is due to the O–H stretching mode. The appearance of two peaks at 570 cm−1 and 617 cm−1 (Fig. 5B) supports the formation of Fe3O4. The absorption peak at 570 cm−1 is attributed to Fe–O vibration of the Fe3O4 MNPs. The formation of Fe3O4@SiO2 was confirmed by the appearance of peaks at 450, 801, 955 and 1097 cm−1 which are assigned to the Si–O–Si bending, Si–O bending, Si–OH stretching and Si–O–Si stretching, respectively (Fig. 5B). The peak at 1619 cm−1 may be attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N vibrations of the tetrazolium moiety of the ionic liquid (Fig. 5E).
N vibrations of the tetrazolium moiety of the ionic liquid (Fig. 5E).
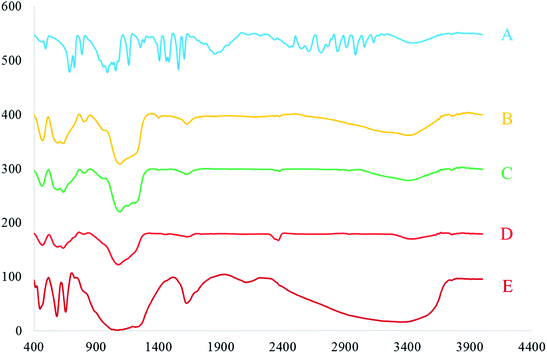 | ||
| Fig. 5 The FT-IR spectra of 5-phenyl-1H-tetrazole (A), Fe3O4@SiO2 (B), Fe3O4@SiO2@(CH2)3Cl (C), Fe3O4@SiO2@(CH2)35-phenyl-1H-tetrazole (D) and [FSTet-SO3H]Cl (E). | ||
To determine the crystallographic structure of Fe3O4@SiO2, Fe3O4@SiO2@(CH2)3Cl, Fe3O4@SiO2@(CH2)35-phenyl-1H-tetrazole and [FSTet-SO3H]Cl, XRD analysis was carried out (Fig. 6). As shown in Fig. 6, peaks of 2θ at 30.00°, 35.30°, 42.80°, 53.00°, 56.80° and 62.50° can be indexed as (hkl) to the (220), (311), (400), (422), (511) and (440) planes of cubic iron oxide.
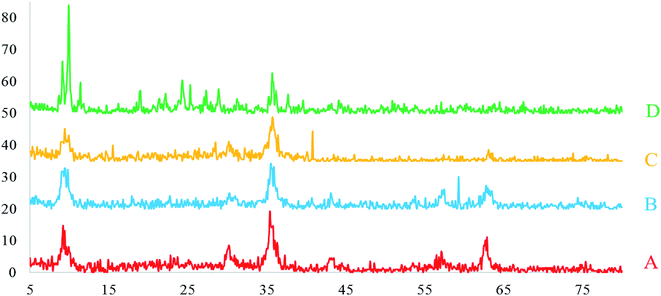 | ||
| Fig. 6 The XRD patterns of Fe3O4@SiO2 (A), Fe3O4@SiO2@(CH2)3Cl (B), Fe3O4@SiO2@(CH2)35-phenyl-1H-tetrazole (C) and [FSTet-SO3H]Cl (D). | ||
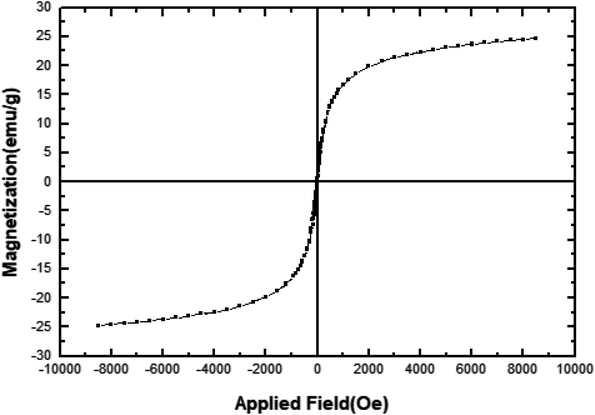
In order to study the magnetic properties of [FSTet-SO3H]Cl, a vibrating sample magnetometer (VSM) was used to characterize the as-prepared catalyst with a magnetometer at 298 K and with field sweeping from −10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to +10
000 to +10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe. Fig. 7 shows a typical room temperature magnetization curve of [FSTet-SO3H]Cl. As demonstrated in Fig. 7, the magnetization curves of the as-prepared ionic liquid display no hysteresis loop which demonstrates its superparamagnetic characteristics. Therefore, at the end of the reaction, [FSTet-SO3H]Cl could simply be collected from the reaction mixture using an external magnet.
