Open Access Article
Open Access ArticleLuminescence properties and energy transfer investigations of Ba2La2.85−xTb0.15Eux(SiO4)3F multicolor phosphor
Xiaoxue Maa,
Libing Liao *a,
Qingfeng Guob,
Haikun Liua,
Tianshuai Zhoua and
Lefu Mei*a
*a,
Qingfeng Guob,
Haikun Liua,
Tianshuai Zhoua and
Lefu Mei*a
aBeijing Key Laboratory of Materials Utilization of Nonmetallic Minerals and Solid Wastes, National Laboratory of Mineral Materials, School of Materials Science and Technology, China University of Geosciences, Beijing 100083, China. E-mail: clayl@cugb.edu.cn; mlf@cugb.edu.cn; Fax: +86-10-82322974; Fax: +86-10-8232-1701; Tel: +86-10-82322039 Tel: +86-10-8233-1701
bSchool of Gemmology, China University of Geosciences, Beijing 100083, China
First published on 31st July 2018
Abstract
The Ba2La2.85−xTb0.15Eux(SiO4)3F (BLSOF:0.15Tb3+, xEu3+) multicolor phosphors with apatite structure were synthesized via the solid-state pathway. The crystal structure and luminescence properties of the phosphors were investigated by means of scanning electron microscopy (SEM), X-ray diffraction (XRD), Rietveld refinement, photoluminescence excitation (PLE) and photoluminescence (PL). The luminescence performance of the phosphor was optimum when the concentration of Tb3+ was set to be 0.15 mol and the concentration of Eu3+ was set to be 0.22 mol. Under the accurate excitation of 373 nm near ultraviolet (n-UV) light, the emitting color of the phosphors can be tuned from green to red with increasing Eu3+/Tb3+ ratio. It was further proved that the quadrupole–quadrupole (q–q) interaction is responsible for the energy transfer (ET) in the BLSOF:0.15Tb3+, 0.22Eu3+ phosphor. Owing to the excellent thermal quenching luminescence property, the BLSOF:0.15Tb3+, xEu3+ phosphor can be applied in n-UV white light emitting diodes (w-LEDs) and serve as the warm part of warm white light.
1. Introduction
Light emitting diodes (LEDs) have been used in a wide range of fields owing to their advantages, such as low-carbon emission, long service lifetime, high photoelectric conversion rate, environmentally-friendly performance, and so on.1 The market for LEDs covers a variety of applications, for example, displays, traffic lights, decorative lighting and general lighting. In particular, white light emitting diodes (w-LEDs) have attracted the attention of researchers from all over the world, and have been called the fourth generation of lighting source owing to their outstanding merits in the general lighting field.2–4 One method to produce w-LEDs capable of emitting high-quality white light is to combine tri-color phosphors with near ultraviolet (n-UV)-InGaN chips (350–420 nm).5 In the applications of w-LEDs, several shortcomings of phosphor converted w-LEDs have been noted, such as low color rendering index (CRI), high correlated color temperature (CCT), blue light hazard induced by the former two, and different service lifetime of each part.6,7 Long exposure to light of the blue and purple part of the spectrum can do harm to the retina, which is known as blue light hazard. The lack of a warm component in the light emitted by w-LEDs results in low CRI and high CCT. These studies highlight the importance of exploiting new kinds of phosphor to provide multicolor warm light in order to improve users' visual experience.8–10Much recent research has focused on multicolor single-phase phosphors. Phosphors belonging to this category can avoid the drawbacks of inconsonant decay, inconsistent service lifetime and so on.11 Generally, there are two methods to obtain multicolor single-phase phosphors: structure regulation, and energy transfer from sensitizer to activator.12,13 There has been widespread application of energy transfer from sensitizers to activators, using an abundant range of host materials. Numerous sensitizer → activator ion pairs have been reported, including Eu2+ → Mn2+, Tb3+ → Eu3+, Ce3+ → Tb3+, and so on.14–16 Eu3+ can emit red light due to its electric dipole 5D0 → 7F2 transition, making it a suitable activator of the phosphor for w-LED requirements.17–20 Terbium is usually used with europium to produce multicolor warm light, such as in LaBWO6:Tb3+, Eu3+; Zn2GeO4:Tb3+, Eu3+; Sr3GdNa(PO4)3F:Tb3+, Eu3+; Sr2Zr(PO4)6:Tb3+, Eu3+; KCaY(PO4)2:Tb3+, Eu3+ and so on.21–25
An appropriate activator and appropriate matrix are necessary to synthesize good phosphors. Outstanding photoluminescence can be achieved by doping rare earth (RE) elements in apatite. Apatite is a common choice for the matrix because of its good chemical stability and rich crystal field environment.26 The general formula of apatite compounds can be expressed as M10(XO4)6Y2, in which M represents Ca, Sr, Ba, La, RE and so on; X represents Si, Ge, P and so on; Y represents F, Cl, Br, O and so on. It is widely known that there are two non-equivalent crystallographic sites in the apatite structure: the nine-coordinated 4f site with C3 point symmetry and the seven-coordinated 6h site with Cs point symmetry, both of which are usually occupied by alkali metal, alkaline earth metal, and RE ions. Apatite compounds have been widely investigated for various applications, such as Ca9La(PO4)5(SiO4)F2, Sr8La2(PO4)6O2, Ba3LaK(PO4)3F, and so on.27–29 However, there is no report of the Ba2La3–x–yEuxTby(SiO4)3F(BLSOF:Tb3+, Eu3+) phosphor with apatite structure and oxyfluoride phase to the best of our knowledge.30,31 In the crystal field environment of Ba2La3–x–yEuxTby(SiO4)3F, the La, Tb, and Eu ions should occupy the X sites, which include both 4f and 6h sites according to the theory mentioned above.
