Open Access Article
Open Access ArticleDevelopment, characterization and application of hydroxypropylmethylcellulose films enriched with cypress seed extract
Wafa Rhimi,
Abdennacer Boulila,
Rim Gheribi and
Khaoula Khwaldia *
*
Laboratoire des Substances Naturelles, Institut National de Recherche et d'Analyse Physico-chimique (INRAP), Pôle Technologique de Sidi Thabet, 2020 Sidi Thabet, Tunisia. E-mail: khaoula_khwaldia@yahoo.fr
First published on 28th June 2018
Abstract
The phenolic profile of cypress seed extract (CSE) was investigated by means of liquid chromatography with photodiode array and electrospray ionisation mass spectrometric detection (LC/PDA/ESI-MS). The total phenolic (TP) and flavonoid (TF) contents as well as the antioxidant capacity of CSE were determined. The effects of CSE concentration (0.1, 0.3, 0.5, 1, and 2% (w/v)) on the functional properties of hydroxypropylmethylcellulose (HPMC) films were studied. Results showed that CSE presents a good antioxidant capacity due to its high phenolic/flavonoid contents and particularly the presence of bi-flavonoid compounds including cupressuflavone and amentoflavone derivatives. The incorporation of CSE in HPMC films led to a significant decrease in their water vapor permeability (WVP) and enhanced their mechanical strength. The lowest WVP value, the greatest opacity and the highest antioxidant capacity were obtained with the highest CSE concentration. HPMC films with and without CSE were applied on virgin olive oil to study their effect on the oxidative stability of olive oil during accelerated storage by periodically analyzing changes in FTIR spectra and peroxide values. HPMC-2% CSE films were the most effective in lowering light transmission, and consequently decreasing peroxide formation and delaying oxidation of olive oil.
1. Introduction
Lipid oxidation is one of the main causes of the alteration of the appearance, texture, sensory properties, and nutritional quality of fatty food products, which may negatively affect their commercial value, limit their shelf life and cause consumer rejection.1 As a result of inadequate processing or storage factors (e.g. air, light and temperature), the oxidation reactions are initiated by hydrogen removal or inclusion of oxygen radicals in polyunsaturated fatty acid chains, and lead to the generation (formation) of many oxidation products.2During the last decade, several studies demonstrated the effectiveness of antioxidants in inhibiting lipid oxidation by many mechanisms, including free radical scavenging, metal chelating, singlet oxygen quenching and photosensitizer inactivation.3 Natural antioxidants from medicinal plants have attracted much attention as safe alternatives to synthetic ones such as butylated hydroxytoluene (BHT) and t-butyl-4-hydroxyanisole (BHA) which are suspected to have hazardous and carcinogenic effects on consumer health.4 Recent studies pointed out that in addition to other biological activities, plant-derived antioxidants have favorable redox potentials and free radical scavenging activity, are able to form less reactive phenoxyl radicals, and possess a protective capacity against UV radiation.5
It is well known that the incorporation of active agents, including antioxidants, into food packaging materials may offer many advantages when compared to their direct addition to foods due to the ability of biopolymer films and coatings to maintain a predetermined level of active substance concentration mainly localized on the food surface during long storage.6 Moreover, high barrier packaging materials are required to preserve food products against oxidation and thus improve their shelf life.7 Among many (polysaccharidic) biopolymers used for preparation of edible films and coatings, hydroxypropylmethylcellulose (HPMC) has received the attention of many researchers due to its availability, edibility, good film-forming properties resulting in transparent films with suitable mechanical performance and excellent gas and grease barrier properties as well as high retention ability of active compounds.8,9 In this context active films and coatings based on HPMC incorporating natural antioxidants were proven efficient in delaying oxidation and improving food quality attributes.10,11
Cupressus sempervirens L. (cypress) the evergreen tree native to North America, Mediterranean region, and Western Asia is used in Tunisia as a windbreak and/or ornamental plant. It is considered as medicinal plant for its effects to treat wounds, skin eruption, ulcers, and inflammation.12 Rawat et al. reported its use as antispasmodic, astringent, antiseptic, deodorant, and diuretic agent, together with its capacity to promote venous circulation to the kidneys and bladder area.13 The paste of cones and young branches are used externally for tightening up the blood vessels in haemorrhoids.14 Today the demand for cypress is increasing for the quality of its essential oil used in perfumery. The isolated essential oils contain mainly monoterpenes and sesquiterpenes. Moreover, the species contains a wide range of other compounds including phenolic acids, flavonoids and bi-flavonoids.15
In this research, novel active films based on HPMC and extracts of cypress (C. sempervirens) cone seeds were prepared. To the best of our knowledge, there have been no studies that have investigated the antioxidant potential of cypress seed extract (CSE) and its use to functionalize food packaging materials. Therefore, the main aims of this work were to evaluate the effect of CSE incorporation on the functional properties of HPMC films in terms of antioxidant, mechanical, optical, and water vapor barrier properties, and to study their ability to control virgin olive oil photo-oxidation.
