Open Access Article
Open Access ArticleEvaluation of anti α-D-Glcp-(1→4)-α-D-Glcp (GAGA4) IgM antibodies as a biomarker for multiple sclerosis†
Chriselle D. Braganzaab,
Kristiana T. Santosoab,
Emma M. Dangerfieldab,
Anne C. La Flammebc,
Mattie S. M. Timmer *ab and
Bridget L. Stocker*ab
*ab and
Bridget L. Stocker*ab
aSchool of Chemical and Physical Sciences, Victoria University of Wellington, P. O. Box 600, Wellington 6140, New Zealand
bCentre for Biodiscovery, Victoria University of Wellington, P. O. Box 600, Wellington 6140, New Zealand. E-mail: mattie.timmer@vuw.ac.nz; bridget.stocker@vuw.ac.nz
cMalaghan Institute of Medical Research, Victoria University of Wellington, P. O. Box 7060, Wellington 6242, New Zealand
First published on 6th August 2018
Abstract
The correct diagnosis of multiple sclerosis (MS) remains challenging due to the complex pathophysiological and clinical characteristics of the disease. Consequently, there has been immense interest in finding a non-invasive diagnostic test for MS. Recent studies found that serum anti-α-D-Glcp-(1→4)-α-D-Glcp (GAGA4) IgM antibodies were upregulated in MS patients, and this finding led to the development of a commercial diagnostic test (gMS® Dx test), although the test has poor selectivity and has not been independently validated. Herein, we developed an enzyme-linked immunosorbent assay (ELISA) to evaluate the use and reliability of several anti-glucose IgM antibodies, including those against GAGA4, as diagnostic biomarkers for MS. In contrast to previous studies, our results show that serum anti-GAGA4 IgM antibody levels are not significantly higher in MS patients, which could potentially explain the poor selectivity of the commercial test.
Introduction
Multiple sclerosis (MS) is a chronic autoimmune disease characterized by damage to the protective myelin sheaths that insulate nerve fibers.1 Demyelination of the nerve fibers impairs or prevents the transduction of signals throughout the central nervous system (CNS) resulting in an array of symptoms such as fatigue, blurred vision, muscle weakness and cognitive impairment.2 Symptoms can vary widely among individuals and consequently, MS diagnosis is often difficult and requires a combination of blood tests, magnetic resonance imaging, and evoked potential tests, which measure the electrical conduction of nerves.3 Cerebrospinal fluid, obtained via lumbar puncture, can also be analyzed for the presence of oligoclonal IgG bands that are indicative of inflammation in the CNS,4 however this procedure is non-specific to MS and is difficult to repeat regularly due to its invasive nature.5To develop a non-invasive diagnostic tool for MS, recent research has focused on serum-derived antibodies as potential biomarkers for the disease.6,7 In some studies, high levels of anti-myelin oligodendrocyte glycoprotein and anti-myelin basic protein IgM antibodies have been suggested to predict early relapse in MS patients,8 however in other studies, no such correlation was observed.9 A multitude of other antibodies have also been studied for their potential use as biomarkers for MS, including antibodies against the Epstein Barr nuclear antigen,10 heat shock proteins,11,12 and complement regulators,13,14 although none have been successfully validated in a clinical setting.7,15