It is important to discover novel phosphors that can be excited by n-UV light (350–420 nm) to satisfy the needs of the market.32 In this paper, the energy transfer mechanism in apatite compounds was investigated, and the performance of the studied phosphor under high temperature was found to be satisfactory. Under the accurate excitation of 373 nm n-UV light, the phosphor can emit light of different wavelengths depending on the doping ratio of Tb3+ and Eu3+. It is concluded that the BLSOF:0.15Tb3+, xEu3+ multicolor phosphor can be applied in n-UV w-LEDs and provide the warm part of warm light.
2. Experimental details
2.1 Synthesis
The desired phosphors Ba2La2.85−xTb0.15Eux(SiO4)3F were prepared via the conventional solid-state method under high temperature. The raw materials included BaCO3 (A.R.), La2O3 (99.99%), SiO2 (A.R.), NH4HF2 (A.R.), Tb4O7 (99.99%) and Eu2O3 (99.99%), which were used directly without any other treatment. The selected starting materials were weighed according to the calculated precursor stoichiometry and mixed in an agate mortar. After thorough grinding, the stoichiometric mixture was transferred into an alumina crucible. The crucible was sealed into a high temperature tube furnace and annealed at 1350 °C for 5 h. After that, the sample was cooled to room temperature gradually, and ground into powder again for the series of tests that followed.2.2 Characterization
XRD spectra were obtained on an X-ray powder diffractometer (XD-3, PGENERAL, China) with Cu-Kα radiation (λ = 0.15406 nm) operated at 40 kV and 30 mA. Powder diffraction data were fitted by the Rietveld method using the computer software TOPAS 3.0 package. PLE and PL spectra under room temperature were characterized on a Hitachi F-4600 fluorescence spectrophotometer. A PL system equipped with a xenon lamp (400 V, 150 W) acted as the excitation source. The thermal quenching luminescence properties were measured on the same spectrophotometer, which was combined with a self-made heating attachment and a computer-controlled electric furnace. A 400 nm cut off filter was applied in the experiment to remove the second-order emission of source radiation. Decay curves were monitored by an EDINBURGH FS5 Combined Fluorescence Lifetime & Steady State Spectrometer. Emission spectra of BLSOF:yTb3+ were measured on the same device with a 400 nm cut off filter. Quantum yield was measured by an EDINBURGH FLS980 Spectrometer. Morphological analysis of the compounds was observed by SEM (JSM-6701F, Hitachi, Japan). Before the morphology was observed, the powder was coated on the conducting resin directly without any other measurement and sprayed with nano-platinum on the surface.3. Results and discussions
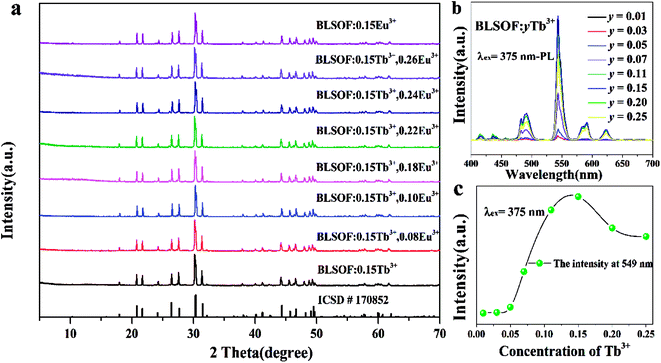
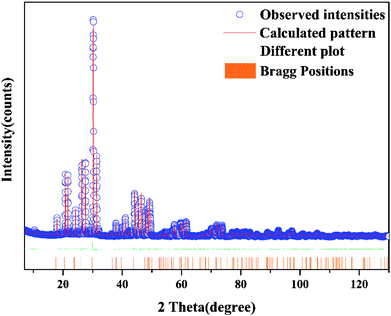
Fig. 1(a) shows the typical XRD patterns of the as-prepared samples including the BLSOF:0.15Tb3+, BLSOF:0.15Eu3+ and BLSOF:0.15Tb3+, xEu3+ (x = 0.08, 0.1, 0.18, 0.22, 0.24, 0.26) phosphors. It is clear that the XRD peaks of the BLSOF phosphors can be accurately assigned to the phase of BLSOF (ICSD no. 170852), which belongs to the apatite structure with the hexagonal system and space group P63/m.33 Fig. 1(b) shows the emission spectra of the BLSOF:yTb3+ phosphors and Fig. 1(c) shows the concentration–intensity relationship of the BLSOF:yTb3+ phosphors. The emission intensity reaches the maximum when the concentration of Tb3+ ions is 0.15 mol, above which the luminescence intensity decreases resulting from concentration quenching as widely noted in the literature.34 Terbium ions act as the sensitizer and europium ions act as the activator in the