Open Access Article
Open Access ArticleElucidating the mechanism behind the laccase-mediated modification of poly(ethersulfone)†
Sjoerd Slagman a,
Wendy A. Jonkers
a,
Wendy A. Jonkers a,
Han Zuilhof
a,
Han Zuilhof ab and
Maurice C. R. Franssen
ab and
Maurice C. R. Franssen *a
*a
aLaboratory of Organic Chemistry, Wageningen University, Stippeneng 4, 6708 WE Wageningen, The Netherlands. E-mail: maurice.franssen@wur.nl
bSchool of Pharmaceutical Sciences and Technology, Tianjin University, 92 Weijin Road, Tianjin, People's Republic of China
First published on 30th July 2018
Abstract
Laccase-mediated oligomerisation of 4-hydroxybenzoic acid (4-HBA) derivatives and simultaneous in situ surface modification has proven to be a cost-effective, easily applicable and eco-friendly strategy for preventing biofouling of poly(ethersulfone) (PES) water filtration membranes. Modification of the membrane surface has previously been hypothesised to occur through covalent bonding of enzymatically generated phenolic radicals to the polymeric membrane. The current study shows, however, that in situ formation of soluble phenolic oligomers does not result in covalent membrane modification. We studied in situ laccase-mediated oligomerisation of custom-synthesised positively charged and commercially available negatively charged monomeric phenols, and demonstrated that their mode of binding to PES is not covalent. In addition, soluble, non-soluble and on-resin PES model compounds were synthesised and used in the laccase-mediated oligomerisation of 4-HBA. Covalent bond formation between these model compounds and (oligomeric) 4-HBA could not be observed either. Furthermore, extensive washing of PES membranes modified through laccase-mediated oligomerisation of 4-HBA resulted in substantial discolouration of the membrane surface, showing that the layer of oligomerised phenolics could easily be removed. Altogether, it was concluded that laccase-assisted modification of PES membranes resulted from strong physical adsorption of phenolic oligomers and polymers rather than from covalent bonding of those.
Introduction
Fouling of synthetic membranes is a major concern in the water purification industry.1–3 Fouling results in membrane deterioration and decline of flux (water throughput), factors which are eventually leading to less efficient water purification and higher operating costs. The main cause underlying these issues is biological fouling (biofouling), which is the adherence of biological material, typically initiated by the process of non-specific adsorption of proteins and other biopolymers, followed by biofilm formation.4 Biofouling can, however, be minimised in various ways: by plasma-assisted coating,5 chemical grafting6 or mere blending for example.7 However, all these methods are either laborious or require harsh, toxic and costly chemicals.8As enzymes can be employed at ambient temperature to perform reactions in aqueous media, the use of enzymes to perform complex chemical modifications is continually gaining interest. Apart from their use in the synthesis of small-molecules9 and polymers,10,11 they have also been employed in material surface modification. Within our group we have developed a laccase-mediated modification strategy to alter the surface of commercially available poly(ethersulfone) membranes (PES membranes).12 The enzyme laccase generates reactive phenolic radicals from 4-hydroxybenzoic acid (4-HBA) derivatives under ambient conditions; these radicals then undergo dimerisation and subsequent oligomerisation, while concomitantly covering the membrane surface. The resulting overlayer prevents the adsorption of polysaccharides, polyphenols, proteins and microorganisms, while maintaining a high membrane porosity.13,14 Since laccase uses oxygen as oxidant and produces water as the only by-product, this is also an environmentally benign process.15
It has been shown that laccase has a low reactivity towards 4-HBA (26% conversion over 24 h) due to the presence of an electron-withdrawing carboxylic acid substituent on the phenol ring.16 After slow initial dimerisation of 4-HBA, the formed dimers (C3–C3′-, C3–O- and C1–C3′-bound) are rapidly converted to oligomers. The presence of additional electron-donating substituents thus increases phenol reactivity. Additionally, the abundance of these dimers is mainly governed by the rate of subsequent oligomerisation. While these observations only concern solution-phase chemistry, initial NMR studies on dissolved membranes modified with oligomeric 4-HBA revealed the occurrence of an additional set of peaks that differ slightly from the peaks corresponding to non-bound 4-HBA.12 These new peaks were attributed to 4-HBA that is covalently bound (grafted) to the PES surface. Additional molecular modelling studies further suggested the likelihood of O-bound and C-bound phenolic species being covalently bound to the surface. Altogether, it was deemed highly likely that laccase-mediated functionalisation of PES is the result of covalent binding.
Apart from their use in the water filtration industry, synthetic polymeric membranes (like PES) are often used as support for layer-by-layer (LbL) deposition of polyelectrolytes. These LbL membranes have smaller pore sizes,17–19 lower molecular weight cut-off values,18,19 increased chemical resistance,17,20,21 are selective towards certain ions21–23 and/or have specific separatory characteristics,24,25 which makes them useful in a wide range of (nanofiltration) applications. These support membranes are either non-charged (like PES) or charged (like sulfonated PES). Non-charged supports are often inexpensive, commercially available membranes, but the deposited polyelectrolyte does not bind to it strongly. This can be overcome by choosing a charged support membrane.26 These are, however, often less readily available, costly specialty products. A low-cost post-production alternative towards charged support membranes would thus allow for the development of more durable LbL technologies. Additionally, installing permanently positively charged quaternary ammonium moieties is likely to induce anti-bacterial properties to the membrane.27
We envisioned that laccase's ability to generate highly reactive radicals under mild conditions could also be used to functionalise PES membranes with charged phenolics, analogous to the modification of PES with oligomeric 4-HBA. Here we report on the synthesis of a series of positively charged phenolic monomers and their oligomerisation in the presence of PES membranes or non-porous sheets. The modification process was followed by X-ray photoelectron spectroscopy (XPS) and static water contact angle (SWCA) measurements. The results of these experiments prompted us to additionally study the laccase-mediated conversion of phenolics in the presence of (resin-bound) PES model compounds. All the results taken together shed new light on the nature of the binding modes resulting from laccase-mediated modification of PES, which appeared to be different from what was proposed in earlier research.