Recently, anti-glycan IgM antibodies have been described as potential biomarkers for MS.16,17 In 2005, Lolli et al. observed high IgM autoantibody titers against the N-glucosylated peptide CSF114(Glc) in patients with RRMS.16 It was determined that a native N-linked β-D-glucopyranose-asparagine moiety was the minimal epitope required for antibody binding. More recently, it was observed that purified N-glucosylated glycoproteins from Haemophilus influenzae were preferentially recognized by antibodies from a subpopulation of MS patients, thereby providing the first example of an N-glucosylated native antigen for antibodies in MS.18 In 2003, Schwarz et al. immobilized a range of glycans onto a glass chip via a cyanuric 1,8-diamino-3,6-dioxaoctane linker19 and used this glycan array to test for serum anti-glycan antibodies.17,19–21 From this work, it was determined that serum anti-α-glucose (α-Glc) IgM antibodies, specifically those against α-D-Glcp-(1→4)-α-D-Glcp (GAGA4) (Fig. 1), were significantly up-regulated in MS patients. These findings then led to the development of a commercial blood test known as the gMS® Dx test, which claims to have a high positive predictive value for MS and the ability to differentiate MS from other neurological disorders.17,20,21 However, the test has a low sensitivity rate of 33.7%,21 and has not been widely validated. Moreover, others have identified antibodies against several α-glucosyl antigens in healthy donors.22–24
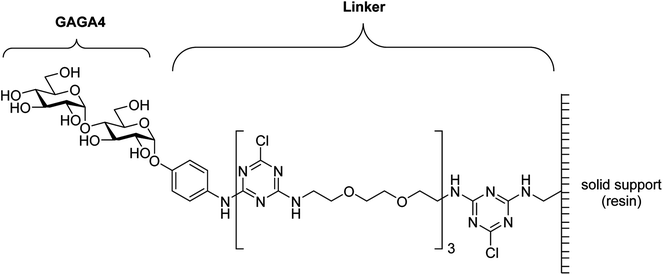 | ||
| Fig. 1 Structure of GAGA4 with the cyanuric linker.19 | ||
Given the poor selectivity of the gMS® Dx test, we sought to independently validate the potential of a variety of glucosyl antigens for the detection of IgM antibodies as diagnostic biomarkers for MS. To this end, we synthesized a range of α- and β-glucose–glycoprotein conjugates, which contain native glycosidic linkages at their reducing ends to avoid potential non-specific antibody responses. These conjugates were then used in an enzyme-linked immunosorbent assay (ELISA) with serum samples from healthy donors and patients with relapsing-remitting multiple sclerosis (RRMS) to ascertain whether differences in specific glycan IgM responses were observed between the two patient populations. Our results indicate that anti-glycan IgM antibody levels vary tremendously among individuals and as a result, no specific anti-glycan IgM response was seen for patients with RRMS compared to healthy controls. The findings of our study provide insight into the poor selectivity observed with the commercial gMS® Dx test.
Results and discussion
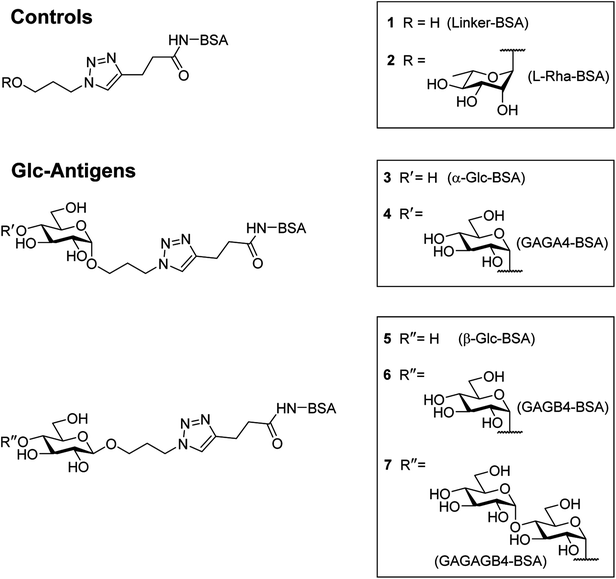
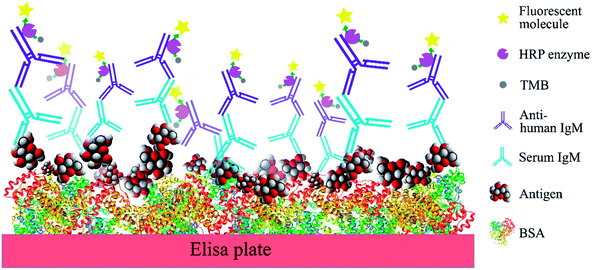
