Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Controlled organocatalyzed D,L-lactide ring-opening polymerizations: synthesis of low molecular weight oligomers†
M. R. Newman ab,
S. G. Russellc and
D. S. W. Benoit
ab,
S. G. Russellc and
D. S. W. Benoit *abc
*abc
aDepartment of Biomedical Engineering, University of Rochester, Rochester, NY 14627, USA. E-mail: benoit@bme.rochester.edu
bCenter for Musculoskeletal Research, University of Rochester Medical Center, Rochester, NY 14642, USA
cDepartment of Chemical Engineering, University of Rochester, Rochester, NY 14627, USA
First published on 14th August 2018
Abstract
A systematic approach to the synthesis of organocatalyzed oligo(D,L-lactide) demonstrates that choice of initiator, catalytic ratio, and reaction time yields well-controlled oligomers. Ring-opening polymerization of D,L-lactide with the initiator α-methyl propargyl alcohol, a secondary alcohol, used in excess of 4-dimethylaminopyridine catalyst mitigates cyclicization, transesterification, and catalyst-initiated side reactions. This approach enables the design of uniform lactide oligomers for controlled release applications, such as delivery systems for drugs, prodrugs, and molecular sensors.
Poly(lactide) (PLA) is a versatile polymer with properties suitable for a range of controlled release applications. Molecular weight and copolymer composition control degradation and drug release, with degradation yielding biocompatible lactic acid products. PLA and copolymers of lactide and glycolide (PLGA) have been used pre-clinically and clinically for release of drugs,1–3 prodrugs,4–6 and molecular sensors.7–9 Highly reproducible PLA chemistries are particularly important to ensure control over the release of drugs with narrow therapeutic windows and to enhance efficacy by reducing dependence on patient compliance.10–13 Stannous octoate is commonly employed as an organometallic catalyst of lactide ring-opening polymerization (ROP).14,15 However, tin catalysts, which are challenging to fully remove during purification, can result in toxicity.16 Alternatives such as strongly basic amine organocatalysts are favored, particularly 4-dimethylaminopyridine (DMAP), which was pioneered by Nederberg et al.17 DMAP-mediated ROP is used for one-pot PLA polymerizations and conjugations,18 diblock or triblock copolymerizations with lactide as a first or second block,19–21 grafting lactide to cellulose polymer fibers,22 and synthesizing star-shaped/cross-linked PLA networks.23 PLA has also been exploited to tether and release drugs from linear polymers and hydrogel depots.24 Drug tethers are typically low molecular weight oligo(lactide) composed of fewer than seven lactide repeat units to avoid crystallinity and associated challenges with solubility and control over degradation rates.25 Self-catalysis, or direct polycondensation, of lactic acid at increased temperature and reduced pressure for an extended time yields low-molecular weight (800–3200 Da) oligo(lactide), in contrast to organocatalysts that are commonly employed for high molecular weight PLA synthesis. However, extensive setup and environmental control decrease the accessibility of these reactions.26,27 Furthermore, with neither an initiator nor a catalyst used in polycondensation, mixtures of α-hydroxy and ω-carboxy PLA are formed, limiting the versatility of post-polymerization drug functionalization.28 Alternatively, click chemistry29 may be exploited to control subsequent modification of oligo(lactide) by employing a ‘clickable’ alcohol to initiate oligo(lactide) synthesis.30
Here, we explored bulk ROP of D,L-lactide by propargyl alcohol initiator and DMAP catalyst to synthesize low molecular weight oligo(lactide) linkers. To enable subsequent click reactions and to mitigate crystallinity, propargyl alcohol (PA) and D,L-lactide (L) were used.27 Although not studied here, similar approaches have shown products do not epimerize, and we expect oligomers to be atactic and amorphous.17 To investigate the molecular weight and polydispersity of oligo(lactide), an initial polymerization was designed similar to that of Nederberg et al.17 and Coulembier and Dubois18 using a PA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMAP ratio of 1