Results and discussion
Synthesis of positively charged phenolic monomers
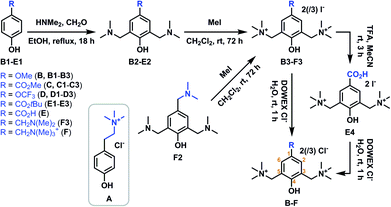
Laccase-assisted modification of PES membranes with (oligomers of) charged phenolics would grant easy access to antibacterial LbL supports. Multiple permanently positively charged phenolics were designed and synthesised for this purpose (Scheme 1). These phenols were expected to possess diverse reactivity patterns based on the presence or absence of substituents on the C3 and C5 positions of the phenol ring.The simplest positively charged phenol (N,N,N-trimethyltyramine chloride (A)) was based on tyramine. Laccase-mediated oligomerisation of A, and its binding to PES, is expected to result in an inhomogeneous mixture of products (Scheme 2), as A is not substituted at its C3 and C5 positions. Tyramine itself is only a poor laccase substrate,28 it is therefore interesting to see whether quaternising its nitrogen atom will increase reactivity. Initial attempts towards A, based on the direct methylation of tyramine, resulted in an inseparable mixture (results not shown).29 Methylation of commercially available N,N-dimethylamine followed by ion exchange did, however, afford compound A in excellent yield.
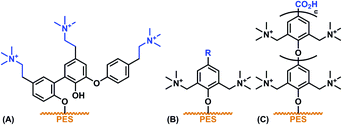 | ||
| Scheme 2 Anticipated modes of laccase-mediated surface functionalisation, (A) with phenolic monomer A, (B) with phenolic monomers B–D or F and (C) with phenolic monomer E. | ||
Laccase-mediated conversion of phenolics B, C and D is expected to exclusively result in phenolic monomers binding to the surface through C–O bond formation with the oxygen-centred radical (Scheme 2). This approach would thereby thus not introduce multilayered oligomers on the surface (as was the case for 4-HBA). B and D are furthermore likely to be more reactive due to the presence of additional electron-donating substituents. For the synthesis of these doubly charged monomers, para-substituted phenols (B1–D1) were first converted to their respective Mannich bases.30,31 Subsequent methylation of both tertiary nitrogen atoms followed by ion exchange afforded B, C and D. OCF3-substituted compound D is of special interest as the presence of multiple fluorine atoms would allow for easy detection of surface-bound D through X-ray photoelectron spectroscopy (XPS).
Syringic acid was shown to be effectively polymerised (MW up to 18 kDa) by laccase through C1–O bond formation.32 It was anticipated that laccase-mediated conversion of E would, analogously, result in polymer formation, and that E could thus, concomitantly, be used to decorate PES membranes with a multilayer of these highly ordered polymers (Scheme 2). Initial attempts to obtain E, in a manner analogous to the synthesis of B–D, through Mannich base formation of 4-HBA resulted in a mixture of meta- and ortho-substituted phenol derivatives (results not shown). None of the components of this mixture proved to be compatible with the methylation conditions employed thereafter. Mannich base formation from methyl 4-hydroxybenzoate followed by methylation and deprotection using hydrochloric acid resulted in decarboxylation (results not shown). Finally, E could be obtained by starting from tert-butyl 4-hydroxybenzoate (E1) and deprotection of the tert-butyl ester with trifluoroacetic acid.
The use of triply charged derivative F is expected to locally introduce a very high charge density on the PES membrane. F was obtained through methylation of commercially available 2,4,6-tris(dimethylaminomethyl)phenol (F2), followed by ion exchange. Ion exchange was performed for all phenolics A–F in order to replace the easily oxidisable iodide ion, as it was expected that its laccase-mediated oxidation would interfere with oxidation of A–F.33 Intermediate F3 could be purified substantially through precipitation from methanol, the success of which was demonstrated using XPS-analysis (ESI Fig. S15†). XPS N 1s narrow scans of the purified compound showed that conversion towards the triply charged species was nearly quantitative, as almost no neutral (tertiary) nitrogen (400.4 eV) could be observed.
In summary, six phenols (A–F) with distinct charge densities were synthesised. Their structural variety should allow for rigorous testing of the mechanism of the laccase-mediated oligomerisation: A is likely resulting in an inhomogeneous mixture of products due to the free C3 and C5 positions, where B, C, D and F can only bind to the surface through C–O bond formation, while the use of E is expected to result in polymers in which the monomeric units will consistently be C1–O-bound (Scheme 2).
Laccase-mediated conversion of charged monomers in the presence of PES membranes
Initial attempts at functionalising PES membranes using laccase and positively charged N,N,N-trimethyltyramine chloride (A) resulted in a coloured (faintly yellow) reaction medium. Colouration could indicate extension of a π-system, which thus hinted at oligomerisation through C–C bond formation. NMR analysis confirmed successful conversion of the starting material to a plurality of products (Fig. 1).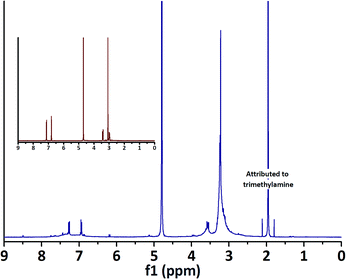 | ||
| Fig. 1 1H NMR spectrum of the reaction medium containing laccase (1 U ml−1) and A after 24 h, showing conversion of A and the appearance of a new singlet at 1.95 ppm. Inset: 1H NMR spectrum of A. | ||
Oligomerisation of A did, however, not result in membrane colouration as was observed for the laccase-mediated oligomerisation of 4-HBA.12 Furthermore, XPS analysis of the membrane surface did not reveal the presence of a peak corresponding to quaternary (positively charged) nitrogen at ∼502 eV (Table 1, entry 1, Fig. 2). In an attempt to enhance conversion and subsequent coupling of A to the surface, laccase concentrations were quadrupled. As expected, this resulted in stronger colouration of the reaction medium. Formation of a precipitate further indicated conversion towards (less soluble) higher-order oligomers. Evolution of a strong amine-like odour, combined with the observation of an intense singlet at 1.95 ppm in NMR, however, indicated the formation of trimethylamine (Fig. 1). It was hypothesised that formation of trimethylamine resulted from deamination of (oligomers of) A with concomitant formation of 4-vinylphenol (derivatives). Although oligomerisation did not result in membrane colouration, XPS analysis revealed the presence of a peak at 401.6 eV corresponding to quaternary nitrogen (N+, Table 1, entry 2, Fig. 2). Thus, even though deamination seems to have occurred, successful surface functionalisation had been achieved.