In order to compare the level of anti-glycan IgM antibodies in patients with RRMS and healthy controls (HC), we aim to prepare carbohydrate antigens and control glycoprotein conjugates 1–7 (Fig. 2). Here, the free azidopropyl linker-BSA conjugate (1) will be used as a negative control to measure background titers. L-Rha-BSA (2) will be used as the positive control as previous studies on human anti-glycan antibodies revealed that anti-L-rhamnose (L-Rha) antibodies were present in high levels in the majority of subjects.22,25,26 The synthesis of a selection of α- and β-linked glucose-containing antigens (3–7) will also be undertaken, including glycoconjugate 4, which contains the GAGA4-motif previously suggested to be up-regulated in patients with MS.17 Similarly, synthesis and analysis of glycoconjugate 7 containing D-maltotriose will allow for investigations into whether this glycoconjugate can elicit a similar IgM antibody response to that seen with GAGA4, since 7 also possesses the GAGA4 moiety at its terminus. Use of the simple triazole-functionalized linker is envisioned to eliminate the high levels of non-specific binding observed with more complex linkers.27To measure serum anti-glycan IgM levels, we will use an ELISA protocol that presents the antigens in such a way that their carbohydrate moieties are exposed for antibody binding (Fig. 3). This will be achieved by first coating the plates with the antigen–BSA conjugates, followed by incubation with serum samples. Incubation with the secondary anti-human IgM antibody that is coupled to horseradish peroxidase will then allow for the transformation of 3,3′,5,5′-tetramethylbenzidine (TMB) and thus, determination of the level of bound anti-glycan IgM antibodies.
Synthesis of antigens
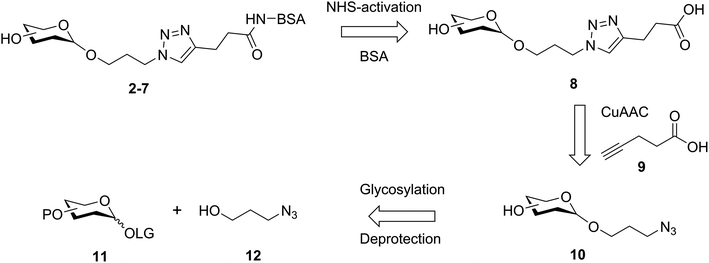
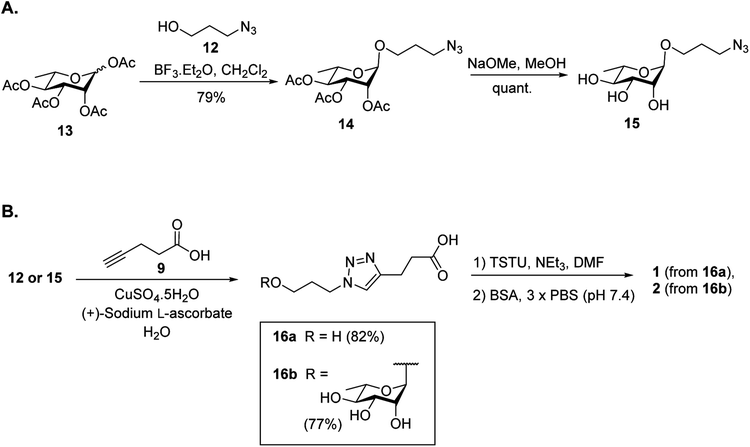
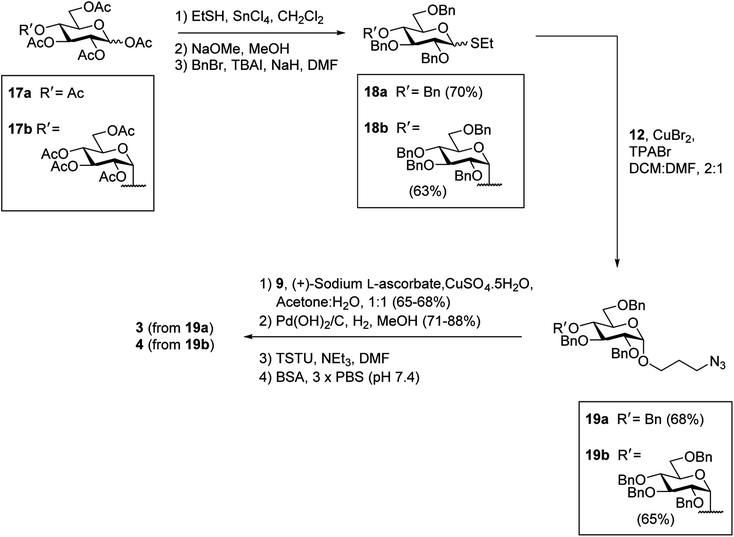
It was proposed that glyconconjuates 2–7 could be prepared from the corresponding free carboxylic acid 8 via the formation of N-hydroxysuccinimide (NHS)-activated ester and subsequent conjugation to bovine serum albumin (BSA) (Scheme 1). Carboxylic acid 8, in turn, should be accessible by way of a copper catalyzed azide-alkyne Huisgen 1,3-dipolar cycloaddition (CuAAC)28 of 4-pentynoic acid (9) with azide-functionalized glycan 10, whereby the glycan would be prepared via a glycosylation reaction of suitably protected sugar 11 with 3-azidopropanol (12). Here, the stereochemistry at the anomeric position would be controlled through the use of coupling conditions that favor the formation of the theromodynamic product (α-anomer), or through neighboring group participation (β-anomer). Preparation of the free azidopropyl linker-BSA conjugate (1) would be achieved via the CuAAC of 3-azidopropanol (12) with 4-pentynoic acid (9) and subsequent protein conjugation.With a suitable synthetic strategy in place, we first undertook the synthesis of the free azidopropyl linked-BSA conjugate (1) and the positive control Rha-BSA (2) (Scheme 2). Glycosylation of peracetylated L-rhamnose (13) with 3-azidopropanol (12) using BF3·Et2O as