DMAP ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
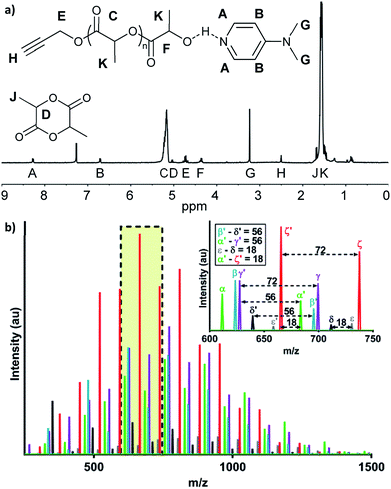
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (Scheme 1; see Table S1, ESI†). The neat polymerization was stirred at 130 °C under a nitrogen environment. After 5, 10, 15, 30, and 60 minutes, reaction vials were opened to atmosphere and cooled before dissolution in dichloromethane (DCM) and precipitation in hexanes. 1H-NMR spectroscopy identified successful synthesis of PA-functional oligo(D,L-lactide) (PA-ODLA) with ∼99% conversion of D,L-lactide after only 5 minutes of polymerization (Fig. 1a; see Fig. S3, ESI†). Integration of peaks C, E, and F indicated linkers were ∼19 lactide units, or an average Mn of 2825 Da. However, matrix assisted laser desorption ionization time of flight mass spectrometry suggested Mn was 752 Da. As Mn determined by NMR was based on average end-group analysis and assumed PA-initiated oligo(lactide), and Mn determined by MALDI analysis represented all species present, these data indicated that not all oligo(lactide) chains were initiated by PA. Rather, distinct series of peaks periodically separated by 144 Da, the Mn of lactide, were formed during polymerization (α, β, γ, δ, ε, and ζ; Fig. 1b; see Table S2, ESI†). Peaks 72 Da less than these peaks were also identified (α′–ζ′). Additional reactions were conducted to identify the formed products: one with the initiator α-methyl propargyl alcohol (αMPA; see Fig. S4, ESI†), and another with only D,L-lactide and DMAP but no initiator (see Fig. S9, ESI†).
4 (Scheme 1; see Table S1, ESI†). The neat polymerization was stirred at 130 °C under a nitrogen environment. After 5, 10, 15, 30, and 60 minutes, reaction vials were opened to atmosphere and cooled before dissolution in dichloromethane (DCM) and precipitation in hexanes. 1H-NMR spectroscopy identified successful synthesis of PA-functional oligo(D,L-lactide) (PA-ODLA) with ∼99% conversion of D,L-lactide after only 5 minutes of polymerization (Fig. 1a; see Fig. S3, ESI†). Integration of peaks C, E, and F indicated linkers were ∼19 lactide units, or an average Mn of 2825 Da. However, matrix assisted laser desorption ionization time of flight mass spectrometry suggested Mn was 752 Da. As Mn determined by NMR was based on average end-group analysis and assumed PA-initiated oligo(lactide), and Mn determined by MALDI analysis represented all species present, these data indicated that not all oligo(lactide) chains were initiated by PA. Rather, distinct series of peaks periodically separated by 144 Da, the Mn of lactide, were formed during polymerization (α, β, γ, δ, ε, and ζ; Fig. 1b; see Table S2, ESI†). Peaks 72 Da less than these peaks were also identified (α′–ζ′). Additional reactions were conducted to identify the formed products: one with the initiator α-methyl propargyl alcohol (αMPA; see Fig. S4, ESI†), and another with only D,L-lactide and DMAP but no initiator (see Fig. S9, ESI†).
 | ||
| Scheme 1 Ring-opening polymerization of lactide by alcohol initiator. X = H, propargyl alcohol; CH3, α-methyl propargyl alcohol. | ||
The desired product, PA-ODLA “α” (Fig. 1a), was formed via DMAP base activation of PA, which initiated ROP (see Fig. S5, ESI†). Base activation of the alcohol was confirmed similar to previous reports20,31 using 1H-NMR of PA mixed with DMAP in CDCl3 to identify ppm shifts for hydroxyl groups (see Fig. S6, ESI†). Peak α′, 72 Da less than α, was the result of transesterification of PA-ODLA (see Fig. S7, ESI†), which is a common side-reaction during ROP of lactide.18,20 Two undesired products, peaks ζ and ζ′, were cyclic PA-ODLA with and without transesterification, respectively. Cyclicization increases over time during ROP of lactide32–34 and is undesired because hydroxyl end groups are not available for subsequent conjugation. These peaks were due to radical-mediated dehydration with hydroxyl end group participation,35 as the addition of hydroquinone (HQ), a radical scavenger, eliminated peaks ζ and ζ′ (see Fig. S8, ESI†). However, a peak appeared that corresponded to HQ-DMAP-ODLA and lacked alkyne functionalities for subsequent click reactions.