000 Oe. Fig. 7 shows a typical room temperature magnetization curve of [FSTet-SO3H]Cl. As demonstrated in Fig. 7, the magnetization curves of the as-prepared ionic liquid display no hysteresis loop which demonstrates its superparamagnetic characteristics. Therefore, at the end of the reaction, [FSTet-SO3H]Cl could simply be collected from the reaction mixture using an external magnet.
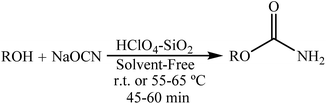
Following the synthesis of 1-arylureas8 and in a further study on the preparation of nitrogen-containing compounds, we focussed on the synthesis of 1-carbamoyl-1-phenylureas as nitrogen-rich compounds from arylcyanamides and sodium cyanate (NaOCN) as starting materials. In 2008, our research group reported the synthesis of primary carbamates from the reaction between phenol or alcohols with NaOCN in the presence of HClO4–SiO2 at room temperature or 55–65 °C for an appropriate time in high yields and under solvent-free conditions (Scheme 4).16
Herein, in the course of our research on the synthesis of the nitrogen-containing compounds,15 we now wish to report the preparation of novel 1-carbamoyl-1-phenylureas by the reaction of NaOCN with arylcyanamides in the presence of a [FSTet-SO3H]Cl catalyst in water under reflux conditions. Apparently, to the best of our knowledge, so far no methodology has been reported where NaOCN is used as an effective salt in the synthesis of the 1-carbamoyl-1-phenylureas.
We applied our catalyst to the synthesis of 1-carbamoyl-1-phenylureas under reflux conditions and 3-bromophenylcyanamide was chosen as a model substrate. Various reaction conditions including the amount of the [FSTet-SO3H]Cl catalyst and temperature were varied to examine the influence on the compositions in the reaction mixture (Table 1). We observed that 1-(3-bromophenyl)-1-carbamoylurea (1) was not produced without using the [FSTet-SO3H]Cl catalyst (Table 1, entry 1). However, with the presence of a 3-bromophenylcyanamide, NaOCN, [FSTet-SO3H]Cl catalyst and H2O mixture at room temperature, product (1) was obtained in 23% yield. We found that the yield and reaction rate were improved significantly by increasing the reaction temperature. In general, the best result was obtained with 0.2 g of the [FSTet-SO3H]Cl catalyst under reflux conditions (Table 1, entry 4), whereas no significant improvements in the reaction yield and time were observed by further increasing the amount of the [FSTet-SO3H]Cl catalyst from 0.1 g to 0.3 g (Table 1, entry 5).