the activator gave glycoside 14 as the α-anomer [J1,2 = 1.5 Hz] exclusively (Scheme 2A). The acetyl protecting groups in 14 were subsequently removed under Zemplén conditions29 to give deprotected azidopropyl L-Rha glycoside 15. Installation of a carboxylic acid group onto 3-azidopropanol (12) or azidopropyl rhamnopyranoside 15 was then achieved via CuAAC28 with 4-pentynoic acid (9) to give the corresponding carboxy-modified triazoles 16a and 16b in good yields of 82% and 77%, respectively. Each derivative was then converted to its corresponding NHS ester using an activated form of NHS, N,N,N′,N′-tetramethyl-O-(N-succinimidyl)uronium tetrafluoroborate (TSTU),26 in the presence of NEt3 (Scheme 2B). The NHS esters were then cross-linked to BSA26 to yield free azidopropyl linked-BSA conjugate 1 and L-rha-BSA conjugate 2. Analysis of the final conjugates was then undertaken using MALDI-TOF spectrometry to determine the average molecular weight of the protein after conjugation to the sugar, which was used to estimate the number of antigens per protein molecule (Fig. S1†). Here, conjugate 1 was observed to contain an average of 42 linker molecules per BSA, and conjugate 2 contained 35 rhamnose molecules per BSA.
Having successfully synthesized the azidopropyl linked-BSA conjugate 1 and the L-Rha-BSA conjugate 2, we then used a similar strategy to prepare the glucose-functionalized antigens. Synthesis of the α-linked antigens began with the preparation of the thioglycosides30 of per-acetylated D-glucose (17a) and per-acetylated D-maltose (17b), with subsequent deacetylation29 and benzylation31 to give the fully protected thioglycosides 18a and 18b, respectively, in good overall yields (Scheme 3). To achieve good α-selectivity during the ensuing glycosylation reactions with 3-azidopropanol (12), we optimized glycosylation reaction conditions previously reported by Sato et al.,32 and used CuBr2 (3 equiv.) and tetrapropylammonium bromide (TPABr) (3 equiv.) as mild activators. Here, the glycosylation reactions occurred via the corresponding glycosyl bromide intermediates, which underwent in situ anomerisation33 to form the desired benzylated glycosides in 85% yields with a 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 α
1 α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β ratio. The protected α-glycosides were readily separated from their β-glycoside counterparts via silica gel flash column chromatography to give the target products 19a [J1,2 = 3.8 Hz] and 19b [J1,2 = 3.7 Hz] in 68% and 65% isolated yields, respectively. Carboxylic acid groups were then installed on the benzylated α-glycosides through the use of CuAAC reactions with 4-pentynoic acid (9), followed by hydrogenolysis using H2 gas and Pd(OH)2 and treatment with TSTU to afford the NHS esters. Conjugation of the activated esters to BSA then gave the target α-linked Glc-antigens α-Glc-BSA (3) and GAGA4-BSA (4). MALDI-TOF analysis of glycoconjugates 3 and 4 showed average loadings of 31 and 25 glycans per BSA, respectively (Fig. S1†).