Another undesired product, DMAP-ODLA, or peak γ, formed via DMAP nucleophilic attack of lactide (see Fig. S9, ESI†). Peak γ′, separated by 72 Da from peak γ, resulted from transesterification of DMAP-ODLA. By conducting reactions of only DMAP and lactide, only PA and lactide, and only αMPA and lactide, it was confirmed that DMAP can both initiate and catalyze lactide ROP, but PA and αMPA cannot (see Fig. S10, ESI†). DMAP-ODLA formed due to PA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMAP ratios less than 1, as previously described,18 and is undesired because ‘clickable’ propargyl end groups are not present. Finally, peaks β, δ, and ε are likely ion fragments, as these peaks were only present when using MALDI in linear, but not reflector, mode (see Fig. S11, ESI†).
DMAP ratios less than 1, as previously described,18 and is undesired because ‘clickable’ propargyl end groups are not present. Finally, peaks β, δ, and ε are likely ion fragments, as these peaks were only present when using MALDI in linear, but not reflector, mode (see Fig. S11, ESI†).
To increase the amount of oligo(lactide) with alkyne and hydroxyl functionalities, side reactions were systematically addressed. It was noted that transesterification increased with time of polymerization and was greater for PA than for αMPA, as less nucleophilic secondary alcohols are unable to participate in transesterification reactions.30 Thus, reactions were conducted at 130 °C for 5 minutes using αMPA as the initiator to optimize desired product (αMPA-ODLA, peak α).
Ratios of αMPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMAP were investigated to mitigate undesired DMAP-initiated and cyclic ODLA (Table 1). Holding αMPA
DMAP were investigated to mitigate undesired DMAP-initiated and cyclic ODLA (Table 1). Holding αMPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L constant and increasing αMPA
L constant and increasing αMPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMAP increased the intensity of cyclic αMPA-ODLA ζ relative to DMAP-ODLA γ, but αMPA-ODLA α became negligible (Fig. 2a; see Fig. S12, ESI†). Increasing αMPA
DMAP increased the intensity of cyclic αMPA-ODLA ζ relative to DMAP-ODLA γ, but αMPA-ODLA α became negligible (Fig. 2a; see Fig. S12, ESI†). Increasing αMPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L at a constant αMPA
L at a constant αMPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMAP increased the intensity of α relative to both ζ and γ (Fig. 2b; see Fig. S13, ESI†). Similar behavior was identified when holding L
DMAP increased the intensity of α relative to both ζ and γ (Fig. 2b; see Fig. S13, ESI†). Similar behavior was identified when holding L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMAP constant and increasing αMPA
DMAP constant and increasing αMPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L (Fig. 2c; see Fig. S14, ESI†). These results suggest that αMPA
L (Fig. 2c; see Fig. S14, ESI†). These results suggest that αMPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L controls cyclicization, as the distribution of ζ is similar among reactions in Fig. 2a, and the ratio of ζ
L controls cyclicization, as the distribution of ζ is similar among reactions in Fig. 2a, and the ratio of ζ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) α decreases between ratios of 1
α decreases between ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and 1
10 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in Fig. 2b. αMPA
2 in Fig. 2b. αMPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMAP controls DMAP-ODLA generation, as the distribution of γ is similar within Fig. 2b and smallest at a ratio of 2
DMAP controls DMAP-ODLA generation, as the distribution of γ is similar within Fig. 2b and smallest at a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Finally, lower ratios of L
1. Finally, lower ratios of L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMAP appear to increase α, with 2
DMAP appear to increase α, with 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 yielding the greatest amount of desired product α. Altogether, higher αMPA
1 yielding the greatest amount of desired product α. Altogether, higher αMPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L, higher αMPA
L, higher αMPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMAP, and lower L
DMAP, and lower L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMAP ratios yield higher levels of α relative to γ and ζ.
