Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rh(III)-catalyzed synthesis of tetracyclic isoquinolinium salts via C–H activation and [4+2] annulation of 1-phenyl-3,4-dihydroisoquinolines and alkynes in ethanol†
Xinxin Danga,
Yu Hea,
Yingtian Liua,
Xuehong Chena,
Jun-Long Li b,
Xian-Li Zhou*a,
Hezhong Jiang*a and
Jiahong Li
b,
Xian-Li Zhou*a,
Hezhong Jiang*a and
Jiahong Li *a
*a
aSchool of Life Science and Engineering, Southwest Jiaotong University, Chengdu 610041, China. E-mail: jiahongljh@163.com; jianghz10@home.swjtu.edu.cn; zhouxl@swjtu.edu.cn
bAntibiotics Research and Re-evaluation Key Laboratory of Sichuan Province, Sichuan Industrial Institute of Antibiotics, Chengdu University, Chengdu, 610052, China
First published on 24th August 2018
Abstract
An efficient and convenient method to construct tetracyclic isoquinolinium salts via [Cp*RhCl2]2 catalyzed C–H activation and [4 + 2] annulation reactions in ethanol is described. This reaction is very fast and highly efficient in the green solvent ethanol. The reaction works with a broad substrate scope affording the products in good to excellent yields in a short time. Moreover, a ratio of S/C up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 could be achieved with gram scale synthesis.
000 could be achieved with gram scale synthesis.
Introduction
N-Heterocyclic quaternary ammonium salts and their derivatives are versatile heterocyclic compounds found in many natural1 and synthetic products2 and are well-known for their potent biological activities3 (Fig. 1). Therefore, the development of improved methodologies to synthesize new N-heterocyclic quaternary ammonium salts still remains highly desirable.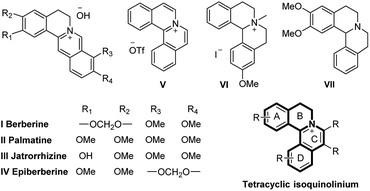 | ||
| Fig. 1 Representative tetracyclic isoquinolinium salts and their derivatives in medicinal chemistry and natural products. | ||
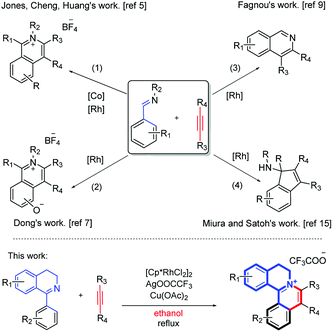
In recent years, significant advancements have been made in transition-metal-catalyzed C–C bond formation via C–H activation, among which rhodium-catalyzed direct C–H bond activations are powerful strategies to synthesize various polycyclic skeletons and N-heterocyclic scaffolds, due to their high efficiency and atom economy.4 Particularly, using aldehyde imine/ketimine substrates to construct an isoquinoline skeleton via C–H annulation has been documented. Earlier, methods for the synthesis of isoquinolinium salts by C–H activation and [4 + 2] annulation of various imines have been studied (Scheme 1, eqn (1) and (2)),5 such as by Cheng's group5b,6 and Xu's group.7 Recently, You's group reported a Rh-catalyzed cascade C–H activation/[4 + 2] annulation of aldoximes with alkynes to synthesize multisubstituted protoberberine skeletons.8 In the meantime, the synthesis of isoquinoline compounds by the [4 + 2] annulation of open-ring imines has also been reported (Scheme 1, eqn (3)).9 Fagnou's group used [Cp*Rh(MeCN)3][SbF6]2 to catalyze the formation of isoquinoline compounds from N-tert-butylbenzaldimines and internal alkyne.9 Similar work has also been reported by Lade,10 Dong,11 Chiba,12 Cheng,13 Zhao14 et al. In addition, there are some reports about the reaction of Rh, Ir and Ru-catalyzed [3 + 2] annulation using imine as a directing group (Scheme 1, eqn (4)).15
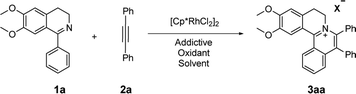
A close look at the literature precedents revealed that all the previously elegant examples regarding the isoquinolinium salts syntheses mainly focused on the use of acyclic aldimines or ketimines, while not even a single example, starting from the cyclic imines, such as 3,4-dihydroisoquinoline, has been documented. Inspired by these work, we proposed that it was possible to use the imine group of dihydroisoquinoline as a directing group to furnish C–H activation and [4 + 2] annulation to construct tetracylic isoquinolinium salts. Herein, we report Rh-catalyzed [4 + 2] annulations of cyclic-imine of 1-phenyl-3,4-dihydroisoquinolines to synthesis tetracyclic isoquinolinium salts in ethanol. Notably, ethanol is safer and more environmentally friendly compared with some other organic solvents, especially poisonous DCE. Noteworthily, this reaction proceeds with excellent efficiency. What's more, the ratio of S/C could achieve up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.