β ratio. The protected α-glycosides were readily separated from their β-glycoside counterparts via silica gel flash column chromatography to give the target products 19a [J1,2 = 3.8 Hz] and 19b [J1,2 = 3.7 Hz] in 68% and 65% isolated yields, respectively. Carboxylic acid groups were then installed on the benzylated α-glycosides through the use of CuAAC reactions with 4-pentynoic acid (9), followed by hydrogenolysis using H2 gas and Pd(OH)2 and treatment with TSTU to afford the NHS esters. Conjugation of the activated esters to BSA then gave the target α-linked Glc-antigens α-Glc-BSA (3) and GAGA4-BSA (4). MALDI-TOF analysis of glycoconjugates 3 and 4 showed average loadings of 31 and 25 glycans per BSA, respectively (Fig. S1†).
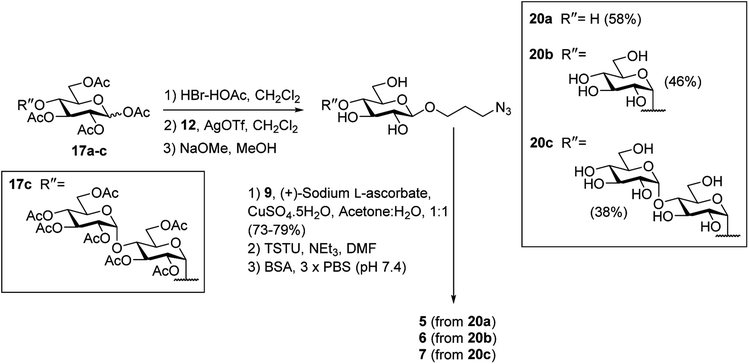
To prepare the β-Glc antigens, per-acetylated D-glucose (17a), per-acetylated D-maltose (17b), and peracetylated D-maltotriose (17c) were converted to the corresponding α-glycosyl bromides using HBr-HOAc,34 before undergoing glycosylation with acceptor 12 in the presence of AgOTf to selectively produce the β-linked products (Scheme 4). Deacetylation29 then furnished the deprotected β-azidopropyl glycosides 20a–c in good overall yields (38–58%). Here, selective formation of the β-product was confirmed by 1H NMR [J1,2 = 7.9 Hz (20a), 8.0 Hz (20b) and 8.0 Hz (20c)]. Formation of the NHS-activated esters was then undertaken as described above, with conjugation to BSA affording the target antigens β-Glc-BSA (5), GAGB4-BSA (6) and GAGAB4-BSA (7). Average glycan loadings of 25 glycans per BSA for glycoconjugates 5 and 6, and 21 glycans per BSA for glycoconjugate 7 were determined by MALDI-TOF analysis (Fig. S1†).