| # | Phenol | [C]a | [lac]b | Tc | SWCAd | N+e | Nf |
|---|---|---|---|---|---|---|---|
| a Concentration of respective phenol in the reaction mixture (mM).b Concentration of laccase in the reaction mixture (U ml−1).c Reaction temperature.d Static water contact angle of a 3 μl water droplet on the (modified) surface.e Presence of positively charged quaternary nitrogen based on the XPS N 1s narrow scan.f Presence of neutral nitrogen based on the XPS N 1s narrow scan.g n.d. = not determined.h Surfaces were wiped after modification.i Blank PES membrane.j Blank PES sheet. | |||||||
| 1 | A | 28.8 | 1 | rt | n.d.g | No | Major |
| 2 | A | 28.8 | 4 | rt | n.d. | Minor | Major |
| 3 | B | 28.8 | 4 | rt | 51° | Minor | Major |
| 4 | B | 57.6 | 4 | rt | 60° | n.d. | n.d. |
| 5 | B | 28.8 | 4 | 40 °C | 57° | n.d. | n.d. |
| 6 | B | 57.6 | 4 | 40 °C | 60° | Major | Major |
| 7 | Bh | 57.6 | 4 | 40 °C | 72° | No | Minor |
| 8 | C | 28.8 | 4 | rt | 59° | No | Major |
| 9 | C | 57.6 | 4 | rt | 57° | No | Major |
| 10 | C | 28.8 | 4 | 40 °C | 61° | n.d. | n.d. |
| 11 | C | 57.6 | 4 | 40 °C | 56° | n.d. | n.d. |
| 12 | D | 28.8 | 4 | rt | 58° | No | Major |
| 13 | D | 28.8 | 0 | rt | 77° | No | No |
| 14 | F | 28.8 | 4 | rt | 55° | Minor | Major |
| 15 | Fh | 28.8 | 4 | rt | 78° | n.d. | n.d. |
| 16 | None | 0 | 4 | rt | 56° | No | Major |
| 17 | None | 0 | 4 | 40 °C | 58° | n.d. | n.d. |
| 18i | None | 0 | 0 | — | n.d. | No | Major |
| 19j | None | 0 | 0 | — | 74° | No | No |
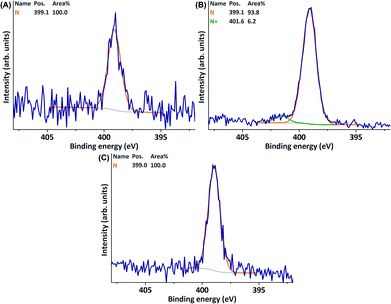 | ||
| Fig. 2 XPS N 1s narrow scans of PES membranes modified through oligomerisation of A at (A) a laccase concentration of 1 U ml−1 and (B) a laccase concentration of 4 U ml−1. (C) A blank PES membrane. | ||
The use of (PES) membranes adds additional challenges to the analysis of their surface properties. Firstly, since membranes are porous they do not allow for static water contact angle (SWCA) measurements. This assessment of surface wettability would be highly valuable as surface modification with charged phenolics was anticipated to result in a significantly lower SWCA. Secondly, PES membranes contain 10–20% poly(vinylpyrrolidone) as pore former.34 The presence of this nitrogen-containing polymer (Table 1, entry 18, Fig. 2) hampered further analysis, because functionalisation with the charged phenolics would thus not introduce a unique element. In order to ease analysis, the experiments described below have been conducted on PES sheets rather than on membranes.
Conversion of doubly charged phenols B and C at high laccase concentrations (4 U ml−1) resulted in a deeply red and deeply yellow reaction medium, respectively, without concomitant formation of odours. Where the use of B did lead to the presence of a peak corresponding to quaternary nitrogen (401.6 eV) in the XPS N 1s narrow scan (Table 1, entry 3, ESI Fig. S2†), the use of C did not (Table 1, entry 8, ESI Fig. S6†). This difference is likely to be caused by the fact that B is more reactive, as it bears a stronger electron-donating (methoxy) substituent than C (methyl ester) does. Further increasing the concentration of B (from 28.8 mM to 57.6 mM) and increasing the reaction temperature to 40 °C resulted in a more intense N+ signal (Table 1, entry 6, Fig. 3). Increasing the reaction temperature to 40 °C did not lead to the presence of quaternary nitrogen on the surface when using C (Table 1, entry 9, ESI Fig. S7†). For the use of both B and C, the presence of a strong (neutral) nitrogen signal (∼399 eV) was observed (Table 1, entry 3, 6, 8 and 9, Fig. 3). As the used PES sheets did not contain any form of nitrogen (Table 1, entry 19, Fig. 3), this must have resulted from laccase-assisted surface modification. It was hypothesised that the observed nitrogen originates from unintentional enzyme immobilisation, in other words: protein fouling.
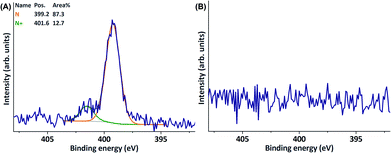 | ||
| Fig. 3 XPS N 1s narrow scans of (A) a PES sheet modified through the laccase-mediated oligomerisation of B (57.6 mM) at 40 °C and (B) a blank PES sheet. | ||
As with the use of C, the use of OCF3-substituted D did not result in successful surface functionalisation; neither fluorine (688 eV), nor quaternary nitrogen were present on the surface after modification (Table 1, entry 12, ESI Fig. S8 and S9†). Similar to all previous experiments, the use of D resulted in the presence of surface-bound neutral nitrogen-containing species. As a control experiment, D was also used without providing laccase to the reaction mixture (Table 1, entry 13, Fig. 4), which, obviously, did not lead to surface functionalisation with the phenolic monomer. Additionally, no nitrogen was observed on the PES sheets used in these control experiments. Furthermore, experiments in which PES sheets were merely agitated in buffer in which laccase was dissolved did result in the observation of surface-bound nitrogen (399.4 eV, Table 1, entry 16, Fig. 4).