000.
Results and discussion
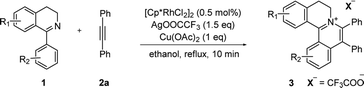
At the outset of our study, [Cp*RhCl2]2 was used to catalyze 6,7-dimethoxy-1-phenyl-3,4-dihydroisoquinoline 1a with diphenylacetylene 2a to investigate the catalytic performance of additives, solvents, and oxidants (Table 1). When [Cp*RhCl2]2 was used as a catalyst, without any additives, 3aa was barely formed in dioxane (entry 1). Then we explored various silver salts, among which AgOOCCF3 performed most remarkably, and provided 68% yield in 4 h (entry 3). Other silver salts showed little poor performance (entries 2, 4). Meanwhile, the effects of different solvents were investigated (entries 5–10). It is noteworthy that the reaction could get almost quantitative yield in ethanol, with 99% yield in 4 h (entry 10). Meanwhile, we compared the effects of additives, such as copper salts, K2S2O8, C6H5I(O2CCH3)2 and 2,3-dicyano-5,6-dichlorobenzoquinone (DDQ) (entries 11–16). Among these additives, Cu(OAc)2 gave the best yield of 99% for 3aa in 10 minutes (entry 12). However, the reaction could not proceed when only AgOOCCF3 and Cu(OAc)2 were used without Rh catalyst. (entry 17). What is noteworthy is that even when the S/C ratio was 1000, the reaction could finish completely in 10 minutes with 99% isolated yield (entry 18).| Entry | Oxidant | Additive | Solvent | Time | 3aa (%)b |
|---|---|---|---|---|---|
| a Reaction conditions unless otherwise specified: 1a (0.32 mmol), 2a (1 eq.), 0.5 mol% of [Cp*RhCl2]2, 1.0 eq. of oxidants, 3 mL of solvent, reflux, ND = Not Detected.b Isolated yield.c 120 °C.d 1 eq. of additives.e No [Cp*RhCl2]2 was added.f S/C = 1000, 1a (1.6 mmol), 2a (1 eq.), 1.5 eq. of oxidants, 1 eq. of Cu(OAc)2. | |||||
| 1 | — | — | Dioxane | 4 h | ND |
| 2 | AgOTf | — | Dioxane | 4 h | 40 |
| 3 | AgOOCCF3 | — | Dioxane | 4 h | 68 |
| 4 | AgOAc | — | Dioxane | 4 h | Trace |
| 5 | AgOOCCF3 | — | DCE | 4 h | 70 |
| 6 | AgOOCCF3 | — | Toluene | 4 h | 95 |
| 7 | AgOOCCF3 | — | DCM | 4 h | 25 |
| 8c | AgOOCCF3 | — | DMF | 4 h | 70 |
| 9c | AgOOCCF3 | — | DMSO | 4 h | 45 |
| 10 | AgOOCCF3 | — | EtOH | 4 h | 99 |
| 11d | AgOOCCF3 | CuCl2·2H2O | EtOH | 10 min | 25 |
| 12d | AgOOCCF3 | Cu(OAc)2 | EtOH | 10 min | 99 |
| 13d | AgOOCCF3 | Cu(CF3COO)2 | EtOH | 10 min | 92 |
| 14d | AgOOCCF3 | K2S2O8 | EtOH | 10 min | 81 |
| 15d | AgOOCCF3 | C6H5I(O2CCH3)2 | EtOH | 10 min | 90 |
| 16d | AgOOCCF3 | DDQ | EtOH | 10 min | Trace |
| 17d,e | AgOOCCF3 | Cu(OAc)2 | EtOH | 10 min | ND |
| 18f | AgOOCCF3 | Cu(OAc)2 | EtOH | 10 min | 99 |
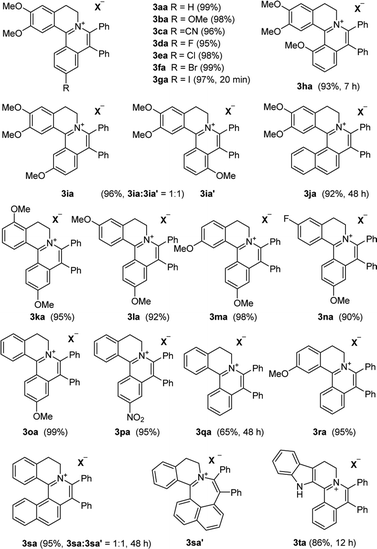
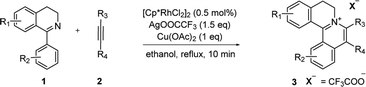
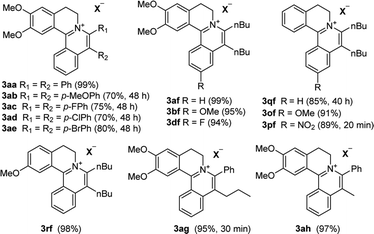