DMAP ratios yield higher levels of α relative to γ and ζ.
αMPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMAP DMAP |
X | Mn (NMR) | Mn (MALDI) | PDI |
|---|---|---|---|---|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
96% | 2040 Da | 721 Da | 1.16 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
87% | 3620 Da | 782 Da | 1.20 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
88% | 3150 Da | 826 Da | 1.23 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
65% | 3120 Da | 881 Da | 1.23 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
92% | 2200 Da | 759 Da | 1.25 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
97% | 1470 Da | 763 Da | 1.16 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
97% | 1160 Da | 656 Da | 1.10 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
96% | 2050 Da | 772 Da | 1.11 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
97% | 1550 Da | 725 Da | 1.12 |
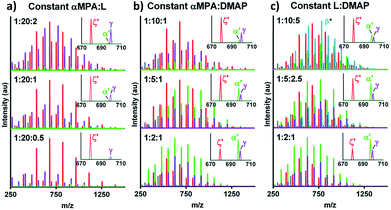 | ||
Fig. 2 Initiator, catalyst, and monomer relationships control ratios of formed products. (a) MALDI-TOF with (top to bottom) increasing αMPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMAP and L DMAP and L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMAP. (b) MALDI-TOF with increasing αMPA DMAP. (b) MALDI-TOF with increasing αMPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L and decreasing L L and decreasing L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMAP. (c) MALDI-TOF with increasing αMPA DMAP. (c) MALDI-TOF with increasing αMPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMAP and αMPA DMAP and αMPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L. *β is a temporary ion fragment (see Fig. 1). L. *β is a temporary ion fragment (see Fig. 1). | ||
Increasing the stoichiometry of lactide to initiator is commonly exploited to form linkers of various lengths to achieve differential release rates.24 To explore the utility of this approach using optimized reaction conditions, αMPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L ratios of 1
L ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, and 1
5, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 were investigated using 1 mol% DMAP (Table 2, see Fig. S15–S17, ESI†). With an αMPA
10 were investigated using 1 mol% DMAP (Table 2, see Fig. S15–S17, ESI†). With an αMPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMAP ratio of 33 or greater, γ was absent in all reactions. The polymerization with the highest αMPA
DMAP ratio of 33 or greater, γ was absent in all reactions. The polymerization with the highest αMPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L ratio, 1
L ratio, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03, had the greatest overall α
0.03, had the greatest overall α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ζ ratio. Interestingly, the peak η appeared, corresponding to αMPA-ODLA with a K+ ion, in contrast to α with a DMAP-H+ ion adduct. There was a transition from η to α over time, and η was most abundant in 1
ζ ratio. Interestingly, the peak η appeared, corresponding to αMPA-ODLA with a K+ ion, in contrast to α with a DMAP-H+ ion adduct. There was a transition from η to α over time, and η was most abundant in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03 reactions. This was explored further with polymerization times of 4 minutes and less (see Fig. S18, ESI†). One minute reactions showed negligible conversion of lactide monomer. After 2 minutes, conversion was 68% and the major product was η. By 4 minutes, α and ζ exceeded η, suggesting that shorter reaction times are necessary to isolate linear αMPA-ODLA without DMAP adducts.
0.03 reactions. This was explored further with polymerization times of 4 minutes and less (see Fig. S18, ESI†). One minute reactions showed negligible conversion of lactide monomer. After 2 minutes, conversion was 68% and the major product was η. By 4 minutes, α and ζ exceeded η, suggesting that shorter reaction times are necessary to isolate linear αMPA-ODLA without DMAP adducts.
αMPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMAP DMAP |
Time | X | α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ζ ζ |
Mn (MALDI) |
|---|---|---|---|---|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03 0.03 |
5 min | 82% | 1.1 | 619 Da |
| 10 min | 99% | 1.3 | 685 Da | |
| 15 min | 99% | 1.6 | 661 Da | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.06 0.06 |
5 min | 73% | 0.13 | 753 Da |
| 10 min | 89% | 0.23 | 727 Da | |
| 15 min | 94% | 0.24 | 758 Da | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.11 0.11 |
5 min | 32% | 0.25 | 772 Da |
| 10 min | 71% | 0.10 | 736 Da | |
| 15 min | 83% | 0.08 | 747 Da |
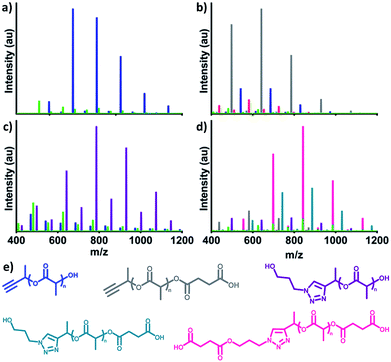
Finally, proof of concept conjugations demonstrated the utility of αMPA-ODLA as a heterobifunctional linker (Fig. 3; see Fig. S19, ESI†). Following 90 seconds of polymerization of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03 αMPA
0.03 αMPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMAP (Fig. 3a), αMPA-ODLA was modified on either propargyl or hydroxyl end groups. 3-Azido-1-propanol (see Fig. S20, ESI†) was added to propargyl moieties via Huisgen 1,3-dipolar cycloaddition (Fig. 3b). Interestingly, only η was shifted by 101 Da, the Mn of 3-azido-1-propanol, by MALDI analysis, suggesting that DMAP adducts in α interfere with propargyl modification. Hydroxyl end groups were modified to carboxylic acids using two-step, one-pot reactions whereby succinic anhydride (SA) was added following 90 seconds of lactide polymerization (Fig. 3c). These reactions were viable in either order when modifying both ends of αMPA-ODLA (Fig. 3d, S19d and e, see ESI†). It is noted that since an alcohol was used as a model azide, both N3-αMPA-ODLA-SA and SA-N3-αMPA-ODLA-SA formed during reactions. Pure N3-αMPA-ODLA-SA could be achieved by forming αMPA-ODLA-SA, removing unreacted SA, and conducting azide–alkyne conjugation.
DMAP (Fig. 3a), αMPA-ODLA was modified on either propargyl or hydroxyl end groups. 3-Azido-1-propanol (see Fig. S20, ESI†) was added to propargyl moieties via Huisgen 1,3-dipolar cycloaddition (Fig. 3b). Interestingly, only η was shifted by 101 Da, the Mn of 3-azido-1-propanol, by MALDI analysis, suggesting that DMAP adducts in α interfere with propargyl modification. Hydroxyl end groups were modified to carboxylic acids using two-step, one-pot reactions whereby succinic anhydride (SA) was added following 90 seconds of lactide polymerization (Fig. 3c). These reactions were viable in either order when modifying both ends of αMPA-ODLA (Fig. 3d, S19d and e, see ESI†). It is noted that since an alcohol was used as a model azide, both N3-αMPA-ODLA-SA and SA-N3-αMPA-ODLA-SA formed during reactions. Pure N3-αMPA-ODLA-SA could be achieved by forming αMPA-ODLA-SA, removing unreacted SA, and conducting azide–alkyne conjugation.
Conclusions
This work presents a systematic design of narrowly dispersed, low molecular weight oligo(lactide) formed without metal catalysts that are difficult to completely remove during purification. Before optimization, observed MALDI Mn disagreed with Mn as determined by NMR, suggesting transesterification reactions were present. Previous studies investigated the singular effects of polymerization temperature, polymerization time, monomer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) initiator ratio, chemical initiator, and reagent degassing time on PLA transesterification.36 This study identified the combined effects of choice of initiator, initiator
initiator ratio, chemical initiator, and reagent degassing time on PLA transesterification.36 This study identified the combined effects of choice of initiator, initiator![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst ratio, initiator
catalyst ratio, initiator![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) monomer ratio, and polymerization time on molecular weight, polydispersity, and relative amounts of desired and undesired products. Temperature was not changed from 130 °C due to the high melting point of D,L-lactide (122 °C). A 90 second reaction of 1
monomer ratio, and polymerization time on molecular weight, polydispersity, and relative amounts of desired and undesired products. Temperature was not changed from 130 °C due to the high melting point of D,L-lactide (122 °C). A 90 second reaction of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03 αMPA
0.03 αMPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L
L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMAP formed oligo(lactide) with alkyne and hydroxyl functionalities available for subsequent click conjugations. Further optimization may be required to increase the reaction efficiency, as theoretical Mn does not match Mn determined by MALDI. Although studies herein investigated ROP of lactide by DMAP, the approach can be employed for similar systems (e.g. catalysis by stannous octoate or ROP of glycolide). Similarly, copper-catalyzed azide–alkyne cycloaddition (CuAAC) may be used in place of Huisgen 1,3-dipolar cycloaddition for applications in which residual copper contamination is acceptable.