Serum anti-glycan IgM levels
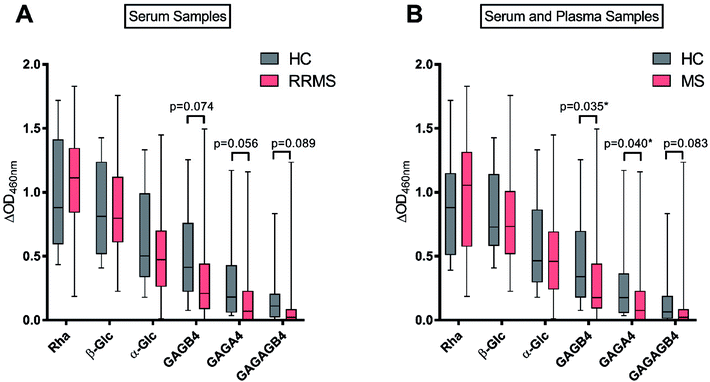
With the antigen–BSA conjugates in hand, we then determined serum anti-glycan IgM levels in RRMS patients and HC. To this end, the sera from 10 HC and 40 RRMS patients were analysed by ELISA for IgM antibodies specific to each of the carbohydrate antigens Rha (2), α-Glc (3), GAGA4 (4), β-Glc (5), GAGB4 (6) and GAGAGB4 (7) (Fig. 4A). To account for non-specific binding, IgM titres against the free azidopropyl linker-BSA conjugate (1) for each subject were subtracted from the IgM titres obtained for each glycan. An additional cohort of plasma samples (6 HC and 8 MS) were also analysed in addition to the previous sera samples in order to evaluate any possible differences in sample type (Fig. 4B).As anticipated, the highest IgM response was observed against the positive control Rha (2), in the sera of both HC and RRMS patients (Fig. 4A), and a similar response was observed when plasma samples were included (Fig. 4B). This result is consistent with previous findings by Huflejt et al., where all 106 tested healthy subjects showed high anti-Rha antibody titres.22 High IgM titres were also observed towards β-Glc (5) for most subjects, which is again consistent with previous studies.22,25 No significant difference was observed between HC and MS patients for Rha- or β-Glc-specific IgM levels in serum or plasma.
In previous studies by Schwarz et al., it was demonstrated that anti-α-Glc serum IgM levels were higher in RRMS patients compared to HC.17 In contrast, we observed no difference between the anti-α-Glc (3) IgM levels of RRMS patients and HC. Brettschneider et al. observed elevated levels of anti-GAGA4 IgM antibodies in RRMS patients and determined that this difference allowed MS patients to be differentiated from HC and patients with other neurological diseases.21 These findings by Brettschneider et al. formed the basis of the gMS® Dx test for the early detection of MS. We, however, did not observe increased levels of anti-GAGA4 (4) IgM antibodies in RRMS patients. Instead, a lower level of these antibodies was observed when compared to HC, with this difference trending towards significance (p = 0.056) when serum samples were used. When combined with results from the plasma samples, however, the difference in anti-GAGA4 IgM levels became significantly lower (p = 0.040).
To investigate whether the reducing-end glycosidic linkage influenced IgM levels, GAGB4 (6) was included in our study. Similar to the results obtained for GAGA4 (4), we observed a decrease in anti-GAGB4 IgM levels in RRMS patients compared to HC, with the difference becoming significant (p = 0.035) when plasma samples were tested. Serum IgM antibody levels to GAGAGB4 (7) were also measured in order to compare these results to those obtained for GAGA4 (4) since both contain the same terminal structural motif. We observed anti-GAGAGB4 IgM levels that were even lower than those measured for GAGA4, with the IgM titres being lower in RRMS patients and the difference trending towards significance (p = 0.083).