BLSOF:Tb3+, Eu3+ phosphors. When y = 0.15, the phosphor presents the strongest emission intensity. Considering this, the concentration of Tb3+ was fixed to be 0.15 mol and the concentration of Eu3+ was variable in the co-doped phosphors. In terms of their positions, the intensities of the diffraction peaks were matched perfectly with the reference ICSD file in Fig. 1(a). According to the substitution principle of isomorphism, Tb3+ and Eu3+ are assumed to replace La3+ in the structure and no charge compensation or vacancies will be formed as this is an isovalent substitution process. We can infer that the doping of Tb3+ and Eu3+ ions did not cause any structural change, which demonstrated that the isomorphic replacement of La3+ with Tb3+ and Eu3+ was successful in the present sample structure.35Fig. 2 shows the Rietveld refinement of BLSOF:0.15Tb3+ at 298 K with λ = 1.5406 Å by the TOPAS program. The crystal structure of BLSOF (apatite-type structure) was taken as the starting model for Rietveld refinement and almost all peaks were indexed with parameters close to BLSOF. Solid red lines are the calculated intensities, and blue circles are the observed intensities. Short vertical lines show the position of Bragg reflections of the calculated pattern. Green solid lines below the profiles stand for the difference between the observed and the calculated intensities. Refinement was stable and gave low R-factors (Table 1, Fig. 2). All the experimental peaks were well fitted by the refinement, indicating that all of those peaks are Bragg reflections from the BLSOF structure and converge to Rexp = 3.737%, Rwp = 12.969%, Rp = 9.280% and χ2 = 3.470. In the BLSOF compound, the lattice parameters are determined to be a = b = 9.89093(21) Å, c = 7.36654(19) Å and V = 624.120(32) Å3. The refined atomic positions and isotropic temperature factors for all atoms are also shown in Table 2.36,37
| Formula | Ba2La3(SiO4)3F |
| Space group | P63/m |
| Symmetry | Hexagonal |
| a/b (Å) | 9.89093(21) |
| c (Å) | 7.36654(19) |
| α/β | 90° |
| γ | 120° |
| V (Å3) | 624.120(32) |
| R-Bragg | 3.42909 |
| Rexp (%) | 3.737 |
| Rwp (%) | 12.969 |
| Rp (%) | 9.280 |
| χ2 | 3.470 |
| Atoms | x | y | z | Occ | Beq |
|---|---|---|---|---|---|
| La2 | 0.23946(23) | 0.98545(25) | 0.25 | 0.67(65) | 1 |
| Ba2 | 0.23946(23) | 0.98545(25) | 0.25 | 0.08(67) | 1 |
| Tb2 | 0.23946(23) | 0.98545(25) | 0.25 | 0.25(18) | 1 |
| La1 | 0.6666667 | 0.3333333 | 0.00082(56) | 0.91(66) | 1 |
| Ba1 | 0.6666667 | 0.3333333 | 0.00082(56) | 0.08(67) | 1 |
| Tb1 | 0.6666667 | 0.3333333 | 0.00082(56) | 0.00(20) | 1 |
| Si1 | 0.4080(11) | 0.37362(99) | 0.25 | 1 | 1 |
| O1 | 0.5970(24) | 0.4536(22) | 0.25 | 1 | 1 |
| O2 | 0.3482(24) | 0.5038(22) | 0.25 | 1 | 1 |
| O3 | 0.3557(13) | 0.2672(13) | 0.0697(16) | 1 | 1 |
| F | 0 | 0 | 0.25 | 1 | 2.81 |
The microstructure of BLSOF:0.15Tb3+, 0.22Eu3+ was determined and is shown in Fig. 3(a). The sample is composed of numerous smaller and larger blocks. The observation of Ba, La, Si, O, F, Tb, and Eu peaks in the EDX spectrum in Fig. 3(b) further implied the formation of the sample with the desired composition. In addition, the elemental mapping results as shown in Fig. 3(c) demonstrated that all the elements in the sample were homogeneously distributed throughout each block.38
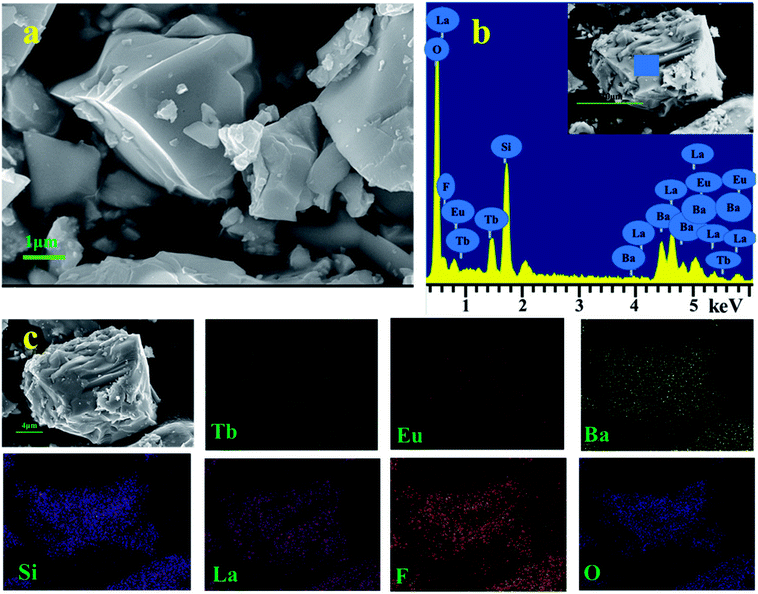 | ||
| Fig. 3 (a) SEM image of BLSOF:0.15Tb3+, 0.22Eu3+ phosphor. (b) EDX spectrum of BLSOF:0.15Tb3+, 0.22Eu3+. (c) Elemental mapping of BLSOF:0.15Tb3+, 0.22Eu3+ phosphor. | ||
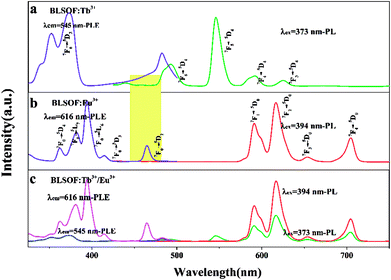