DMAP formed oligo(lactide) with alkyne and hydroxyl functionalities available for subsequent click conjugations. Further optimization may be required to increase the reaction efficiency, as theoretical Mn does not match Mn determined by MALDI. Although studies herein investigated ROP of lactide by DMAP, the approach can be employed for similar systems (e.g. catalysis by stannous octoate or ROP of glycolide). Similarly, copper-catalyzed azide–alkyne cycloaddition (CuAAC) may be used in place of Huisgen 1,3-dipolar cycloaddition for applications in which residual copper contamination is acceptable.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the National Science Foundation (NSF DGE1419118 and CBET1450987) for funding this work. We thank Prof. Wyatt Tenhaeff at the University of Rochester for advice on manuscript revisions.Notes and references
- C. Guan, B. Xu, S. Qiao, L. Qin, Y. Li, Z. Li, Y. Guo, Z. Sun, L. Song, R. Gao and P. I. Investigators, Cathet. Cardiovasc. Interv., 2017, 89, 520–527 CrossRef PubMed.
- A. H. Salama, A. A. Mahmoud and R. Kamel, AAPS PharmSciTech, 2016, 17, 1159–1172 CrossRef PubMed.
- S. Gholizadeh, J. Kamps, W. E. Hennink and R. J. Kok, Int. J. Pharm, 2017 DOI:10.1016/j.ijpharm.2017.10.032.
- K. Ren, M. Zhang, J. He, Y. Wu and P. Ni, ACS Appl. Mater. Interfaces, 2015, 7, 11263–11271 CrossRef PubMed.
- M. Veurink, L. Asmus, M. Hennig, B. Kaufmann, L. Bagnewski, A. Heiligenhaus, E. Mendrinos, C. J. Pournaras, R. Gurny and M. Moller, Eur. J. Pharm. Sci., 2013, 49, 233–240 CrossRef PubMed.
- M. Sobczak, E. Witkowska, E. Oledzka and W. Kolodziejski, Molecules, 2008, 13, 96–106 CrossRef PubMed.
- L. R. Jaidev, D. R. Chellappan, D. V. Bhavsar, R. Ranganathan, B. Sivanantham, A. Subramanian, U. Sharma, N. R. Jagannathan, U. M. Krishnan and S. Sethuraman, Acta Biomater., 2017, 49, 422–433 CrossRef PubMed.
- W. Lin, Y. Li, W. Zhang, S. Liu, Z. Xie and X. Jing, ACS Appl. Mater. Interfaces, 2016, 8, 24426–24432 CrossRef PubMed.
- Q. Zhang, Y. Du, L. Jing, X. Liang, Y. Li, X. Li, Z. Dai and J. Tian, J. Biomed. Nanotechnol., 2016, 12, 491–502 CrossRef PubMed.
- J. Xie, A. Li and J. Li, Macromol. Rapid Commun., 2017, 38(23), 1700413 CrossRef PubMed.
- L. G. Leahy, J. Psychosoc. Nurs. Ment. Health Serv., 2017, 55, 19–23 Search PubMed.
- Y. L. Fan, H. W. Hou, H. M. Tay, W. M. Guo, P. O. Berggren and S. C. Loo, AAPS PharmSciTech, 2017, 18(7), 2648–2657 CrossRef PubMed.