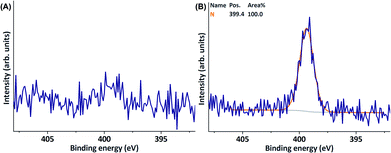 | ||
| Fig. 4 XPS N 1s narrow scans of (A) a PES sheet after incubation with D (without addition of laccase) and (B) a PES sheet after incubation with laccase only. | ||
As the commercial laccase enzyme is highly impure, it was purified in order to unambiguously determine the cause of these nitrogen-containing contaminations. Purification (ESI Fig. S34†) of the brown laccase powder resulted in a clear blue solution due to the presence of copper(II) ions in the enzyme active site. However, also experiments in which the laccase-mediated conversion of A was performed using purified laccase led to the presence of additional nitrogen-containing species on the membrane (ESI Fig. S12 and S13†). It can thus be concluded that laccase-mediated conversion of phenolics also leads to immobilisation of the enzyme itself.
Laccase-mediated surface functionalisation using triply charged F is expected to introduce a high charge density per immobilised molecule. F is hypothesised to be less reactive than B due to the poor electron-donating capacity of the CH2N(CH3)3+ substituent. Nonetheless, a (minor) N+ peak was observed after laccase-mediated modification of PES sheets (Table 1, entry 14, ESI Fig. S10†).
To summarise, successful laccase-mediated surface functionalisation was achieved for A, B and F. For the use of all three, however, the peak corresponding to N+ (∼402 eV) was observed to be relatively small. As was shown for pure F, the presence of quaternary nitrogen atoms on the surface would have resulted in an intense peak in XPS (ESI Fig. S15†). It was therefore concluded that surface coverage after laccase-mediated oligomerisation was minimal in all cases.
The use of PES sheets rather than membranes allowed for SWCA measurements to be conducted. Due to the strongly hydrophilic nature of charged molecules, the SWCA was anticipated to drop significantly after surface functionalisation. Whichever charged phenol was employed, the SWCA dropped from 74° (Table 1, entry 19) to approximately 50–60° (Table 1, entry 3–6, 8–12, and 14). This was, however, also observed in cases where no N+ was observed after surface modification, and also in a control experiment without phenolics in the reaction medium (Table 1, entry 8, 9, 12, 16 and 17). Experiments in which laccase was not added to the reaction mixture (Table 1, entry 13) did not result in a drop in SWCA. It was therefore concluded that unintentional adsorption of the laccase enzyme caused the observed change in wettability. To remove the adsorbed enzyme, the surface of the modified PES sheets was gently wiped with a soft cotton swab. This indeed resulted in a significant lowering of the amount of nitrogen on the surface (Table 1, entry 7, ESI Fig. S4 and S5†). Correspondingly, an increase in SWCA was observed, back to the level of unmodified PES. However, as shown through N 1s XPS spectra, wiping also resulted in the complete removal of any species containing quaternary nitrogen.
Gentle wiping was not expected to result in the removal of covalently bound small organics such as the phenolics used in this study. The removal of nitrogen-containing species would thereby thus indicate that surface modification is not covalent in these cases, which is in contrast with previous research that indicated that laccase-mediated surface modification of PES using 4-hydroxybenzoic acid derivatives is the result of covalent bonding.12 As the results stated above suggest that surface functionalisation using charged phenolics occurs through an alternative mechanism, it was decided to conduct more research towards the nature of the linkage between PES and 4-HBA (derivatives). By doing so we hope to clarify why 4-HBA adheres to the surface covalently and charged phenolics do not.
Laccase-mediated oligomerisation of 4-hydroxybenzoic acid derivatives in the presence of PES model compounds
Laccase-mediated functionalisation of PES membranes using 4-hydroxybenzoic acid derivatives has proven to be a mild, eco-friendly, easily applicable and cost-effective way of inducing antifouling properties to these membranes.13 It is assumed that this is the result of covalent surface functionalisation. It is, however, difficult to directly observe the bond between the membrane and phenolic compounds, as (I) this bond is very similar to bonds between two 4-HBA units, and (II) the bulk will still consist mainly of unmodified PES.Probing the modes of binding between PES and (oligomeric) 4-HBA required us to first study comprehensible molecules (model compounds) that have been subjected to the same conditions. Subsequent experiments in which laccase-mediated surface modification has been performed using negatively charged phenols act as positive controls. Additionally, washing membranes that have undergone laccase-mediated modification using 4-HBA should subsequently give insight into the degree of grafting.
Studying how laccase-mediated oligomerisation alters lignin model compounds has proven to be an indispensable tool for studying grafting on lignin.35,36 Lignin model compounds are (soluble) lignin-like monomers or oligomers that bear some of the functional groups present in lignin. These model compounds are small enough to be separated and/or studied by LC-MS and NMR spectroscopy. Analogously, we aimed to study laccase-mediated functionalisation of (soluble) PES model compounds to mimic laccase-mediated grafting on PES.
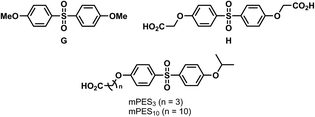
For this purpose, two PES model compounds were synthesised; water-insoluble 4,4′-sulfonylbis(methoxybenzene) (G, Scheme 3) and water-soluble 2,2′-((sulfonylbis(4,1-phenylene))bis(oxy))-diacetic acid (H). G was afforded in one step through methylation of 4,4′-sulfonyldiphenol. Etherification of both alcohol moieties of 4,4′-sulfonyldiphenol using ethyl chloroacetate followed by basic hydrolysis of the methyl esters afforded H in good yield.
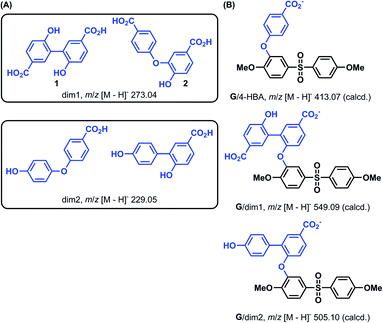
4-HBA, laccase and either of the PES model compounds G or H were agitated at pH 5, followed by LC-MS analysis. Successful grafting on these PES model compounds would be indicated by the formation of “PES model compound/(oligomeric) 4-HBA conjugates”. Laccase-mediated oligomerisation of 4-HBA has been shown to firstly result in C–C- or C–O-bound dimers with m/z 273.04 (dim1, Scheme 4) as most abundant products and also in dimers with m/z 229.05 (dim2).16 Our initial targeted search therefore focussed on conjugates of either of the PES model compounds with 4-HBA or its dimers. As an example: a conjugate between model compound G and a dimer with m/z 273.04 will be denoted as G/dim1 (calculated m/z 549.09).