The luminescence properties of the samples were studied in detail in the following work. The emission and excitation spectra of BLSOF:0.15Tb3+, BLSOF:0.15Eu3+ and BLSOF:0.15Tb3+, 0.22Eu3+ were measured. Fig. 4(a) depicts the spectra of the BLSOF:0.15Tb3+ phosphor. As shown in Fig. 4(a), the PLE spectrum of BLSOF:0.15Tb3+ shows typical excitation ranging from 325 to 500 nm with a characteristic excitation peak at 373 nm (7F6 → 5D3), indicating that the excitation wavelength of the as-grown phosphors overlaps well with that of n-UV light LED chips. Upon 373 nm excitation, the phosphor emits sharp peaks of Tb3+ centered at 492, 545, 592 and 624 nm originating from transitions of 5D4 → 7FJ (J = 6, 5, 4, 3). Fig. 4(b) depicts the spectra of the BLSOF:0.15Eu3+ phosphor. There are several peaks in these excitation spectra, which can be ascribed to transitions from the ground state 7F0 to the excited states 5D4 (362 nm), 5L7 (381 nm), 5L6 (394 nm), 5D3 (414 nm), and 5D2 (465 nm).39,40 Upon 394 nm excitation, the BLSOF:0.15Eu3+ phosphor emits sharp peaks of Eu3+ centered at 591, 616, 654 and 700 nm originating from transitions of 5D0 → 7FJ (J = 1, 2, 3, 4). Comparing Fig. 4(a) and (b), there is an overlap of the excitation spectrum of Eu3+ and emission spectrum of Tb3+ highlighted in yellow (about 445–485 nm), which means that energy transfer from Tb3+ to Eu3+ is possible in theory. Fig. 4(c) depicts the spectra of the BLSOF:0.15Tb3+, 0.22Eu3+ phosphor. Using the same test parameters and coordinate range (in vertical and horizontal directions), PLE peaks at 373 nm, 394 nm and PL peaks at 545 nm, 616 nm were obtained and the relative intensity of the Tb3+ and Eu3+ peaks can be estimated from the spectra. Considering the excitation curves, the sample could be monitored by 616 nm and 545 nm, and excitation curve monitored by 616 nm is higher than the one monitored by 545 nm. Upon excitation, the sample shows emission from Eu3+ at an emission wavelength of 616 nm and from Tb3+ at an emission wavelength of 545 nm, and the former is more intense. The phosphor emits sharp peaks of Tb3+ and Eu3+ upon 373 nm excitation, which indicates that Tb3+, Eu3+ co-doped BLSOFs can be used as green and red double-color emitting phosphors under n-UV excitation. Monitored at 394 nm, only red emission of Eu3+ could be observed, similar to the characteristic profile of the Eu3+ singly doped BLSOF sample. When the phosphor is excited by 373 nm, the peak of green curve at 616 nm is not as intense as the red curve (which was excited by 394 nm) in Fig. 4(c), indicating a decrease in emission of Eu3+ at this excitation wavelength. The emission spectrum monitored at 373 nm includes not only the characteristic emission derived from Eu3+ (5D0 → 7F2 at 616 nm), but also the Tb3+ characteristic emission profile (5D4 → 7F6,5), similar to the Tb3+ singly doped sample. In this case, we focused on the emission spectra monitored at 373 nm to characterize the energy transfer properties from Tb3+ to Eu3+. The results showed that the relative intensities of green and red emissions can be varied and multicolor emission may be achieved by adjusting the concentrations of Tb3+ and Eu3+ through energy transfer.41
An energy level diagram with all the relevant transitions is shown in Fig. 5 to visually illustrate the mechanism of luminescence and energy transfer. When the BLSOF:Tb3+, Eu3+ phosphor is exposed to UV light, Tb3+ ions in the phosphor are excited from the ground state 7F6 to the excited state 5D3 and emit green light when they leap back from 5D4 to 7FJ (J = 6, 5, 4, 3) after nonradiative processes from 5D3 to 5D4. Energy transfer from the 5D4 energy level of Tb3+ to the 5D0 energy level of Eu3+ can also occur through a nonradiative process because the former is higher in energy. Furthermore, the Tb3+ emission curve, which is attributed to the transitions 5D4 → 7F6,5,4,3, is greatly overlapped with the PLE curve of Eu3+, which originates from the 7F0 → 5D2,3 transition in Fig. 4. Red light is produced when Eu3+ ions return to the ground states 7F1,2,3,4 through radiative transition.42–44