Overall, for each of the antigens, the HC group demonstrated either comparable or higher IgM levels compared to the RRMS population. In addition, we observed a reduction in IgM levels as the length of the carbohydrate scaffold was increased, which highlights the impact of the carbohydrate chain length on IgM binding. No significant upregulation of anti-glycan IgM antibody responses was detected for RRMS patients in this study, which is in contrast to earlier studies by Schwarz et al.17 and Brettschneider et al.21 Moreover, as illustrated in our work, and that of others,22 anti-glycan IgM levels vary strongly amongst the human population. Due to this extensive variability in human antibody levels, it is challenging to use IgM antibodies as biomarkers for disease, and our findings may go some way in explaining the poor selectivity of 33.7% observed when using the gMS® Dx test.21
Conclusion
In summary, the results of this study show that the IgM antibody response to the simple GAGA4 moiety is not upregulated in RRMS patients. In contrast, we observed a lower level of anti-GAGA4 IgM antibodies in RRMS patients, thereby highlighting the challenge in finding a suitable antibody biomarker for MS. Thus, there still remains an unmet need for a non-invasive, early diagnostic tool for MS. Whether a suitable antigen with a more robust and reproducible antibody response can be found to meet this need, remains to be seen.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors would like to thank NZ Lottery Health Research (340915/13) and the Health Research Council (Hercus Fellowship, BLS, 2013/33) for financial support. We are extremely grateful to Liz Goode, Kathryn Hally, Dr Lisa Johnston and Carl Beyers for collecting the blood samples used in this study. We would like to thank Sven Sondhauss for helping with the MALDI-TOF and Vimal Patel for helping with the ELISA protocol. We would also like to thank Dr Lisa Woods for assistance with statistical analysis of the data.References
- W. Brück, J. Neurol., 2005, 252, v3–v9 CrossRef PubMed.
- A. Huntley and E. Ernst, Complement. Ther. Med., 2000, 8, 97–105 CrossRef PubMed.
- D. Karussis, J. Autoimmun., 2014, 48–49, 134–142 CrossRef PubMed.
- G. P. Owens, J. L. Bennett, D. H. Gilden and M. P. Burgoon, Neurol. Res., 2006, 28, 236–244 CrossRef PubMed.
- U. Ziemann, M. Wahl, E. Hattingen and H. Tumani, Prog. Neurobiol., 2011, 95, 670–685 CrossRef PubMed.
- M. Reindl, M. Khalil and T. Berger, J. Neuroimmunol., 2006, 180, 50–62 CrossRef PubMed.
- J. J. Graber and S. Dhib-Jalbut, J. Neurol. Sci., 2011, 305, 1–10 CrossRef PubMed.
- T. Berger, P. Rubner, F. Schautzer, R. Egg, H. Ulmer, I. Mayringer, E. Dilitz, F. Deisenhammer and M. Reindl, N. Engl. J. Med., 2003, 349, 139–145 CrossRef PubMed.
- J. Kuhle, C. Pohl, M. Mehling, G. Edan, M. S. Freedman, H.-P. Hartung, C. H. Polman, D. H. Miller, X. Montalban, F. Barkhof, L. Bauer, S. Dahms, R. Lindberg, L. Kappos and R. Sandbrink, N. Engl. J. Med., 2007, 356, 371–378 CrossRef PubMed.
- K. Munger, L. Levin, E. O'Reilly, K. Falk and A. Ascherio, Mult. Scler. J., 2011, 17, 1185–1193 CrossRef PubMed.
- S. Chiba, S. Yokota, K. Yonekura, S. Tanaka, H. Furuyama, H. Kubota, N. Fujii and H. Matsumoto, J. Neurol. Sci., 2006, 241, 39–43 CrossRef PubMed.
- F. J. Quintana, M. F. Farez, V. Viglietta, A. H. Iglesias, Y. Merbl, G. Izquierdo, M. Lucas, A. S. Basso, S. J. Khoury, C. F. Lucchinetti, I. R. Cohen and H. L. Weiner, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 18889–18894 CrossRef PubMed.
- C. Pintér, S. Beltrami, D. Caputo, P. Ferrante and A. Clivio, J. NeuroVirol., 2000, 6, S42–S46 Search PubMed.
- G. Ingram, S. Hakobyan, N. P. Robertson and B. P. Morgan, Clin. Exp. Immunol., 2009, 155, 128–139 CrossRef PubMed.
- G. Ingram, J. J. Bugert, S. Loveless and N. P. Robertson, Eur. J. Neurol., 2010, 17, 1386–1389 CrossRef PubMed.
- F. Lolli, B. Mulinacci, A. Carotenuto, B. Bonetti, G. Sabatino, B. Mazzanti, A. M. D'Ursi, E. Novellino, M. Pazzagli, L. Lovato, M. C. Alcaro, E. Peroni, M. C. Pozo-Carrero, F. Nuti, L. Battistini, G. Borsellino, M. Chelli, P. Rovero and A. M. Papini, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10273–10278 CrossRef PubMed.