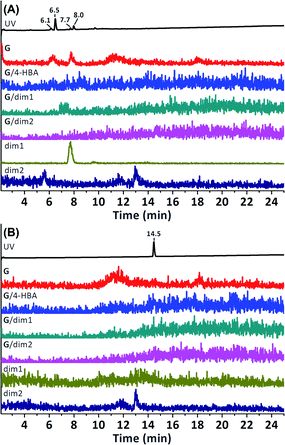
Attachment of 4-HBA, or that of any of its dimers, to G could not be observed (Fig. 5). The use of H was expected to result in increased conjugate formation due to better mass transfer, as H is soluble in water. However, coupling of 4-HBA, or its dimers, to H could not be detected either (ESI Fig. S16†). Products that could be observed were merely the result of laccase-mediated oligomerisation of 4-HBA. Attempts at increasing the conversion of 4-HBA by adding a mediator (TEMPO) did not result in H/dim1, H/dim2 or H/4-HBA conjugates either (ESI Fig. S17†). Performing LC-MS analysis at higher concentrations allowed for easier (semi-)untargeted detection of conjugates. In this way the mass spectra at retention times corresponding to significant peaks in the UV chromatogram were analysed for plausible conjugates in which the bound (oligomeric) 4-HBA fragment would have undergone additional transformations e.g. further oligomerisation, hydroxylation or oxidation (ESI Fig. S18–S26†). However, none of the observed peaks corresponded to a possible conjugate between H and 4-HBA derivatives/oligomers. In a final attempt to induce conjugation, a more reactive phenol (vanillic acid) was subjected to laccase-mediated oligomerisation in the presence of PES model compound H. Analogous to the laccase-mediated dimerisation of 4-HBA, vanillic acid (VA) is known to form C3–C3′-bound dimers (VAdim1, calculated m/z 333.06).37 Two other abundantly present dimers were the result of monomer coupling and decarboxylative hydroxylation followed by oxidation (H/VAdim2, calculated m/z 303.05). However, none of the expected conjugates H/VA, H/VAdim1 or H/VAdim2 were observed (ESI Fig. S27†). Similar to the laccase-mediated oligomerisation of 4-HBA in the presence of H, the products that were observed, were vanillic acid oligomers.
It appeared that neither 4-HBA nor VA were reactive towards the model compounds H or G. Either the proposed conjugates were not formed and untargeted search was not effective, or the quantities of these conjugates were too low to observe against the background of other molecules. To test whether the latter statement is correct we aimed to separate bulk non-conjugate molecules from the formed conjugates.
Laccase-mediated oligomerisation of 4-hydroxybenzoic acid derivatives in the presence of resin-bound PES model compounds
Laccase-mediated oligomerisation in the presence of (soluble) PES model compounds did not lead to the observation of PES model compound/(oligomeric) 4-HBA conjugates. To ease analysis, separation between the conjugates and non-conjugates (starting material and oligomeric 4-HBA) would be desirable. Due to a high similarity between both, and the low abundance of conjugates, chromatographic separation after laccase-mediated oligomerisation would be nearly impossible. We therefore envisioned immobilising a PES model compound on a resin in order for it to be isolable from the rest of the reaction medium through simple washing and filtering. This is accompanied with the additional advantage that the resin-bound PES model compound (R-mPES) would more accurately resemble solid PES fibres that are restricted in their mobility. As depicted in Scheme 5, the monohydroxy-functionalised PES model compound was first functionalised with a carboxylic acid to enable coupling to the resin to afford the resin-bound model compound; R-mPES. Laccase-mediated oligomerisation in the presence of R-mPES took place subsequently, which was followed by extensive washing of R-mPES to remove all non-bound starting material and oligomeric 4-HBA. Cleavage of the model compound and its reaction products from the resin, followed by LC-MS analysis allowed for the detection of possible conjugates and was expected to thereby reveal the nature of the bond between PES and 4-HBA.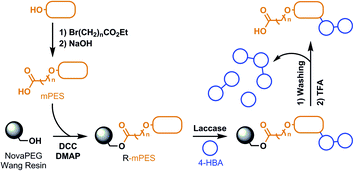 | ||
| Scheme 5 Schematic overview of the anticipated laccase-mediated oligomerisation of 4-HBA in the presence of resin-bound PES model compounds. | ||
Since, to the best of our knowledge, enzymatic transformations on resin-bound model compounds to mimic surface chemistry, have not been studied before, this methodology was first verified by studying a well-known reaction: the laccase-mediated oligomerisation of 4-HBA derivatives. Protection of the hydroxy moiety of 4-HBA as a tetrahydropyranyl (THP) ether afforded 4-(OTHP)BA. 4-(OTHP)BA was subsequently coupled to a hydroxy-functionalised NovaPEG Wang resin using DCC and DMAP. NovaPEG resins have a backbone entirely constituted of PEG units (ChemMatrix), allowing for excellent swelling properties in both water and organic solvents.38 Approximation of reaction completion was accomplished through FT-IR analysis, as coupling resulted in the appearance of a distinct carbonyl stretching vibration signal at ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 1713 cm−1 corresponding to ester formation (Fig. 6). When the intensity of this signal did not intensify any further, the reaction was deemed complete. On-resin deprotection could subsequently be achieved with pyridinium para-toluenesulfonate. Resin-bound 4-HBA was thereafter added to a mixture of laccase and 3-fluoro-4-hydroxybenzoic acid (3-F-4-HBA) and this suspension was incubated for a short period of time. 3-F-4-HBA was chosen as phenol in solution to be able to differentiate solution-phase dimerisation from solid-phase dimerisation; 3-F-4-HBA would form heterogeneous dimers with resin-bound 4-HBA where it would form homogeneous dimers of 3-F-4-HBA in solution. After laccase-mediated oligomerisation, washing of the resin was followed by trifluoroacetic acid-mediated cleavage.