2. Materials and methods
2.1. Chemicals and reagents
Folin–Ciocalteu, gallic acid, quercetin, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,4,6-tripyridyl-S triazine (TPTZ), AlCl3, FeCl3, BHT, trolox and solvents of analytical grade were purchased from Sigma Aldrich (Steinheim, Germany). Hydroxypropyl methylcellulose (HPMC, Methocel E-19, Food grade, molecular weight ∼50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da, Dow Chemical Co. Midland, USA) was used to prepare HPMC-based films.
000 Da, Dow Chemical Co. Midland, USA) was used to prepare HPMC-based films.
2.2. Seed material
Cones of C. sempervirens were collected in March 2015 from wild population in the health trail of El Menzah located in the North of Tunis (latitude of 36°83′ N; longitude of 10°18′ E) at an altitude of 70 m. Plants grow wild in calcareous soil under the upper semi arid bioclimate according to Emberger's Q2 pluviometric coefficient.16 Q2 = 2000P/(M2 − m2), where P is the mean of annual rainfall (550 mm), M is the mean of maximum temperature [K°] for the warmest month, and m is the mean of minimum temperature [K°] for the coldest month. P, M, and m values were calculated as the average for a period of 50 years.The plant material was botanically characterized by Prof. Nadia Ben Brahim (Département de botanique et des plantes d'ornement, Institut National de Recherche Agronomique de Tunis) where a voucher specimen was deposited. The isolated C. sempervirens seeds were oven-dried at 40 °C for 48 h and milled in a Basic IKA Werke Mill (MF10, IKA Works Inc., Wilmington, NC, USA).
2.3. Preparation of ethanol extracts
Dried and ground seeds were defatted with hexane using a soxhlet apparatus and extracted three times by maceration with 95% ethanol. The macerated ethanol extracts were filtred through Whatman no. 541filter papers (Whatman International Paper Ltd., Maidstore, England) and concentrated under reduced pressure in a Heidolph rotary evaporator (Schwabach, Germany). The dried ethanol extracts were flushed with nitrogen and stored at 4 °C until analysis.2.4. Characterization of cypress seed extract (CSE)
All experiments relative to CSE characterization were repeated three times.The mobile phase consisted of 0.1% formic acid in methanol (solvent B) and water with 0.1% formic acid in water (solvent A). The following multi-step linear solvent gradient was employed: 0–5 min 2% B, 5–65 min 100%B, 65–68 min 100% B, 68–68.1 min 2% B, and 68–75 min 2% B. Photodiode array detector was set at 280 nm for acquiring chromatograms. The injection volume was 20 μl.
UV spectra were recorded from 190 to 800 nm and the mass spectra were recorded under the following operating conditions: capillary voltage, 4.5 kV; cone voltage, 60 V; probe temperature, 300 °C; ion source temperature, 120 °C. The spectra were acquired in the m/z range of 135–1000 amu.
Peak identification was performed by comparing the retention time and the UV and MS spectra of the phenolic constituents with those of pure standards when available. Further identification was assessed by comparison of UV and MS spectra with literature.5,19
2.4.4.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay. Free radical scavenging activity of CSE was determined using the stable DPPH radical according to the method of Brand-Williams et al. with a slight modification.20 Briefly, 1 ml of methanol solution containing different amounts of seed extract (0.01–1 mg ml−1) was added to 2 ml of daily prepared methanol DPPH solution (0.1 mM). The mixture was vortexed and incubated in the dark at ambient temperature for 1 h. The absorbance was then measured at 517 nm. BHT and trolox were used as positive controls. The percentage of free radical scavenging was calculated according to the formula:
% DPPH radical scavenging = [(A0 − A1)/A0] × 100, where A0 is the absorbance of the control and A1 is the absorbance of the sample. Sample concentration (mg extract/ml) providing 50% radical inhibition (EC50) was defined from a graph plotting free radical scavenging activity (%) against extract concentration. The antioxidant capacity of the samples was expressed as EC50.
2.4.4.2. ABTS (azinobis(ethylbenzothialzoline 6-sulphonic acid)) assay. The method of Re et al. was used to perform ABTS assay.21 Summarily, 7 mM ABTS aqueous solution was reacted with 2.4 mM potassium persulfate for 12 h in the dark to produce ABTS radical (ABTS+). The ABTS solution, previously diluted with ethanol until an absorbance of 0.7 at 734 nm was reached, was mixed with 150 μl of seed extracts (0.01–1 mg ml−1) or trolox standard and kept in the dark for 15 min before measuring absorbance at 734 nm. Trolox was used as positive control. The antioxidant capacity of samples was expressed as EC50, the concentration necessary to 50% reduction of ABTS+.
2.4.4.3. Ferric reducing antioxidant power (FRAP). FRAP assay was performed according to the method of Benzie and Strain with some modifications.22 FRAP reagent, containing 300 mM acetate buffer (pH 3.6), 40 mM hydrochloric acid, 10 mM TPTZ and 20 mMFeCl3, was incubated at 37 °C for 30 min. 150 μl of seed extracts (0.01–1 mg ml−1) was mixed with 2850 μl of FRAP reagent and kept at room temperature for 30 min in the dark before determining absorbance at 593 nm.