| Entry | Cat. (g) | Temperature (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a The reactions were performed with 1.0 mmol of 3-bromophenylcyanamide and 1.0 mmol NaOCN in H2O (10 mL).b Yield refers to the pure isolated product. | ||||
| 1 | 0.0 | Reflux | 5 | 0.0 |
| 2 | 0.05 | Reflux | 10 | 43 |
| 3 | 0.1 | Reflux | 7 | 70 |
| 4 | 0.2 | Reflux | 5 | 90 |
| 5 | 0.3 | Reflux | 5 | 90 |
| 6 | 0.2 | R.T. | 10 | 23 |
With the optimized conditions in hand, we next tested the substrate scope of this transformation. Excellent yields could be achieved regardless of the substituents associated with the arylcyanamides (Table 2). Different substituents such as Br, Cl, OMe and Me groups were compatible, and achieved a yield up to 90% (1–7). Both electron-withdrawing and electron-donating groups in the cyanamides were compatible. As shown in Table 2, the arylcyanamides with electron-donating groups were completed under reflux conditions after 3 h and the corresponding products were obtained in shorter reaction times because of their greater ability to attack the NaOCN. However, the species bearing electron withdrawing groups required higher reaction times. As shown in Table 2, 1,4-phenylenecyanamide interestingly afforded the double-addition product (7), due to presence of two CN groups. The products were characterized by IR, 1H NMR and 13C NMR spectroscopy, elemental analysis and melting point determination.
| a The reactions were performed with 1.0 mmol arylcyanamide, 1.0 mmol NaOCN and 0.2 g [FSTet-SO3H]Cl catalyst in H2O (10 mL) under reflux conditions. Yield refers to the pure isolated product. |
|---|
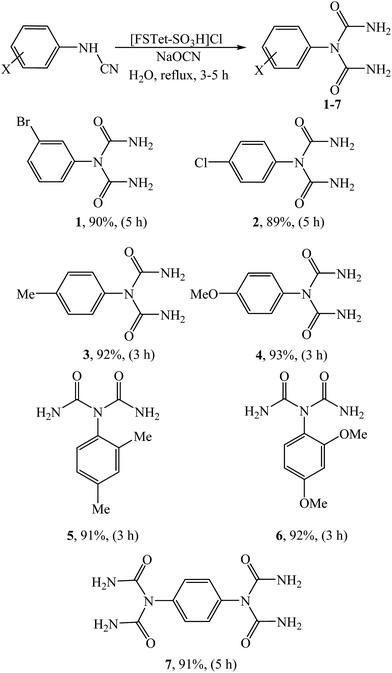 |
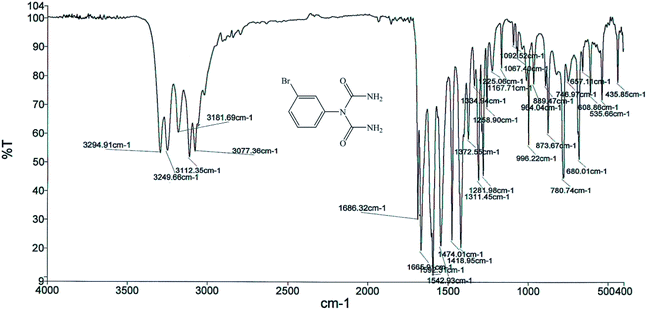
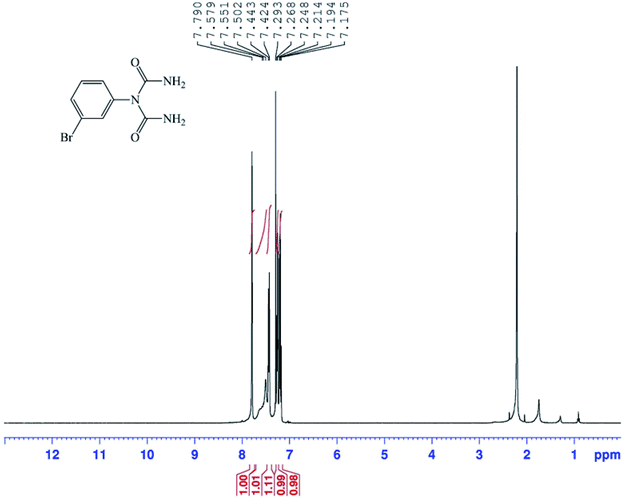
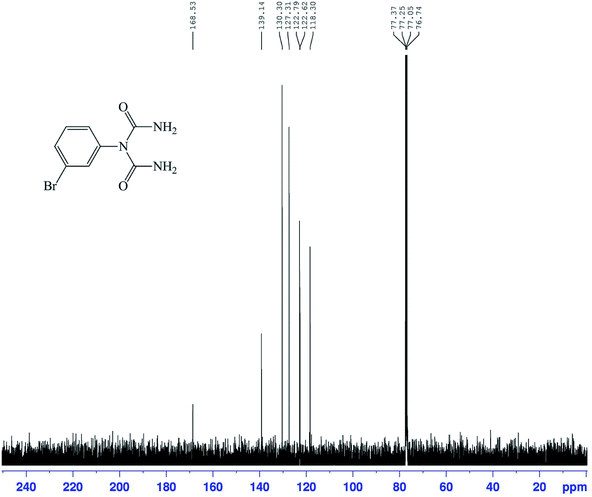