- P. Rafiei and A. Haddadi, Pharm. Nanotechnol., 2017, 5, 3–23 CrossRef PubMed.
- S. Kaihara, S. Matsumura, A. G. Mikos and J. P. Fisher, Nat. Protoc., 2007, 2, 2767–2771 CrossRef PubMed.
- J. W. Leenslag and A. J. Pennings, Makromol. Chem., 1987, 188, 1809–1814 CrossRef.
- M. C. Tanzi, P. Verderio, M. G. Lampugnani, M. Resnati, E. Dejana and E. Sturani, J. Mater. Sci.: Mater. Med., 1994, 5, 393–396 CrossRef.
- F. Nederberg, E. F. Connor, M. Moller, T. Glauser and J. L. Hedrick, Angew. Chem., Int. Ed. Engl., 2001, 40, 2712–2715 CrossRef PubMed.
- O. Coulembier and P. Dubois, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 1672–1680 CrossRef.
- K. Makiguchi, S. Kikuchi, K. Yanai, Y. Ogasawara, S. Sato, T. Satoh and T. Kakuchi, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 1047–1054 CrossRef.
- J. Kadota, D. Pavlovic, H. Hirano, A. Okada, Y. Agari, B. Bibal, A. Deffieux and F. Peruch, RSC Adv., 2014, 4, 14725–14732 RSC.
- I. V. Dimitrov, I. V. Berlinova and V. I. Michailova, Polym. J., 2013, 45, 457–461 CrossRef.
- C. H. Yan, J. M. Zhang, Y. X. Lv, J. Yu, J. Wu, J. Zhang and J. S. He, Biomacromolecules, 2009, 10, 2013–2018 CrossRef PubMed.
- M. Eldessouki, G. Buschle-Diller and Y. Gowayed, Des. Monomers Polym., 2016, 19, 180–192 CrossRef.
- D. S. W. Benoit, C. R. Nuttelman, S. D. Collins and K. S. Anseth, Biomaterials, 2006, 27, 6102–6110 CrossRef PubMed.
- S. J. de Jong, S. C. De Smedt, J. Demeester, C. F. van Nostrum, J. J. Kettenes-van den Bosch and W. E. Hennink, J. Controlled Release, 2001, 72, 47–56 CrossRef PubMed.
- C. S. Proikakis, P. A. Tarantili and A. G. Andreopoulos, J. Elastomers Plast., 2002, 34, 49–63 CrossRef.
- H. Fukuzaki, M. Yoshida, M. Asano and M. Kumakura, Eur. Polym. J., 1989, 25, 1019–1026 CrossRef.
- Y. Liu, W. Hou, H. Sun, C. Cui, L. Zhang, Y. Jiang, Y. Wu, Y. Wang, J. Li, B. S. Sumerlin, Q. Liu and W. Tan, Chem. Sci., 2017, 8, 6182–6187 RSC.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed. Engl., 2001, 40, 2004–2021 CrossRef PubMed.
- F. Nederberg, E. F. Connor, T. Glausser and J. L. Hedrick, Chem. Commun., 2001, 2066–2067, 10.1039/b106125a.
- C. Bonduelle, B. Martin-Vaca, F. P. Cossio and D. Bourissou, Chemistry, 2008, 14, 5304–5312 CrossRef PubMed.
- H. R. Kricheldorf, S. M. Weidner and F. Scheliga, Polym. Chem., 2017, 8, 1589–1596 RSC.
- V. Katiyar and H. Nanavati, Polym. Chem., 2010, 1, 1491–1500 RSC.
- G. Montaudo, M. S. Montaudo, C. Puglisi, F. Samperi, N. Spassky, A. LeBorgne and M. Wisniewski, Macromolecules, 1996, 29, 6461–6465 CrossRef.
- V. T. Lipik, L. K. Widjaja, S. S. Liow, M. J. M. Abadie and S. S. Venkatraman, Polym. Degrad. Stab., 2010, 95, 2596–2602 CrossRef.
- G. Schwach, J. Coudane, R. Engel and M. Vert, Biomaterials, 2002, 23, 993–1002 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra05306e |
| This journal is © The Royal Society of Chemistry 2018 |