2.5. Preparation of HPMC-based films incorporating CSE
Preparation of HPMC-based film forming solutions was adapted from Akhtar et al.10 5 g of HPMC was dissolved in 35% ethanol solution heated at 65 °C with constant agitation, until all particles were thoroughly dispersed. CSE was dissolved separately in 35% ethanol solution at ambient temperature. HPMC and CSE solutions were then mixed to obtain final concentrations of 0, 0.1, 0.3, 0.5, 1, 2 (w/v) of CSE in the HPMC film-forming solution. The resulting solutions were homogenized at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 rpm for 4 min, using a rotator homogenizer (Ultra-Turrax Model T25 IKA; Labortechnik GmbH, Munich, Germany) and degassed at room temperature using a vacuum pump to remove the entrapped air bubbles. 16 g of each film forming solution was cast onto a Petri dish cover and dried at ambient temperature. Before testing functional properties, the dried films were conditioned in an environmental chamber at 25 °C and 50% RH for 48 h.
500 rpm for 4 min, using a rotator homogenizer (Ultra-Turrax Model T25 IKA; Labortechnik GmbH, Munich, Germany) and degassed at room temperature using a vacuum pump to remove the entrapped air bubbles. 16 g of each film forming solution was cast onto a Petri dish cover and dried at ambient temperature. Before testing functional properties, the dried films were conditioned in an environmental chamber at 25 °C and 50% RH for 48 h.
2.6. Characterization of HPMC-CSE films
The light barrier properties of HPMC films were determined by measuring the film absorbance at 600 nm according to the method of Siripatrawan and Harte.23 The films were cut into rectangular pieces and placed in a UV-vis spectrophotometer test cell and an empty test cell was used as the reference. UV-vis spectra of films were recorded at wavelengths between 200 and 800 nm. The transparency was calculated using the following equation:
Transparency = −log(T600)/X, where T600 is the transmittance at 600 nm and X is the film thickness (mm).
A gloss meter Elcometer (407 Statistical gloss meter, Manchester, UK) was used to measure the gloss of the films at an incidence angle of 60° as described by Khwaldia.9,10 replicates were performed for each sample and 3 films were taken for each film formulation.
WVP (g mm (m2 d kPa)−1) was calculated as follows:
WVP = (WVTR × X)/Δp, where WVTR is the water vapor transmission rate defined as the slope (g d−1) divided by the transfer area (m2), X is the film thickness (mm), Δp is the difference of partial water vapor pressure across the film (p(RH2 − RH1) = 5.942 kPa, where p is the saturation vapor pressure of water at 38 °C, RH2 = 90%, RH1 = 0%).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 10 min at 20 °C. The supernatant was used for the determination of the stability of antioxidant activity.26
000 × g for 10 min at 20 °C. The supernatant was used for the determination of the stability of antioxidant activity.26The assessment of the stability of the antioxidant activity of HPMC-based films was performed by determining the percentage of free radical scavenging activity and ferric reducing power during storage at 25 ± 1 °C and 50% RH for 5, 35 and 210 days.
2.7. Application of active films for controlling olive photo-oxidation
HPMC films with and without CSE were applied at the top of Petri dishes previously filled with 12 g of commercial virgin olive oil to study their effect on the oxidative stability of olive oil over a storage period of 23 days. Olive oil stored in Petri dishes without application of films was used as a control. The securely closed Petri dishes were kept under fluorescent light as described by Akhtar et al. in a controlled experimental cabinet stored in the dark at 23 °C and 50% RH.10 The oxidative stability of olive oil was monitored by periodically analyzing changes in FTIR spectra and peroxide values.2.8. Statistical analysis
Data were subjected to analysis of variance using SYSTAT (Systat Software, San Jose, CA) with a 95% significance level. Means comparisons were performed through Fisher's LSD test.3. Results and discussion
3.1. Chemical composition and antioxidant activity of CSE
The TP and TF values are expressed as mg of gallic acid equivalent (GAE) per gram of dry weight and mg of quercetin equivalent (QE) per gram of dry weight, respectively. The results showed that CSE present a high phenol (387.12 ± 7.13 mg GAE/g) and flavonoid (151.42 ± 1.29 mg QE/g) contents (Table 1). TP and TF values in CSE reached higher values than those published by Sezer Senol et al. in ethanol extract of C. sempervirens needles (43.8 mg GAE/g and 56.8 mg QE/g, respectively),28 but lesser than those obtained by Al-Qaraleh and Tarawneh in the methanol extract of Jordanian C. sempervirens cones.29 Phenolic compounds are the product of the secondary metabolism and their amount shows a wide qualitative and quantitative variability according to genetic and/or environmental factors such as temperature, altitude, amount of rainfall, and soil properties.30| Total phenols (mg GAE/g dry extract) | Total flavonoids (mg QE/g dry extract) | DPPH EC50 (μg ml−1) | ABTS EC50 (μg ml−1) | FRAP (μmol TE/g) | |