The structures of the 1-carbamoyl-1-phenylureas were in agreement with their FT-IR and NMR spectra. In the FT-IR spectra of the 1-carbamoyl-1-phenylureas, three new peaks appeared corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and NH2 groups absorption vibrations and the CN peak had disappeared (Fig. 8). The 1H NMR spectra showed one characteristic peak belonging to the NH2 group (Fig. 9). The appearance of a carbon signal corresponding to a carbonyl group in the 13C NMR spectra is evidence of the formation of 1-carbamoyl-1-phenylureas (Fig. 10).
O and NH2 groups absorption vibrations and the CN peak had disappeared (Fig. 8). The 1H NMR spectra showed one characteristic peak belonging to the NH2 group (Fig. 9). The appearance of a carbon signal corresponding to a carbonyl group in the 13C NMR spectra is evidence of the formation of 1-carbamoyl-1-phenylureas (Fig. 10).
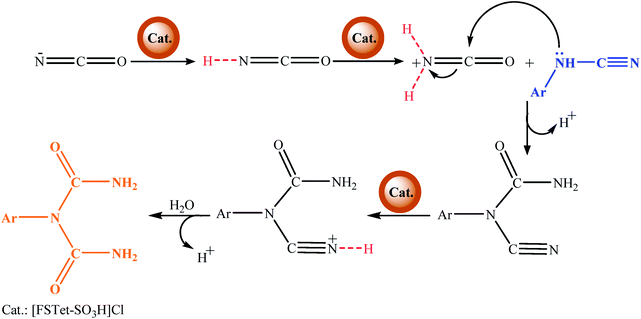
The possible mechanism for the synthesis of 1-carbamoyl-1-phenylureas under reflux conditions is shown in Scheme 5. The [FSTet-SO3H]Cl catalyst as a Brønsted acidic ionic liquid has an important role in the synthesis of 1-carbamoyl-1-phenylureas. [FSTet-SO3H]Cl is supposed to convert the −N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O to a +H2N
O to a +H2N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O electrophile and enhance its reactivity with cyanamide as a nucleophile. In the first step, the presence of the [FSTet-SO3H]Cl catalyst leads to the protonation of sodium cyanate. Next, arylcyanamides attack the protonated cyanate through a pair of nitrogen electrons. Eventually, due to the presence of the [FSTet-SO3H]Cl catalyst and water in the reaction medium, 1-aryl-1-cyanourea was hydrolyzed and the final product was produced.
O electrophile and enhance its reactivity with cyanamide as a nucleophile. In the first step, the presence of the [FSTet-SO3H]Cl catalyst leads to the protonation of sodium cyanate. Next, arylcyanamides attack the protonated cyanate through a pair of nitrogen electrons. Eventually, due to the presence of the [FSTet-SO3H]Cl catalyst and water in the reaction medium, 1-aryl-1-cyanourea was hydrolyzed and the final product was produced.