= 1713 cm−1 corresponding to ester formation (Fig. 6). When the intensity of this signal did not intensify any further, the reaction was deemed complete. On-resin deprotection could subsequently be achieved with pyridinium para-toluenesulfonate. Resin-bound 4-HBA was thereafter added to a mixture of laccase and 3-fluoro-4-hydroxybenzoic acid (3-F-4-HBA) and this suspension was incubated for a short period of time. 3-F-4-HBA was chosen as phenol in solution to be able to differentiate solution-phase dimerisation from solid-phase dimerisation; 3-F-4-HBA would form heterogeneous dimers with resin-bound 4-HBA where it would form homogeneous dimers of 3-F-4-HBA in solution. After laccase-mediated oligomerisation, washing of the resin was followed by trifluoroacetic acid-mediated cleavage.
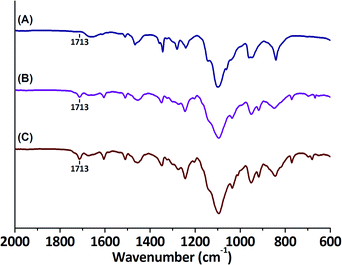 | ||
| Fig. 6 FT-IR spectra of (A) NovaPEG Wang resin, (B) resin-bound 4-(OTHP)BA and (C) resin-bound 4-HBA. | ||
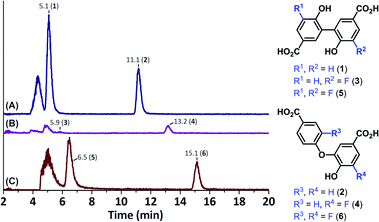
The cleaved material was compared to oligomerised 4-HBA and oligomerised 3-F-4-HBA by means of LC-MS. The use of 3-F-4-HBA not only introduced a moiety with a distinct mass (because of its fluorine atom), but it also proved to be significantly more hydrophobic than 4-HBA itself, which assured good LC separation. The main products of the laccase-mediated oligomerisation of 4-HBA are two dimers with m/z 273.04 (1 and 2).16 Analogously, dimerisation of 3-F-4-HBA was expected to result in a C3–C3′-bound dimer (5) and a C3–O-bound dimer (6), both having a calculated m/z of 309.02. Oligomerisation of 3-F-4-HBA indeed resulted in the formation of two products with m/z 309.02 eluting at 6.5 min (5) and 15.1 min (6, Fig. 7). As expected, corresponding dimeric products of the laccase-mediated oligomerisation of 4-HBA eluted earlier: at 5.1 min (1) and 11.2 min (2). Hetero-dimers 3 and 4 (calculated m/z 291.03), resulting from the coupling of resin-bound 4-HBA and 3-F-4-HBA in solution, were expected to elute halfway. Two peaks, corresponding to a molecule with m/z 291.03, were indeed found. Detectability of the first compound, eluting at 5.9 min, was substantially obscured by the presence of 3-F-4-HBA adducts (4.9 min), but indeed proved to elute exactly halfway between 1 and 5 and was therefore designated as 3. Formation of 4 proved more apparent, as a clear peak, eluting at 13.2 min, was present, again eluting exactly halfway 2 and 6. The observation of these heterogeneous dimers (3 and 4) thereby proved the eligibility of the proposed methodology to perform enzyme-mediated transformations on resin-bound small-molecules.
In order for the PES model compound to be bound to the NovaPEG Wang resin, it had to be functionalised with a carboxylic acid. Therefore, commercially available 4-((4-isopropoxyphenyl)sulfonyl)phenol was etherified using ethyl 4-bromobutanoate, followed by hydrolysis of its ethyl ester. Initially, coupling of the PES model compounds was conducted in pure dichloromethane, however, switching to a mixture of dichloromethane and dimethylformamide allowed for more reproducible coupling. The carboxylic acid-functionalised PES model compound (mPES3) could directly be subjected to enzymatic catalysis after coupling it to the resin. Laccase-mediated oligomerisation of 4-HBA in the presence of the resin-bound PES model compound (R-mPES3) was conducted similarly to the oligomerisation of 3-F-4-HBA in the presence of resin-bound 4-HBA. A targeted search for R-mPES3/4-HBA, R-mPES3/dim1 and R-mPES3/dim2 conjugates by means of LC-MS was conducted after cleavage from the resin. However, no conjugates could be observed (Fig. 8); the peak observed for molecules with a calculated m/z of 649.14 (corresponding to mPES3/4-HBA) proved to be an adduct of mPES3 itself as this peak was also observed when mPES3 was individually subjected to LC-MS analysis. Laccase-mediated oligomerisation of the, more reactive, phenols vanillic acid and syringic acid in the presence of R-mPES3 also did not result in conjugate formation (ESI Fig. S28–S32†). As accessibility of the PES model compound might be limited due to immobilisation near the resin's matrix, formation of conjugates could be hampered. The three-carbon spacer was replaced for a ten-carbon spacer to enhance accessibility of the resin-bound PES model compound (R-mPES10). Laccase-mediated oligomerisation of 4-HBA in the presence of R-mPES10 did, however, not result in covalent bonding between 4-HBA (dimers) and R-mPES10 either (ESI Fig. S33†). It can therefore be stated that, although laccase-assisted transformations on resin-bound 4-HBA proved to be possible, laccase-mediated conjugation of 4-HBA and resin-bound PES model compounds could not be demonstrated.