|---|---|---|---|---|---|
| a (a–c) Different superscripts within a column indicate significant differences among films (P < 0.05). | |||||
| Seeds | 387.12 ± 7.13 | 151.42 ± 1.29 | 13.91 ± 0.44a | 110.73 ± 2.66c | 121.96 ± 1.75 |
| BHT | — | — | 23.00 ± 0.19c | 41.07 ± 1.09a | — |
| Trolox | — | — | 17.45 ± 0.12b | 68.17 ± 1.75b | — |
Although LC-PDA and LC-MS were proven to be an efficient technique for the analysis of plant extracts mainly phenolic enriched ones, their use in the case of C. sempervirens was scarce. Moreover, investigations on the phenolic composition of the species are relatively few.19 In the present study ethanol extract of C. sempervirens was investigated for its chemical composition by the use of HPLC-PDA-MS technique. The data of the retention time, λmax, pseudomolecular ion, main fragment ions and tentative identification are presented in Table 2 and the typical LC-MS-TIC profile of phenolic components from C. sempervirens cones can be observed in Fig. 1. The identification of phenolics was assessed by comparing their UV and MS spectra with those from the literature.5,19
| Peak no. | Rt (min) | UVmax (nm) | [M − H]− | Other fragments | Tentative identification |
|---|---|---|---|---|---|
| 1 | 16.55 | 280 | 577 | 425, 289 | Procyanidin dimer isomer 1 |
| 2 | 25.20 | 280 | 577 | 425, 289 | Procyanidin dimer isomer 2 |
| 3 | 25.80 | 280 | 865 | 465, 289 | Procyanidin trimer |
| 4 | 33.54 | 228, 270, 330 | 537 | 375 | Cupressuflavone |
| 5 | 35.45 | 226, 268, 336 | 537 | 375 | Amentoflavone |
| 6 | 36.05 | 210 | 311 | — | |
| 7 | 37.15 | 210 | 311 | — | |
| 8 | 38.84 | 210 | 325 | — | |
| 9 | 39.66 | 210 | 325 | 293 | — |
| 10 | 40.68 | 228, 270, 330 | 551 | 537 | Methylcupressuflavone |
| 11 | 43.10 | 226, 268, 336 | 551 | 537 | Methylamentoflavone |
| 12 | 43.91 | 234 | 319 | Cupressic acid | |
| 13 | 55.70 | 234 | 301 | Communic acid | |
| 14 | 57.73 | 240 | 277 | — | |
| 15 | 60.95 | 234 | 279 | 609 | — |
| 16 | 62.50 | 234 | 368 | — |
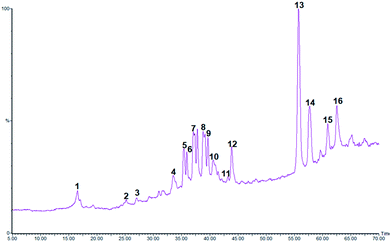 | ||
| Fig. 1 Typical LC-MS chromatogram of CSE extract. The peak assignments are listed in Table 2. | ||
In total, 16 compounds were detected among them 9 major constituents were tentatively identified. The classes of compounds were recognizable from their characteristic UV spectra, which were confirmed by comparison with literature data. In our study, 3 classes of molecules were detected; procyanidins, bi-flavonoids and diterpenes.
Three procyanidin derivatives were found at 16.5, 25.2, and 25.8 min. Analysis revealed the presence of two isomers of procyanidin dimer (compounds 1 and 23) in the ESI(−) mode with a pseudomolecular ion at m/z 577 and an UVmax at 280 nm. They were identified with the same fragmentation ions at m/z 425 (corresponding to the neutral loss of galloyl moiety m/z 152) and 289 (corresponding to catechin unit).31 Peak 3 presented the same UV spectrum as compounds 1 and 3 and a molecular ion [M − H]− at m/z 865 with two major fragments at m/z 465 and m/z 289 indicating the presence of a procyanidin trimer isomer.31
Peaks 4 and 5 eluted at 33.5 and 35.4 min, respectively exhibited a deprotonated molecule at m/z 537 and a fragment ion at m/z 375 [M − 162]− characteristics of the most representative biflavones, cupressuflavone and amentoflavone. Most flavonoids are characterized by the presence of two major absorption bands in the UV-vis region, band I in the 320–385 nm range and band II in the 250–285 nm. In the case of cupressuflavone, the wavelength maxima were detected at 225, 272, and 330 nm while amentoflavone presented two maxima at 268 and 334 nm. The data are consistent with the recorded observations of Ibrahim et al.5 Thus compounds 4 and 5 were identified as cupressuflavone and amentoflavone, respectively.
It has previously been suggested that methyl-amentoflavone, -hinokiflavone, and -robustaflavone occur in cypress leaves.19 In our investigation on cypress cones we detected two methylated bi-flavones eluted at 40.6 and 43.9 min with a deprotonated molecular ion at m/z 551 and fragment ion at m/z 537 indicative of the loss of one methyl unit.19 Based on their UV spectra peaks 10 and 11 were identified as methylcupressuflavone and methylamentoflavone.