- M. Schwarz, L. Spector, M. Gortler, O. Weisshaus, L. Glass-Marmor, A. Karni, N. Dotan and A. Miller, J. Neurol. Sci., 2006, 244, 59–68 CrossRef PubMed.
- M. T. C. Walvoort, C. Testa, R. Eilam, R. Aharoni, F. Nuti, G. Rossi, F. Real-Fernandez, R. Lanzillo, V. Brescia Morra, F. Lolli, P. Rovero, B. Imperiali and A. M. Papini, Sci. Rep., 2016, 6, 39430 CrossRef PubMed.
- M. Schwarz, L. Spector, A. Gargir, A. Shtevi, M. Gortler, R. T. Altstock, A. A. Dukler and N. Dotan, Glycobiology, 2003, 13, 749–754 CrossRef PubMed.
- M. S. Freedman, J. Laks, N. Dotan, R. T. Altstock, A. A. Dukler and C. J. Sindic, Mult. Scler., 2009, 15, 422–430 CrossRef PubMed.
- J. Brettschneider, T. D. Jaskowski, H. Tumani, S. Abdul, D. Husebye, H. Seraj, H. R. Hill, E. Fire, L. Spector, J. Yarden, N. Dotan and J. W. Rose, J. Neuroimmunol., 2009, 217, 95–101 CrossRef PubMed.
- M. E. Huflejt, M. Vuskovic, D. Vasiliu, H. Xu, P. Obukhova, N. Shilova, A. Tuzikov, O. Galanina, B. Arun, K. Lu and N. Bovin, Mol. Immunol., 2009, 46, 3037–3049 CrossRef PubMed.
- D. Bello-Gil, N. Khasbiullina, N. Shilova, N. Bovin and R. Mañez, Front. Immunol., 2017, 8, 1449–1516 CrossRef PubMed.
- S. M. Muthana and J. C. Gildersleeve, Sci. Rep., 2016, 6, 19509–19520 CrossRef PubMed.
- O. Oyelaran, L. M. McShane, L. Dodd and J. C. Gildersleeve, J. Proteome Res., 2009, 8, 4301–4310 CrossRef PubMed.
- W. Chen, L. Gu, W. Zhang, E. Motari, L. Cai, T. J. Styslinger and P. G. Wang, ACS Chem. Biol., 2011, 6, 185–191 CrossRef PubMed.
- R. Adamo, Q.-Y. Hu, A. Torosantucci, S. Crotti, G. Brogioni, M. Allan, P. Chiani, C. Bromuro, D. Quinn, M. Tontini and F. Berti, Chem. Sci., 2014, 5, 4302–4311 RSC.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596–2599 CrossRef PubMed.
- G. Zemplén, A. Gerecs and I. Hadácsy, Ber. Dtsch. Chem. Ges., 1936, 69, 1827–1829 CrossRef.
- C. S. Barry, E. J. Cocinero, P. Çarçabal, D. P. Gamblin, E. C. Stanca-Kaposta, S. M. Remmert, M. C. Fernández-Alonso, S. Rudić, J. P. Simons and B. G. Davis, J. Am. Chem. Soc., 2013, 135, 16895–16903 CrossRef PubMed.
- S. Czernecki, C. Georgoulis and C. Provelenghiou, Tetrahedron Lett., 1976, 17, 3535–3536 CrossRef.
- S. Sato, M. Mori, Y. Ito and T. Ogawa, Carbohydr. Res., 1986, 155, C6–C10 CrossRef.
- R. U. Lemieux, K. B. Hendriks, R. V. Stick and K. James, J. Am. Chem. Soc., 1975, 97, 4056–4062 CrossRef.
- E. Fischer, Ber. Dtsch. Chem. Ges., 1911, 44, 1898–1904 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra04897e |
| This journal is © The Royal Society of Chemistry 2018 |