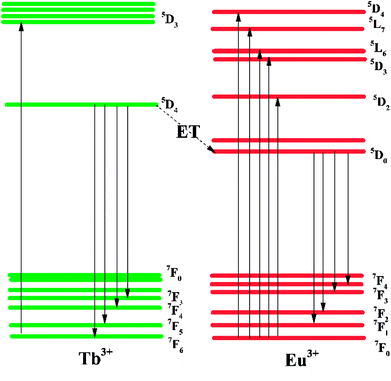 | ||
| Fig. 5 The energy level transitions for PLE and PL of Tb3+ and Eu3+ and the corresponding energy level scheme of energy transfer from to Tb3+ to Eu3+. | ||
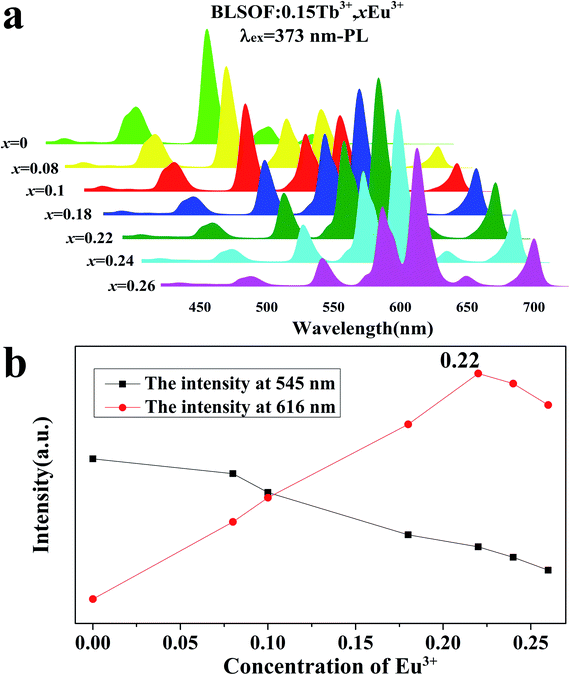
A series of PLE spectra of BLSOF:0.15Tb3+, xEu3+ (x = 0, 0.08, 0.1, 0.18, 0.22, 0.24 and 0.26) under the excitation of 373 nm n-UV light are demonstrated in Fig. 6(a). The emission intensities at 545 and 616 nm with various concentrations of europium(x) are shown in Fig. 6(b). The emission intensity of Tb3+ (545 nm) continually decreases with increasing x and the emission intensity of Eu3+ (616 nm) ions first increases and then reaches the maximum when x = 0.22, after which the emission intensity decreases. Concentration quenching is the likely reason for the decrease in emission intensity above x = 0.22.45 Thus, energy transfer occurs from Tb3+ to Eu3+ in BLSOF.46
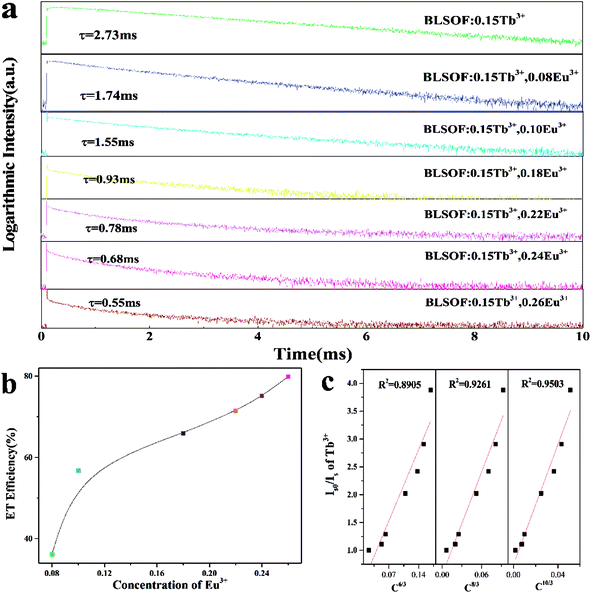
To investigate the energy transfer mechanism, the decay curves of Tb3+ emission for Ba2La2.85−x(SiO4)3F:0.15Tb3+, xEu3+ excited at 373 nm were characterized and are depicted in Fig. 7(a). The decay curves were fitted perfectly based on the following single exponential decay equation:47
I(t) = I0 + A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ) exp(−t/τ)
| (1) |
| η = 1 − (τs/τs0) | (2) |
It is well-known that the mechanism of nonradiative energy transfer among the dopants can be either exchange interaction or electric multipolar interaction. Which of these applies in a particular case can be confirmed by analyzing the critical distance. Based on Blasse's theory, the critical distance Rc can be estimated by using the following equation:49–53
| Rc ≈ 2(3V/4πxcN)1/3 | (3) |
Additionally, Fig. 7(c) shows the dependence of Is0/Is of Tb3+ on C6/3, C8/3 and C10/3 (C is the total concentration of Tb3+ and Eu3+). On the basis of Dexter's energy transfer formula of multipolar interactions, the following relationship can be given:54,55
| (η0/ηs) ∝ Cn/3 | (4) |
| (Is0/Is) ∝ Cn/3 | (5) |
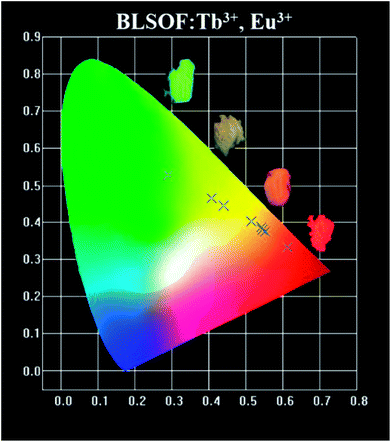
The color coordinates of the BLSOF:0.15Tb3+, xEu3+ phosphors under the excitation of 373 nm were calculated and are presented in Table 3 and Fig. 8. The chromaticity coordinates (x, y) of BLSOF:0.15Tb3+, xEu3+ were calculated based on the corresponding emission spectra. In order to be more intuitive, digital photographs of BLSOF:0.15Tb3+, BLSOF:0.15Tb3+, 0.08Eu3+, BLSOF:0.15Tb3+, 0.22Eu3+ and BLSOF:0.15Eu3+ upon 365 nm UV-lamp excitation are given in the insets of Fig. 8. The series of phosphors were able to be tuned from green (0.29, 0.53), through yellow/orange-red to red (0.61, 0.33) by adjusting the proportion of Tb3+ and Eu3+ concentrations. The results indicate that the prepared phosphors show the merit of multicolor emissions in the visible region when excited by a single wavelength of light, which means that tunable luminescence can be realized in the novel BLSOF:0.15Tb3+, xEu3+ phosphors based on energy transfer.58–60
| Number | Formula | CIE value |
|---|---|---|
| 1 | Ba2La2.85(SiO4)3F:0.15Tb3+ | (0.2908, 0.5282) |
| 2 | Ba2La2.77(SiO4)3F:0.15Tb3+, 0.08Eu3+ | (0.4083, 0.4652) |
| 3 | Ba2La2.75(SiO4)3F:0.15Tb3+, 0.10Eu3+ | (0.4397, 0.4445) |