Two diterpenoids have tentatively identified in the cypress cone extract; cupressic acid (m/z 319) and communic acid (m/z 301). However, peak 6–9 and 14–16 occurring in CSE, remain unidentified.
Phenolics are one of the most studied phytochemicals and their biological properties have been described by panoply of in vitro and in vivo assays. In recent years, many reports have highlighted them as the most important plant antioxidants. This antioxidant capacity was often correlated to TP and TF values. In this study, IC50 values obtained by DPPH and ABTS assay were 13.91 and 110.73 μg ml−1. Both values are less important than those of BHT (IC50 = 23.00 and 41.07 μg ml−1, respectively). These results are supported by the obtained TEAC value (121.96 μmol TE/g) indicating that CSE possess a good antioxidant capacity which was most likely attributed to its higher total phenolic/flavonoid contents32 and its particular richness in biflavones.33
3.2. Characterization of HPMC films incorporating CSE
The properties of biodegradable films are highly influenced by the nature and concentration of film components and film preparation method and structure.34 Physical and mechanical properties of HPMC films are summarized in Table 3. The HPMC films had a thickness value of 79.23 ± 3.41 μm and incorporation of CSE at different concentrations exerted no effect on their thickness (p < 0.05). Similar results were achieved when gelatin films were incorporated with green tea extract.26 In another study, Dashipour et al. reported a significant effect of ZEO concentration on the thickness of carboxymethyl cellulose (CMC) film.35| HPMC films | Thickness (μm) | WVP (gmm (m2 d kPa)−1) | TS (MPa) | % E (%) |
|---|---|---|---|---|
| a (a–c) Different superscripts within a column indicate significant differences among films (P < 0.05). | ||||
| Control | 79.23 ± 3.41a | 5.92 ± 0.19c | 53.02 ± 5.13a | 10.32 ± 1.44bc |
| 0.1% CSE | 79.08 ± 2.40a | 5.33 ± 0.13b | 62.34 ± 5.91b | 11.20 ± 1.75c |
| 0.3% CSE | 80.90 ± 2.26a | 5.14 ± 0.21b | 62.06 ± 5.60b | 11.12 ± 1.42c |
| 0.5% CSE | 81.00 ± 2.12a | 5.00 ± 0.26ab | 61.63 ± 4.60b | 10.66 ± 1.09bc |
| 1% CSE | 81.77 ± 1.27a | 5.08 ± 0.49b | 61.07 ± 2.95b | 9.13 ± 1.03ab |
| 2% CSE | 83.83 ± 5.52a | 4.46 ± 0.26a | 61.04 ± 5.01b | 7.67 ± 0.59a |
The incorporation of CSE in HPMC films led to a significant decrease in their WVP (Table 3). No significant difference (p > 0.05) was found between WVP of HPMC films containing 0.1 to 1% CSE. The lowest WVP value was obtained with the highest CSE concentration (p < 0.05) which reduced WVP by 25% compared with that of HPMC films without CSE. This improvement in water vapor barrier properties may be related the increase in hydrophobic compounds content in films formulated with CSE and the interaction between hydroxyl groups of HPMC and phenolic compounds of CSE, leading to a more cohesive structure and lower free volume for moisture transmission. Likewise, Ghadermazi et al. reported a significant reduction in WVP of HPMC films containing essential oils.36 Conversely, a decrease in water vapor barrier properties of CMC films was noticed after addition of ZEO.35
The TS of HPMC films was 53.02 ± 5.13 MPa. This value tended to increase after addition of CSE (p < 0.05). However, the CSE concentration did not significantly affect TS (Table 3). With the exception of films enriched with 2% CSE, no significant difference (p > 0.05) was found between % E of HPMC films with and without CSE. In agreement with these findings, Wu et al. claimed that the enhanced film strength resulted from the strong interaction of the biopolymer matrix with the natural plant extract through hydrophobic interactions and hydrogen bonding.26 In another study, the highest mechanical properties were achieved by CMC films incorporating 1% ZEO.35
Optical properties such as transparency, color and gloss are key factors controlling the choice of the adequate application of films and coatings and the final product appearance. The range of transparency values of HPMC films was 1.06–3.16 (Table 4) and incorporation of CSE at 0.5–2% concentrations significantly affected this response (p < 0.05). Transparency values of films increased with increasing CSE content and the highest transparency value was obtained with 2% CSE concentration (p < 0.05). The high values of transparency indicate lower film transparency and higher opacity. Consequently, HPMC films containing 2% CSE had the greatest opacity and therefore the best light barrier properties. In this context, Akhtar et al. demonstrated the ability of a natural red color compound to decrease light transmission of HPMC films, and consequently protect them against photo-oxidation.8