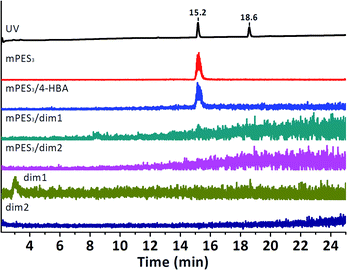 | ||
| Fig. 8 UV trace and extracted ion chromatograms of ions whose m/z ([M − H]− ± 0.50 Da) corresponds to that of mPES3, dim1/dim2 or their conjugates after incubation of 4-HBA, laccase and R-mPES3. | ||
Rewriting the mechanism behind the laccase-assisted surface functionalisation of PES
Although laccase-mediated oligomerisation of 4-HBA proved to be highly effective in inducing antifouling properties to commercially available PES membranes, the mechanism behind the formation of the layer of oligomeric 4-HBA had not been elucidated yet. It was hypothesised that phenolic radicals would bind covalently to PES. We were, however, not able to observe any covalent bonding to the PES membrane or to model compounds mimicking it. Furthermore, the limited amount of charged phenolics present on PES after laccase-mediated oligomerisation of positively charged phenolics could easily be removed by wiping. Therefore, the hypothesis above had to be altered: laccase-assisted functionalisation of PES does not result from covalent binding, but rather from strong, but aspecific physical adsorption. This would clarify why the use of 4-HBA results in more surface functionalisation than the use of charged phenolics does; oligomeric 4-HBA precipitates readily and is, due to its low solubility, not easily washed off, whereas oligomers of charged phenols do not, or barely, form a precipitate and are also readily removed by aqueous washing. The use of other phenols, that are known to be oligomerised by laccase, but do not precipitate, is therefore expected not to result in PES membrane modification. Furthermore, extensive washing of PES membranes that have been functionalised with oligomers of 4-HBA should thus result in further removal of these oligomers.To test whether the use of soluble phenolic oligomers would result in modification of PES, the phenolic monomer guaiacol sulfonate was chosen. Oligomers resulting from the laccase-mediated conversion of guaiacol sulfonate have been shown to be (completely) soluble in acidic aqueous media.39 It was, analogously, anticipated that this would also be true for oligomers of 4,5-dihydroxy-1,3-benzenedisulfonate (Tiron). Oligomerisation of guaiacol sulfonate resulted in a reaction medium being coloured intensely yellow, where oligomerisation of 4,5-dihydroxy-1,3-benzenedisulfonate resulted in a deeply red medium (while both reaction media remained completely transparent by eye). As predicted, the colour of the solution was not transferred to the membranes, which thus indicated that modification did not take place. This was confirmed through XPS analysis: no chemical alterations were observed apart from those resulting from enzyme adsorption (Fig. 9).
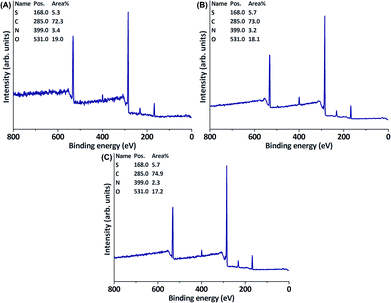 | ||
| Fig. 9 XPS wide scans of a PES membrane after incubation with laccase and (A) guaiacol sulfonate or (B) 4,5-dihydroxy-1,3-benzenedisulfonate. (C) XPS wide scan of a blank PES membrane. | ||
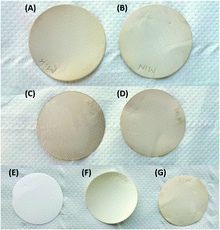
Laccase-assisted modification of PES membranes with oligomeric 4-HBA resulted in intense colouration of the modified membrane, which persists after strong flushing and repeated dipping in water.12 If modification proves indeed to be non-covalent, more extensive washing should result in less intense membrane colouration. Initial (2 min) flushing on one side of the membrane with lukewarm tap water resulted in a distinct loss of colouration (Fig. 10A) compared to the side that had not been flushed (Fig. 10C). Elongating the time of flushing to 5 min resulted in minimal additional colour loss (Fig. 10B and D). A significant effect of further washing could be observed after agitating the membranes in either water, or an aqueous solution of a detergent (TWEEN®, 0.1 wt%) at 40 °C for 72 h. Where washing in water did not alter colouration (Fig. 10G), addition of a detergent resulted in a homogenously tainted membrane of which colouration was significantly less intense than before washing (Fig. 10F). As expected, oligomers of 4-HBA are very poorly soluble in water and removal could therefore not be achieved by mere washing/flushing with water. However, the fact that washing with a detergent resulted in extensive removal of membrane colouration confirmed that laccase-assisted coating of PES membranes occurs through adsorption of, rather than through covalently binding the formed phenolic oligomers.
Conclusions
Laccase-mediated oligomerisation of 4-hydroxybenzoic acid (4-HBA) in the presence of PES membranes has been reported to cover the surface with strongly bound coloured material that induced antifouling properties to the membrane.12 Modification was assumed to result from covalent bonding of laccase-generated 4-HBA radicals to the membrane surface. However, the use of a plurality of positively charged phenolics synthesised in-house (instead of 4-HBA) did not, or barely, result in membrane modification. The use of commercially available negatively charged phenolics that, upon oligomerisation, remained completely soluble did not result in modification of the PES surface either. The use of model compounds to mimic a PES surface allowed for easier detection of covalent binding. LC-MS analysis after incubation of either solid, soluble or resin-bound PES model compounds in a mixture of laccase and 4-HBA (derivatives) did not lead to the observation of any covalently bound conjugates between the PES model compound and a (oligomeric) 4-HBA (derivative). These observations led us to believe that the mechanism behind laccase-initiated coating of PES membranes occurs through physical adsorption of oligomeric 4-HBA, rather than through its covalent binding to the surface. This hypothesis was supported by the fact that membranes modified through laccase-initiated modification with oligomers of 4-HBA were significantly less intensely coloured after extensive washing with a detergent solution. Finally, to inform other researchers that laccase-mediated surface functionalisation could easily be interpreted as the result of covalent grafting rather than strong physical adsorption, we recently also reviewed (attempts at) laccase-assisted grafting and assessed to what degree the induced modification could be the result of covalent grafting.36 By doing so, we have identified multiple other cases in which the intended covalent surface modification seems highly unlikely.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank Judith Firet, Adrie H. Westphal and Mahboubeh Tasviri for their assistance in experimental work. This project was funded by the NanoNextNL project 04A.01 “Biomimetic selective layers”.Notes and references
- R. Zhang, Y. Liu, M. He, Y. Su, X. Zhao, M. Elimelech and Z. Jiang, Chem. Soc. Rev., 2016, 45, 5888–5924 RSC.
- P. Le-Clech, V. Chen and T. A. G. Fane, J. Membr. Sci., 2006, 284, 17–53 CrossRef.
- L. F. Greenlee, D. F. Lawler, B. D. Freeman, B. Marrot and P. Moulin, Water Res., 2009, 43, 2317–2348 CrossRef PubMed.
- H. Flemming, Appl. Microbiol. Biotechnol., 2002, 59, 629–640 CrossRef PubMed.
- V. Moghimifar, A. Raisi and A. Aroujalian, J. Membr. Sci., 2014, 461, 69–80 CrossRef.