| 4 | Ba2La2.67(SiO4)3F:0.15Tb3+, 0.18Eu3+ | (0.5160, 0.4027) |
| 5 | Ba2La2.63(SiO4)3F:0.15Tb3+, 0.22Eu3+ | (0.5413, 0.3872) |
| 6 | Ba2La2.61(SiO4)3F:0.15Tb3+, 0.24Eu3+ | (0.5454, 0.3802) |
| 7 | Ba2La2.59(SiO4)3F:0.15Tb3+, 0.26Eu3+ | (0.5534, 0.3745) |
| 8 | Ba2La2.85(SiO4)3F:0.15Eu3+ | (0.6125, 0.3314) |
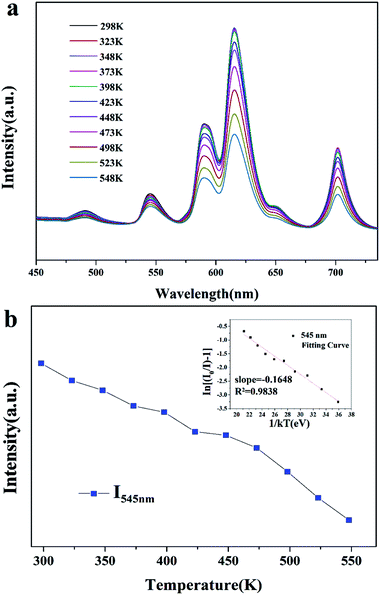
As is well known, the thermal stability of coated phosphors has a critical influence on the performance of LEDs. Fig. 9(a) shows the temperature-dependent emission spectra for the BLSOF:0.15Tb3+, 0.22Eu3+ phosphor and Fig. 9(b) shows the intensity–concentration relationship of Tb3+ (5D4 → 7F5) at 545 nm. It can be seen that the luminescence intensity at 150 °C remains 85.34% of that at room temperature, 25 °C. To understand the temperature-dependent thermal quenching behavior, the activation energy was calculated, as shown in the inset of Fig. 9(b), by means of the following formula:
ln(I0/I − 1) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A − ΔE/kT A − ΔE/kT
| (6) |
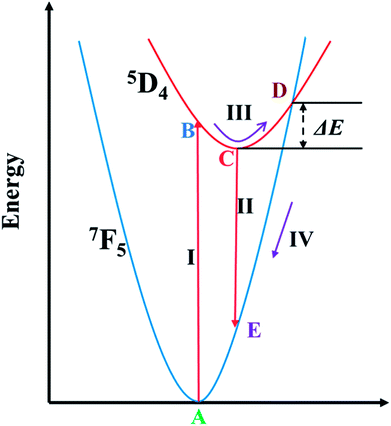
The configuration coordinate diagram for the 7F5 → 5D4 (545 nm) transition of Tb3+ is shown in Fig. 10 to make the thermal quenching process clearer. When the phosphor was exposed to 373 nm light, the electrons of Tb3+ could be pumped from point A in the 7F5 level to point B in the 5D4 level as process I, and then non-radiatively relax to point C, and leap back to point E in the 7F5 level and give out light as process II. The 7F5 and 5D4 states overlapped at point D, and ΔE is the energy difference between D and C. For this phosphor, the energy difference was calculated to be 0.1648 eV as above. At high temperature, the centers will be thermally activated from point B to the junction of point D, corresponding to process III, and then non-radiatively relax to point A as process IV. The higher the temperature, the more electrons can overcome the activation energy. As a result, thermal quenching phenomena occur and the emission intensity of Tb3+ decreases with increasing temperature.62,63
4. Conclusions
In summary, a multicolor warm phosphor, BLSOF:0.15Tb3+, xEu3+, was synthesized by the traditional high-temperature solid-state method. The results reveal that the color of the BLSOF:0.15Tb3+, xEu3+ phosphors can be modulated from green, through yellow/red-orange to red with different Tb3+/Eu3+ ratios. The chromaticity coordinates of the BLSOF:0.15Tb3+, xEu3+ phosphors gradually shift from green (0.29, 0.53) through yellow/red-orange and eventually to red (0.61, 0.33) with increasing Eu3+ concentration. Based on Dexter's energy transfer equation, the quadrupole–quadrupole interaction (q–q) is responsible for the energy transfer from Tb3+ to Eu3+ in BLSOF. The energy level diagram with all the relevant transitions and the configuration coordinate diagram have also been discussed to make the luminescence and thermal quenching process easier to understand. The properties of this phosphor are also stable under high temperature and the applicability of this phosphor is greatly enhanced, suggesting its promising potential. All the observed features indicate that the multicolor phosphor might be a good candidate as a single-phase warm emitter for warm w-LEDs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This present work is supported by the National Natural Science Foundations of China (Grant No. 51672257, 41672044 and 41172053), and the Fundamental Research Funds for the Central Universities (Grant No. 2652017091 and 2652017370).References
- S. Ray, P. Tadge, S. Dutta, T. M. Chen, G. B. Nair and S. J. Dhoble, Ceram. Int., 2018, 44, 8334–8343 CrossRef.