| HPMC films | Transparency values | L* | a* | b* | Gloss (GU) angle 60° |
|---|---|---|---|---|---|
| a (a–c) Different superscripts within a column indicate significant differences among films (P < 0.05). | |||||
| Control | 1.06 ± 0.20a | 93.37 ± 0.82f | −0.13 ± 0.33a | 1.45 ± 0.06 | 22.48 ± 1.74b |
| 0.1% CSE | 1.11 ± 0.19a | 90.07 ± 0.50e | 0.77 ± 0.05b | 4.51 ± 0.01 | 22.34 ± 1.19c |
| 0.3% CSE | 1.54 ± 0.20a | 84.50 ± 0.33d | 2.37 ± 0.11c | 9.02 ± 0.17 | 22.28 ± 1.23bc |
| 0.5% CSE | 1.81 ± 0.30b | 68.35 ± 1.76c | 9.59 ± 0.22d | 15.40 ± 0.18 | 20.77 ± 1.65bc |
| 1% CSE | 2.07 ± 0.28b | 61.37 ± 1.47b | 10.27 ± 0.55e | 16.67 ± 0.98 | 20.51 ± 1.7b |
| 2% CSE | 3.16 ± 0.14c | 54.36 ± 1.08a | 15.57 ± 0.54f | 16.82 ± 0.14 | 18.46 ± 1.7a |
Incorporation of CSE in HPMC films significantly affected (p < 0.05) color parameters (Table 4). Neat HPMC films had the highest L* value and the lowest a* and b* values. Increasing CSE concentration decreased L* values from 93.37 to 54.36 and led to darker films. The increase in a* and b* parameters as CSE content increased indicated the tendency of films to redness and yellowness. Similar results were obtained when gelatin films were incorporated with curcuma ethanol extract.37
The gloss of neat HPMC films was 22.48 ± 1.74 GU. With the exception of films incorporating CSE at 1 to 2%, gloss was not significantly affected by CSE addition (p > 0.05). Increasing CSE concentration from 1 to 2% reduced the gloss of HPMC films (p < 0.05). In agreement with these findings, Bitencourt et al. attributed the decrease in gloss values of gelatin films to the increase in film roughness after incorporation of curcuma extract.37
The stability of the antioxidant activity of HPMC-based films was assessed by DPPH and FRAP assays after 5, 35, and 210 days of storage. The results showed that CSE-HPMC films exhibited significant free radical scavenging activity and ferric reducing antioxidant power in a concentration-dependent manner (Fig. 2). In both assays and for all tested CSE concentrations the antioxidant activity slightly decreased indicating that the antioxidant property of CSE-based films is maintained over storage.
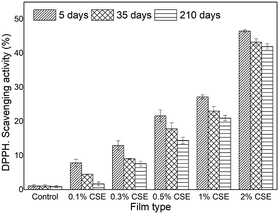 | ||
| Fig. 2 DPPH radical scavenging activity of HPMC films incorporated with CSE and stored at 25 ± 1 °C for 5, 35 and 210 days. | ||
3.3. Effect of active films on the oxidative stability of olive oil
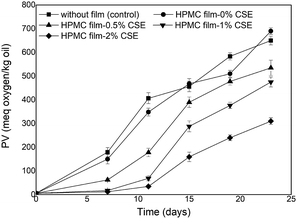
The peroxide value (PV) of virgin olive oil before accelerated storage was 5.83 meq oxygen/kg and significantly increased during storage (Fig. 3) due to the formation of hydroperoxides suggesting the high susceptibility of olive oil to oxidation under fluorescent light. Olive oil stored in Petri dishes without HPMC films or with CSE-free films exhibited the highest PVs throughout storage and therefore the highest oxidation rates. However, the PVs of olive oil samples covered with HPMC films containing 1–2% CSE remained lower than the legal upper limit (20 meq oxygen/kg) after 7 days of fluorescent light storage. Increasing CSE concentration decreased peroxide formation and delayed oxidation of olive oil. Indeed, olive oil samples covered with HPMC films containing 0, 0.5 or 1% CSE showed PVs that were respectively ten times, five times and two times higher than those covered with films incorporating 2% CSE after 11 days of storage. HPMC-2% CSE films exhibited the highest antioxidant activity which was most likely attributed to their higher total phenolic/flavonoid contents and were the most effective in lowering light transmission as previously shown in transparency data, thus decreasing oil peroxidation and oxidation.FTIR spectroscopy has been reported in literature as an effective tool for evaluating quality and composition of edible oils as well as determining their oxidation degree.38 In this study, FTIR spectra of olive oil samples before accelerated storage and those photo-aged with or without HPMC films were collected. However, only changes relative to the control olive oil before and at the end of accelerated storage and to olive oil samples covered with HPMC films incorporating 1% or 2% CSE were reported in Fig. 4.