They are important structural motifs for many arylurea analogs as broad-spectrum antibacterial agents (Fig. 11).17 Nevertheless, there are no reports on the synthesis of 1-carbamoyl-1-phenylureas and investigations of their antibacterial properties. In the present study, for the first time, we report the antibacterial properties of 2-(4-(1-carbamoylureido)phenyl)malonamide (7) as a model substrate.
Escherichia coli is a common inhabitant of the intestinal tract of humans and warm-blooded animals. Most strains of E. coli are harmless and are a part of the normal intestinal microflora. These strains serve a useful function in the body by suppressing the growth of harmful bacteria and by synthesizing appreciable amounts of vitamins. However, several pathogenic E. coli strains have emerged which cause disease in humans. Pathogenic E. coli can be divided into intestinal pathogens causing diarrhoea, and extra intestinal E. coli causing a variety of infections in both humans and animals.18 Through this report we investigated the antibacterial activity of synthesized 2-(4-(1-carbamoylureido)phenyl)malonamide (7) against pathogenic E. coli as following.
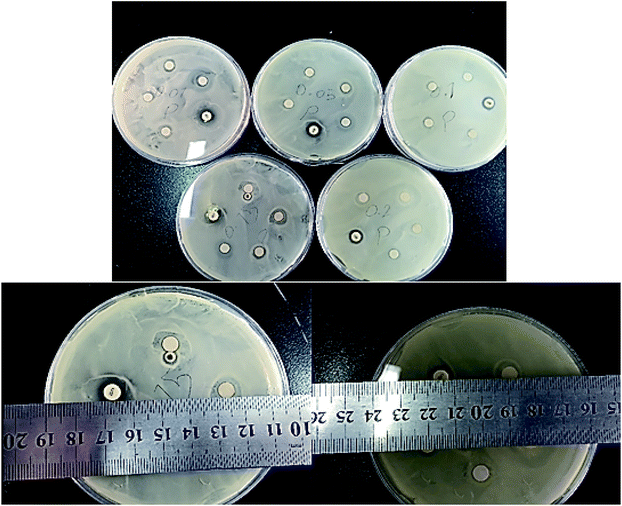
The antibacterial activity of the sample was studied against Escherichia coli bacteria by disk diffusion method using a Muller Hinton agar culture. The concentrations used for investigations of the sample were 1% (10 mg mL−1), 5% (50 mg mL−1), 10% (100 mg mL−1), 15% (150 mg mL−1) and 20% (200 mg mL−1) respectively. The results were compared with chloramphenicol as a positive control. For reporting the results of the antibiogram test the minimum protection zone per millimeter (mm) was reported for each test. Furthermore, the frequency of the test was in triplicate for each concentration of the sample. According to Table 3 and Fig. 12, the sample demonstrated no antibacterial activity against E. coli in concentrations lower than 15% but showed an antibacterial activity with good potential for concentrations equal and greater than 15%.
| Compound name | 20 mg mL−1 | 15% mg mL−1 | 10% mg mL−1 | 5% mg mL−1 | 1% mg mL−1 |
|---|---|---|---|---|---|
| a C+: chloramphenicol disc diameter (positive control), t: test frequency. | |||||
| 2-(4-(1-Carbamoylureido)phenyl)malonamide | t1: 11 mm | t1: 9 mm | t1: no result | t1: no result | t1: no result |
| t2: 9 mm | t2: 11 mm | t2: no result | t2: no result | t2: no result | |
| t3: 9 mm | t3: 14 mm | t3: no result | t3: no result | t3: no result | |
| C+: 12 mm | C+: 12 mm | C+: 12 mm | C+: 12 mm | C+: 12 mm | |
As shown from Fig. 12, for both concentrations of 15% and 20% the sample showed a suitable antibacterial activity but in concentrations lower than 15% no antibacterial results were detected. Therefore, the study confirmed that the concentration of the compound is an important factor concerning its biological activity against E. coli, and in concentrations greater than 15% the sample demonstrated a good antibacterial activity against the mentioned bacteria compared to the positive control.