- X. Y. Huang and H. Guo, RSC Adv., 2018, 8, 17132–17138 RSC.
- B. Li, Q. Sun, S. Y. Wang, H. Guo and X. Y. Huang, RSC Adv., 2018, 8, 9879–9886 RSC.
- Z. Li, Q. L. Pian, L. Li, Y. Sun and S. S. Zheng, Optik, 2018, 161, 38–43 CrossRef.
- Z. M. Wang, J. Zou, C. Y. Zhang, B. B. Yang, M. M. Shi, Y. Li, H. Y. Zhou, Y. M. Liu, M. T. Li and Z. Z. Liu, J. Non-Cryst. Solids, 2018, 489, 57–63 CrossRef.
- G. C. Hu, X. Hu, W. Chen, Y. Z. Cheng, Z. C. Liu, Y. J. Zhang, X. J. Liang and W. D. Xiang, Mater. Res. Bull., 2017, 95, 277–284 CrossRef.
- P. Fulmek, J. Nicolics, W. Nemitz and F. P. Wenzl, Mater. Chem. Phys., 2017, 196, 82–91 CrossRef.
- J. Liu, G. Li, H. J. Guo, D. W. Liu, P. Feng and Y. H. Wang, RSC Adv., 2018, 8, 10246–10254 RSC.
- B. C. Wang, Y. G. Liu, Z. H. Huang and M. H. Fang, RSC Adv., 2018, 8, 15587–15594 RSC.
- D. Kim, S. Park, B. C. Choi, S. H. Park, J. H. Jeong and J. H. Kim, Mater. Res. Bull., 2018, 97, 115–120 CrossRef.
- J. M. Xiang, J. Y. Chen, N. M. Zhang, H. B. Yao and C. F. Guo, Dyes Pigm., 2018, 154, 257–262 CrossRef.
- H. K. Liu, L. B. Liao, Y. Y. Zhang, T. S. Zhou, Q. F. Guo, L. Li and L. F. Mei, Dyes Pigm., 2017, 140, 87–91 CrossRef.
- Q. F. Guo, L. B. Liao and Z. G. Xia, J. Lumin., 2014, 145, 65–70 CrossRef.
- Z. Y. Mao, J. J. Chen, J. Li and D. J. Wang, Chem. Eng. J., 2016, 284, 1003–1007 CrossRef.
- S. L. Yuan, L. T. Wang, Y. X. Yang, F. Cheviré, F. Tessier and G. R. Chen, Ceram. Int., 2016, 42, 12508–12511 CrossRef.
- K. Li, H. Z. Lian, Y. Q. Han, M. M. Shang, R. Van Deun and J. Lin, Dyes Pigm., 2017, 139, 701–707 CrossRef.
- X. Min, Y. K. Sun, L. T. Kong, M. Guan, M. H. Fang, Y. G. Liu, X. W. Wu and Z. H. Huang, Dyes Pigm., 2018, 157, 47–54 CrossRef.
- M. F. Xia, Z. H. Ju, H. Yang, Z. B. Wang, X. P. Gao, F. X. Pan and W. S. Liu, J. Alloys Compd., 2018, 739, 439–446 CrossRef.
- S. Kaur, M. Jayasimhadri and A. S. Rao, J. Alloys Compd., 2017, 697, 367–373 CrossRef.
- J. S. Zhong, H. B. Gao, Y. J. Yuan, L. F. Chen, D. Q. Chen and Z. G. Ji, J. Alloys Compd., 2018, 735, 2303–2310 CrossRef.
- B. Li, X. Y. Huang, H. Guo and Y. J. Zeng, Dyes Pigm., 2018, 150, 67–72 CrossRef.
- Q. Y. Bai, Z. J. Wang, P. L. Li, S. C. Xu, T. Li, J. G. Cheng and Z. P. Yang, Mater. Des., 2016, 108, 597–607 CrossRef.
- N. Guo, Q. M. Liang, S. Li, R. Z. Ouyang and W. Lü, Opt. Mater., 2017, 73, 570–576 CrossRef.
- Y. M. Feng, J. P. Huang, C. M. Li, G. Hu, J. Liu and X. B. Yu, J. Alloys Compd., 2017, 706, 478–484 CrossRef.
- X. G. Zhang, L. Y. Zhou, J. X. Shi and M. L. Gong, Mater. Lett., 2014, 137, 32–35 CrossRef.
- Y. Y. Zhang, L. F. Mei, H. K. Liu, D. Yang, L. B. Liao and Z. H. Huang, Dyes Pigm., 2017, 139, 180–186 CrossRef.
- H. Guo, B. Devakumar, B. Li and X. Y. Huang, Dyes Pigm., 2018, 151, 81–88 CrossRef.
- H. K. Liu, Y. Y. Zhang, L. B. Liao, Q. F. Guo and L. F. Mei, Ceram. Int., 2014, 40, 13709–13713 CrossRef.
- X. X. Ma, L. F. Mei, H. K. Liu, L. B. Liao, K. Nie, Y. Q. Liu and Z. H. Li, Polyhedron, 2016, 119, 223–226 CrossRef.
- P. Hagenmuller, J. Phys. Chem. Solids, 1998, 59, 503–506 CrossRef.