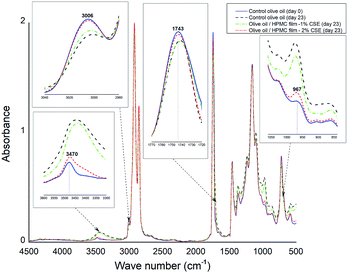 | ||
| Fig. 4 Changes in FTIR spectra of control olive oil samples before and at the end of accelerated storage and those photo-aged at day 23 with HPMC films incorporating 1% or 2% CSE. | ||
For control fresh olive oil, the band at 3006 cm−1 associated with the CH stretching band of the cis double bond decreased during accelerated storage which may be explained by a decrease in the unsaturation degree39 resulting from a reduction in the amount of cis double-bonds over a storage period of 23 days under fluorescent light. However, the band intensity at 3006 cm−1 for photo-aged olive oil samples stored with HPMC-2% CSE films is fairly the same than that for control olive oil at day 0. In the region (3600–3300 cm−1), the band at 3470 cm−1 assigned to O–H hydroperoxides increases in width and in absorbance and is shifted to lower frequencies due to the formation of hydroperoxide products during accelerated storage. In agreement with PV results, the changes observed in this band (Fig. 4) indicate that olive oil stored with HPMC-2% CSE films had the lowest hydroperoxide amount and therefore the lowest oxidation rate. The band at 1743 cm−1 corresponding to carbonyl compounds decreases in intensity and is shifted to lower wavenumbers which may be attributed to the formation of secondary products of oxidation during photoaging of olive oil.40 However, this band remained unchanged for olive oil stored under fluorescent light with HPMC-2% CSE films. The absorption band located at 967 cm−1 in the control fresh olive oil spectrum associated with the CH bending of the trans double bond increases in absorbance and is shifted to higher frequencies at the end of accelerated storage, suggesting the formation of oxidation products (aldehydes, ketones, etc.).38 From Fig. 4, it is possible to notice that the changes in FTIR spectra of olive oil samples photo-aged with HPMC films incorporating the highest CSE concentration (2%) are less pronounced than the other samples, suggesting a protective effect of these active films against oxidation due to their higher phenolic/flavonoid contents, especially their rich biflavone content, as well as their greatest opacity and their best light barrier properties.
4. Conclusions
The effects of CSE concentration on the physical, mechanical and antioxidant properties of HPMC films as well as on the oxidative stability of virgin olive oil during accelerated storage were investigated. HPMC films incorporated with 2% CSE exhibited the highest water vapor barrier properties and antioxidant capacity and the greatest opacity. FTIR spectrum of olive oil samples photo-aged with HPMC films incorporating the highest CSE concentration (2%) was closest to that of fresh control oil at day 0 and showed the lowest oxidation degree and therefore the highest stability during accelerated storage. Indeed, HPMC-2% CSE films, containing the highest active phenolic/flavonoid contents and exhibiting the best light barrier properties were the most effective in lowering light transmission, and consequently decreasing olive oil oxidation. These active films enriched with natural antioxidants can be considered as an effective alternative to synthetic ones and may be successfully used to reduce lipid oxidation of oils, fats as well as foods containing unsaturated fat such as salmon and nuts.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was sponsored by VIPAC Food project which is funded through the ARIMNet2 [2016] Call by the Ministry of Higher Education & Scientific Research (MHESR, Tunisia). ARIMNet2 has received funding from the European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement no. 618127.Notes and references
- J. Gomez-Estaca, C. Lopez-de-Dicastillo, P. Hernandez-Munoz, R. Catala and R. Gavara, Trends Food Sci. Technol., 2014, 35, 42–51 CrossRef.
- L. M. Franklin, D. M. Chapman, E. S. King, M. Mau, G. Huang and A. E. Mitchell, J. Agric. Food Chem., 2017, 65, 2549–2563 CrossRef PubMed.
- E. Choe and D. B. Min, Compr. Rev. Food Sci. Food Saf., 2009, 8, 345–358 CrossRef.
- N. Ito, M. Hirose, A. Hagiwara and S. Takahashi, Carcinogenicity and modification of carcinogenic response by antioxidants, in. Antimutagenesis and Anticarcinogenesis Mechanisms II, ed. Y. Kuroda, D. M. Shankel and M. D. Waters, Claire Wilson & Associates, Springer, Boston, MA, 1990, pp. 183–194 Search PubMed.
- E. A. Ibrahim, S. Y. Desoukey, G. M. Hadad, R. A. A. Salam, A. K. Ibrahim, S. A. Ahmed, M. M. Radwan, A. S. Wanas and M. A. ElSohly, Pharm. Pharmacol. Int. J., 2017, 5, 00134 Search PubMed.
- H. Aloui and K. Khwaldia, Compr. Rev. Food Sci. Food Saf., 2016, 15, 1080–1103 CrossRef.
- F. Vilarinho, M. Andrade, G. G. Buonocore, M. Stanzione, M. F. Vaz and A. Sanches Silva, Eur. Polym. J., 2018, 98, 362–367 CrossRef.
- M. J. Akhtar, M. Jacquot, J. Jasniewski, C. Jacquot, M. Imran, M. Jamshidian, C. Paris and S. Desobry, Carbohydr. Polym., 2012, 89, 1150–1158 CrossRef PubMed.
- K. Khwaldia, Bioresources, 2013, 8, 3438–3452 CrossRef.
- M. J. Akhtar, M. Jacquot, E. Arab-Tehrany, C. Gaïani, M. Linder and S. Desobry, Food Chem., 2010, 120, 395–401 CrossRef.
- S. Ganiari, E. Choulitoudi and V. Oreopoulou, Trends Food Sci. Technol., 2017, 68, 70–82 CrossRef.