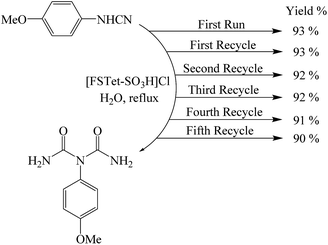
One of the most important points in the area of nanocatalysis is the stability and recyclability of heterogeneous catalysts. In order to show the effectiveness of [FSTet-SO3H]Cl, catalyst recycling experiments were carried out using 1-(4-methoxyphenyl)urea as the model substrate under optimized conditions. After each cycle, [FSTet-SO3H]Cl was separated with an external magnet, washed with ethanol, dried and then reused at least five times without significant loss of catalytic activity (Fig. 13). Easy separation and reusability of the catalysts is one of the most important benefits. As shown in FE-SEM and TEM images of the recycled catalyst (Fig. 14 and 15), no obvious change in the morphology of [FSTet-SO3H]Cl was observed.
Conclusion
In conclusion, we have developed a novel and highly efficient protocol for the preparation of a heterogeneous and recyclable magnetic Brønsted acidic ionic liquid catalyst using 5-phenyl-1H-tetrazole. To the best of our knowledge, this is the first report on the synthesis of a tetrazole-based ionic liquid stabilized on the surface of silica-coated magnetic nanoparticles using 3-chloropropyltriethoxysilane as a linker. In this work, we have developed a new strategy for the synthesis of 1-carbamoyl-1-phenylureas from arylcyanamides via a one-pot procedure in aqueous media under reflux conditions. A wide range of substituted arylcyanamides as substrates were employed and afforded the desired products in excellent yields. The high yield of products, the efficiency, generality, short reaction time, clean reaction profile, the use of water as a green solvent, the use of a relatively inexpensive catalyst, simplicity and easy work-up procedure, recyclability and reusability of the catalyst, and the straightforward isolation of the products are the advantages of this protocol. The wide substrate scope, green reaction conditions, high yield of products and recovery and recyclability of the catalyst offer the potential for scale-up in pharmaceutical applications. Further studies and the development of other methodologies for the arylcyanamides’ and 1-carbamoyl-1-phenylureas’ reactivities are in progress.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the Iranian Nano Council and University of Qom for the support of this work.References
- K. N. Marsh, J. A. Boxall and R. Lichtenthaler, Fluid Phase Equilib., 2004, 219, 93 CrossRef.
- G. Bentivoglio, T. Röder, M. Fasching, M. Buchberger, H. Schottenberger and H. Sixta, Lenzinger Ber., 2006, 86, 154 Search PubMed.
- (a) T. Welton, Chem. Rev., 1999, 99(8), 2071 CrossRef PubMed; (b) C. Yue, D. Fang, L. Liu and T. F. Yi, J. Mol. Liq., 2011, 163, 99 CrossRef; (c) T. L. Greaves and C. J. Drummond, Chem. Rev., 2008, 108, 206 CrossRef PubMed; (d) J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508 CrossRef PubMed.
- G. H. Jonathan, D. W. Heather, P. S. Richard, E. V. Ann and D. R. Robin, Chem. Commun., 1998, 16, 1765 Search PubMed.
- T. P. T. Pham, C. W. Cho and Y. S. Yun, Water Res., 2010, 44, 352 CrossRef PubMed.
- (a) Q. Yan, Y. Wang, H. Zhang, K. Xu, X. Wei, P. Xu and Y. Zhou, Talanta, 2017, 174, 139 CrossRef PubMed; (b) M. J. Trujillo-Rodríguez, V. Pino and J. L. Anderson, Talanta, 2017, 172, 86 CrossRef PubMed; (c) R. Gupta, M. Yadav, R. Gaur, G. Arora and R. K. Sharma, Green Chem., 2017, 19, 3801 RSC.