- P. Hagenmuller, J. Power Sources, 2000, 90, 9–12 CrossRef.
- B. Wang, A. Q. Zhang, X. F. Yang, Q. Wang, Q. Q. Shen, X. G. Liu and H. S. Jia, J. Alloys Compd., 2017, 729, 117–125 CrossRef.
- M. Runowski, S. Goderski, J. Paczesny, M. Księżopolska-Gocalska, A. Ekner-Grzyb, T. Grzyb, J. D. Rybka, M. Giersig and S. Lis, J. Phys. Chem. C, 2016, 120, 23788–23798 CrossRef.
- R. J. Yu, J. Wang, M. Zhang, J. H. Zhang, H. B. Yuan and Q. Su, Chem. Phys. Lett., 2008, 453, 197–201 CrossRef.
- M. Runowski and S. Lis, J. Alloys Compd., 2014, 597, 63–71 CrossRef.
- A. Durugkar, S. Tamboli, N. S. Dhoble and S. J. Dhoble, Mater. Res. Bull., 2018, 97, 466–472 CrossRef.
- Y. Guo, B. K. Moon, B. C. Choi, J. H. Jeong, J. H. Kim and H. Choi, Ceram. Int., 2016, 42, 18324–18332 CrossRef.
- P. Du, X. Y. Huang and J. S. Yu, Chem. Eng. J., 2018, 337, 91–100 CrossRef.
- H. Guo, X. Y. Huang and Y. J. Zeng, J. Alloys Compd., 2018, 741, 300–306 CrossRef.
- K. Binnemans, Coord. Chem. Rev., 2015, 295, 1–45 CrossRef.
- X. M. Zhu and Z. F. Zhou, J. Lumin., 2017, 188, 589–594 CrossRef.
- Z. Chen, X. A. Chen, S. M. Huang and Y. X. Pan, Ceram. Int., 2016, 42, 13476–13484 CrossRef.
- H. Guo, B. Devakumar, B. Li and X. Y. Huang, Dyes Pigm., 2018, 151, 81–88 CrossRef.
- A. G. Bispo-Jr, S. A. M. Lima and A. M. Pires, J. Lumin., 2018, 199, 372–378 CrossRef.
- H. Jing, C. F. Guo, G. G. Zhang, X. Y. Su, Z. Yang and J. H. Jeong, J. Mater. Chem., 2012, 22, 13612–13618 RSC.
- N. Guo, C. Z. Jia, J. Li, Y. F. Zhao, R. Z. Ouyang and W. Lü, J. Am. Ceram. Soc., 2015, 98, 1162–1168 CrossRef.
- P. Du, Z. G. Xia and L. B. Liao, J. Lumin., 2013, 133, 226–229 CrossRef.
- H. K. Liu, Q. F. Guo, L. B. Liao and Z. G. Xia, Opt. Commun., 2013, 309, 64–67 CrossRef.
- L. Qin, P. Q. Cai, C. L. Chen, J. Wang, S. I. Kim, Y. L. Huang and H. J. Seo, J. Alloys Compd., 2018, 738, 372–378 CrossRef.
- J. Zhao, C. F. Guo, T. Li, X. Y. Su, N. M. Zhang and J. Y. Chen, Dyes Pigm., 2016, 132, 159–166 CrossRef.
- P. Du, Z. G. Xia and L. B. Liao, Mater. Res. Bull., 2011, 46, 543–546 CrossRef.
- G. Blasse, Phys. Lett. A, 1968, 28, 444–445 CrossRef.
- G. Blasse, J. Solid State Chem., 1986, 62, 207–211 CrossRef.
- Q. F. Guo, L. B. Liao, L. F. Mei and H. K. Liu, J. Solid State Chem., 2015, 232, 102–107 CrossRef.
- Q. F. Guo, L. B. Liao, L. F. Mei, H. K. Liu and Y. Hai, J. Solid State Chem., 2015, 225, 149–154 CrossRef.
- S. A. Khan, W. W. Ji, L. Y. Hao, X. Xu, S. Agathopoulos and N. Z. Khan, Opt. Mater., 2017, 72, 637–643 CrossRef.
- K. Li, D. L. Geng, M. M.Shang, Y. Zhang, H. Z. Lian and J. Lin, J. Phys. Chem. C, 2014, 118, 11026–11034 CrossRef.
- X. Chen, P. P. Dai, X. T. Zhang, C. Li, S. Lu, X. L. Wang, Y. Jia and Y. C. Liu, Inorg. Chem., 2014, 53, 3441–3448 CrossRef PubMed.
- Z. G. Xia and Q. L. Liu, Prog. Mater. Sci., 2016, 84, 59–117 CrossRef.
- R. Zhang, B. Y. Wang, P. Zhou, X. L. Wu, X. H. Huang and B. Wang, Mater. Lett., 2018, 221, 31–34 CrossRef.
- L. F. Mei, J. Xie, H. K. Liu, L. B. Liao, Y. Y. Zhang and M. L. Li, Opt. Commun., 2015, 335, 90–93 CrossRef.
- S. Mahlik, F. Diaz and P. Boutinaud, J. Lumin., 2017, 191, 18–21 CrossRef.
- H. Ye, M. Y. He, T. S. Zhou, Q. F. Guo, J. L. Zhang, L. B. Liao, L. F. Mei, H. K. Liu and M. Runowski, J. Alloys Compd., 2018, 757, 79–86 CrossRef.
| This journal is © The Royal Society of Chemistry 2018 |