- I. A. Nehdi, Ind. Crops Prod., 2013, 41, 381–385 CrossRef.
- P. Rawat, M. F. Khan, M. Kumar, A. K. Tamarkar, A. K. Srivastava, K. R. Arya and R. Maurya, Fitoterapia, 2010, 81, 162–166 CrossRef PubMed.
- M. Alzweiri, S. Ali-Al, M. Kamal, M. Hudaib and A. Talal, J. Ethnopharmacol., 2011, 137, 127–135 CrossRef PubMed.
- M. F. Khan, T. Ahamad and P. Rawat, Insights Biomed., 2017, vol. 2, pp. 1–5 Search PubMed.
- L. Emberger, Rec. Trav. Lab. Bot. Fac. Sci. Montpellier., 1955, vol. 7, pp. 3–43 Search PubMed.
- M. Mahboubi, N. Kazempour and A. R. Boland Nazar, Jundishapur J. Nat. Pharm. Prod., 2013, 8, 15–19 CrossRef PubMed.
- A. Luximon-Ramma, T. Bahorun, M. A. Soobrattee and O. I. Aruoma, J. Agric. Food Chem., 2002, 50, 5042–5047 CrossRef PubMed.
- A. Romani, C. Galardi, P. Pinelli, N. Mulinacci and D. Heimler, Chromatographia, 2002, 56, 469–474 CrossRef.
- W. Brand-Williams, M. E. Cuvelier and C. Berset, LWT--Food Sci. Technol., 1995, 28, 25–30 CrossRef.
- R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang and C. Rice-Evans, Free Radicals Biol. Med., 1999, 26, 1231–1237 CrossRef PubMed.
- I. Benzie and J. Strain, Anal. Biochem., 1996, 239, 70–76 CrossRef PubMed.
- U. Siripatrawan and B. R. Harte, Food Hydrocolloids, 2010, 24, 770–775 CrossRef.
- ASTM E96/E96M, Standard test methods for water vapor transmission of materials, ASTM International, West Conshohocken, PA, 2016, DOI:10.1520/E0096_E0096M-16.
- ASTM D882, Standard test methods for tensile properties of thin plastic sheeting D882, ASTM International, West Conshohocken, PA, 2012, DOI:10.1520/D0882-12.
- J. Wu, S. Chen, S. Ge, J. Miao, J. Li and Q. Zhang, Food Hydrocolloids, 2013, 32, 42–51 CrossRef.
- ISO 3960, Animal and vegetable fats and oils. Determination of peroxide value; iodometric (visual) endpoint determination, International Organization for Standardization, Geneva, Switzerland, 2007 Search PubMed.
- F. Sezer Senol, I. E. Orhan and O. Ustun, Asian Pac. J. Trop. Med., 2015, 8, 269–275 CrossRef.
- S. Al-Qaraleh and K. A. Tarawneh, Am.-Eurasian J. Agric. Environ. Sci., 2016, 16, 479–486 Search PubMed.
- A. Boulila, A. Sanaa, I. Ben Salem, N. Rokbeni, Y. M'rabet, K. Hosni and X. Fernandez, Ind. Crops Prod., 2015, 76, 616–622 CrossRef.
- H. Du, J. Wu, H. Li, P. X. Zhong, Y. J. Xu, C. H. Li, K. X. Ji and L. S. Wang, Food Chem., 2013, 141, 4260–4268 CrossRef PubMed.
- N. Rokbeni, Y. M'rabet, S. Cluzet, T. Richard, S. Krisa, M. Boussaid and A. Boulila, Indus. Crops Prod, 2016, 94, pp. 505–513 Search PubMed.
- V. S. Gontijo, M. H. Dos Santos Jr and C. Viegas, Mini-Rev. Med. Chem., 2017, 17, 834–862 CrossRef PubMed.
- K. Khwaldia, S. Banon, S. Desobry and J. Hardy, Int. J. Food Sci. Technol., 2004, 39, 403–411 CrossRef.
- A. Dashipour, V. Razavilar, H. Hosseini, S. Shojaee-Aliabadi, J. B. German, K. Ghanati, M. Khakpour and R. Khaksar, Int. J. Biol. Macromol., 2015, 72, 606–613 CrossRef PubMed.
- R. Ghadermazi, J. Keramat and S. A. H. Goli, J. Food Nutr. Res., 2016, 55, 22–32 Search PubMed.
- C. M. Bitencourt, C. S. Favaro-Trindade, P. J. A. Sobral and R. A. Carvalho, Food Hydrocolloids, 2014, 40, 145–152 CrossRef.
- M. D. Guillén and N. Cabo, Food Chem., 2002, 77, 503–510 CrossRef.
- F. S. Lu Henna and P. P. A. Tan, Int. Food Res. J., 2009, 16, 343–354 Search PubMed.
- P. S. Anbinder, P. J. Peruzzo, M. N. Martino and J. I. Amalvy, J. Food Eng., 2015, 151, 43–50 CrossRef.
| This journal is © The Royal Society of Chemistry 2018 |