- (a) Y. Jiang, C. Guo, H. Xia, I. Mahmood, C. Liu and H. Liu, J. Mol. Catal. B: Enzym., 2009, 58, 103 CrossRef; (b) A. A. A. A. Bakhee and X. S. Zhu, J. Mol. Liq., 2017, 242, 900 CrossRef.
- Z. Issaabadi, M. Nasrollahzadeh and S. M. Sajadi, J. Colloid Interface Sci., 2017, 503, 5 CrossRef PubMed.
- (a) V. B. Gondi, M. Gravel and V. H. Rawal, Org. Lett., 2005, 7, 5657 CrossRef PubMed; (b) A. G. Wenzel and E. N. Jacobsen, J. Am. Chem. Soc., 2002, 124, 12964 CrossRef PubMed; (c) T. Akiyama, J. Itoh, K. Yokota and K. Fuchibe, Angew. Chem., Int. Ed., 2004, 43, 1566 CrossRef PubMed.
- A. C. Cole, J. L. Jensen, I. Ntai, K. L. T. Tran, K. J. Weaver, D. C. Forbes and J. H. Davis, J. Am. Chem. Soc., 2002, 124(21), 5962 CrossRef PubMed.
- J. Fraga-Dubreuil, K. Bourahla, M. Rahmouni, J. P. Bazureau and J. Hamelin, Catal. Commun., 2002, 3, 185 CrossRef.
- (a) S. H. Jung and H. Kohn, J. Am. Chem. Soc., 1985, 107, 2931 CrossRef; (b) B. B. Snider and J. R. Duvall, Org. Lett., 2005, 7, 4519 CrossRef PubMed; (c) J. R. Duvall, F. Wu and B. B. Snider, J. Org. Chem., 2006, 71, 8579 CrossRef PubMed; (d) S. H. Kim, B. R. Park and J. N. Kim, Bull. Korean Chem. Soc., 2011, 32, 716 CrossRef.
- D. Schade, K. Topker-Lehmann, J. Kotthaus and B. Clement, J. Org. Chem., 2008, 73, 1025 CrossRef PubMed.
- (a) G.-H. Tao, Y. Guo, Y.-H. Joo, B. Twamley and J. M. Shreeve, J. Mater. Chem., 2008, 18, 5524 RSC; (b) G. Aridoss and K. K. Laali, Eur. J. Org. Chem., 2011, 2011(31), 6343 CrossRef.
- (a) M. Sajjadi, M. Nasrollahzadeh and S. M. Sajadi, J. Colloid Interface Sci., 2017, 497, 1 CrossRef PubMed; (b) M. Tajbakhsh, H. Alinezhad, M. Nasrollahzadeh and T. A. Kamali, Monatsh. Chem., 2016, 147, 2135 CrossRef; (c) D. Habibi and M. Nasrollahzadeh, Monatsh. Chem., 2012, 143, 925 CrossRef; (d) M. Nasrollahzadeh, Y. Bayat, D. Habibi and S. Moshaee, Tetrahedron Lett., 2011, 13, 4435 Search PubMed.
- A. R. Modarresi-Alam, F. Khamooshi, M. Nasrollahzadeh and H. A. Amirazizi, Tetrahedron, 2008, 63, 8723 CrossRef.
- (a) P. P. Seth, R. Ranken, D. E. Robinson, S. A. Osgood, L. M. Risen, E. L. Rodgers, M. T. Migawa, E. A. Jefferson and E. E. Swayze, Bioorg. Med. Chem. Lett., 2004, 14, 5569 CrossRef PubMed; (b) P. Sikka, J. K. Sahu, A. K. Mishra and S. Hashim, Med. Chem., 2015, 5, 479 Search PubMed.
- (a) N. Ahmed, U. Dobrindt, J. Hacker and S. E. Hasnain, Nat. Rev. Microbiol., 2008, 6(5), 387 CrossRef PubMed; (b) M. Z. Alonso, N. L. Padola, A. E. Parma and P. M. Lucchesi, Poult. Sci., 2011, 90(11), 2638 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2018 |