- X. Li, T. Cai and T. Chung, Environ. Sci. Technol., 2014, 48, 9898–9907 CrossRef PubMed.
- E. Celik, H. Park, H. Choi and H. Choi, Water Res., 2011, 45, 274–282 CrossRef PubMed.
- N. Nady, M. C. R. Franssen, H. Zuilhof, M. S. Mohyeldin, R. Boom and K. Schroën, Desalination, 2011, 275, 1–9 CrossRef.
- S. Schmidt, C. Scherkus, J. Muschiol, U. Menyes, T. Winkler, W. Hummel, H. Gröger, A. Liese, H. G. Herz and U. T. Bornscheuer, Angew. Chem., Int. Ed., 2015, 54, 2784–2787 CrossRef PubMed.
- Y. Jiang, A. J. J. Woortman, G. O. R. Alberda van Ekenstein, D. M. Petrović and K. Loos, Biomacromolecules, 2014, 15, 2482–2493 CrossRef PubMed.
- P. Steunenberg, M. Uiterweerd, M. Sijm, E. L. Scott, H. Zuilhof, J. P. M. Sanders and M. C. R. Franssen, Curr. Org. Chem., 2013, 17, 682–690 CrossRef.
- N. Nady, K. Schroën, M. C. R. Franssen, B. van Lagen, S. Murali, R. M. Boom, M. S. Mohyeldin and H. Zuilhof, ACS Appl. Mater. Interfaces, 2011, 3, 801–810 CrossRef PubMed.
- N. Nady, K. Schroën, M. C. R. Franssen, R. Fokkink, M. S. Mohyeldin, H. Zuilhof and R. M. Boom, J. Colloid Interface Sci., 2012, 378, 191–200 CrossRef PubMed.
- S. van der Veen, N. Nady, M. C. R. Franssen, H. Zuilhof, R. M. Boom, T. Abee and K. Schroën, J. Appl. Polym. Sci., 2015, 132, 41576 CrossRef.
- V. Madhavi and S. S. Lele, Bioresources, 2009, 4, 1694–1717 Search PubMed.
- S. Slagman, J. Escorihuela, H. Zuilhof and M. C. R. Franssen, RSC Adv., 2016, 6, 99367–99375 RSC.
- X. Li, S. De Feyter, D. Chen, S. Aldea, P. Vandezande, F. Du Prez and I. F. J. Vankelecom, Chem. Mater., 2008, 20, 3876–3883 CrossRef.
- R. Malaisamy and M. L. Bruening, Langmuir, 2005, 21, 10587–10592 CrossRef PubMed.
- J. Kochan, T. Wintgens, J. E. Wong and T. Melin, Desalination, 2010, 250, 1008–1010 CrossRef.
- D. Saeki, M. Imanishi, Y. Ohmukai, T. Maruyama and H. Matsuyama, J. Membr. Sci., 2013, 447, 128–133 CrossRef.
- Q. Chen, P. Yu, W. Huang, S. Yu, M. Liu and C. Gao, J. Membr. Sci., 2015, 492, 312–321 CrossRef.
- R. Hadj Lajimi, E. Ferjani, M. S. Roudesli and A. Deratani, Desalination, 2011, 266, 78–86 CrossRef.
- L. Y. Ng, A. W. Mohammad, C. Y. Ng, C. P. Leo and R. Rohani, Desalination, 2014, 351, 19–26 CrossRef.
- G. Zhang, W. Gu, S. Ji, Z. Liu, Y. Peng and Z. Wang, J. Membr. Sci., 2006, 280, 727–733 CrossRef.
- U. K. Aravind, J. Mathew and C. T. Aravindakumar, J. Membr. Sci., 2007, 299, 146–155 CrossRef.
- H. Wu, X. Li, C. Zhao, X. Shen, Z. Jiang and X. Wang, Ind. Eng. Chem. Res., 2013, 52, 5772–5780 CrossRef.
- Z. Jia, D. Shen and W. Xu, Carbohydr. Res., 2001, 333, 1–6 CrossRef PubMed.
- T. Kudanga, E. Nugroho Prasetyo, J. Sipilä, P. Nousiainen, P. Widsten, A. Kandelbauer, G. S. Nyanhongo and G. M. Guebitz, Eng. Life Sci., 2008, 8, 297–302 CrossRef.
- L. Servillo, A. Giovane, N. D'Onofrio, R. Casale, D. Cautela, G. Ferrari, M. L. Balestrieri and D. Castaldo, J. Agric. Food Chem., 2014, 62, 2679–2684 CrossRef PubMed.
- D. Skalamera, C. Bohne, S. Landgraf and N. Basaric, J. Org. Chem., 2015, 80, 10817–10828 CrossRef PubMed.
- J. H. Hodgkin, Aust. J. Chem., 1984, 37, 2371–2378 CrossRef.
- R. Ikeda, J. Sugihara, H. Uyama and S. Kobayashi, Polym. Int., 1998, 47, 295–301 CrossRef.
- F. Xu, Appl. Biochem. Biotechnol., 1996, 59, 221–230 CrossRef.
- T. Miyano, T. Matsuura and S. Sourirajan, Chem. Eng. Commun., 1993, 119, 23–39 CrossRef.
- T. Kudanga, E. Nugroho Prasetyo, J. Sipilä, G. S. Nyanhongo and G. M. Guebitz, Enzyme Microb. Technol., 2010, 46, 272–280 CrossRef.
- S. Slagman, H. Zuilhof and M. C. R. Franssen, ChemBioChem, 2018, 19, 288–311 CrossRef PubMed.
- J. Bollag, S. Liu and R. D. Minard, Soil Biol. Biochem., 1982, 14, 157–163 CrossRef.
- F. García-Martín, M. Quintanar-Audelo, Y. García-Ramos, L. J. Cruz, C. Gravel, R. Furic, S. Côté, J. Tulla-Puche and F. Albericio, J. Comb. Chem., 2006, 8, 213–220 CrossRef PubMed.
- M. Lund and A. Ragauskas, Appl. Microbiol. Biotechnol., 2001, 55, 699–703 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: experimental section and supporting figures. See DOI: 10.1039/c8ra04402c |
| This journal is © The Royal Society of Chemistry 2018 |