With the optimized conditions in hand, a range of electronically and sterically diverse of 3,4-dihydroisoquinoline derivatives were employed using 2a as a coupling partner to test the substrate tolerance of the Rh(III)-catalyzed tandem [4 + 2] annulation. And the corresponding tetracyclic isoquinolinium salts were constructed in Table 2. A series of 1-aryl-substituted 3,4-dihydroisoquinoline (1a–g) could be effectively worked with 2a in the catalytic reaction with excellent yields (99–95%). However, when the ortho-position of benzene ring was substituted by methoxy group (1h), the reaction speed went down apparently. The product 3ia and 3ia′ was successfully obtained in 96% yield with the regioselectivity of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
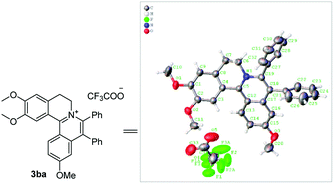
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In addition, using naphthalene-substituted 1j, the reaction proceed slower than 1a, which indicated that steric effect could influence the reaction process. Then, we investigated the effect of steric and electronic influences on the isoquinoline core (1k–o). Notably, the substrates bearing both electron-withdrawing and electron-donating substituted at the ortho-, meta-, and para-positions of the phenyl ring (1b, 1k–o) reacted fast to provide excellent yields. However, the reaction significantly weakened to 65% yield even in 48 hours when 1q was used as a substrate. For naphthalene-substituted substrates, 1s gave mixture product 3sa and 3sa′. Interestingly, 1j only afforded pure product 3ja. It indicated that electronic influences on the isoquinoline core played an important role on the regioselectivity of the reaction process. Notably, the 1t could be converted to corresponding quaternary ammonium salt 3ta in good yield, whose reduction product bears the key hetero-tetracyclic scaffolds of reserpine.16 The structure of the final product 3ba was characterized by X-ray crystallography (Fig. 2).
1. In addition, using naphthalene-substituted 1j, the reaction proceed slower than 1a, which indicated that steric effect could influence the reaction process. Then, we investigated the effect of steric and electronic influences on the isoquinoline core (1k–o). Notably, the substrates bearing both electron-withdrawing and electron-donating substituted at the ortho-, meta-, and para-positions of the phenyl ring (1b, 1k–o) reacted fast to provide excellent yields. However, the reaction significantly weakened to 65% yield even in 48 hours when 1q was used as a substrate. For naphthalene-substituted substrates, 1s gave mixture product 3sa and 3sa′. Interestingly, 1j only afforded pure product 3ja. It indicated that electronic influences on the isoquinoline core played an important role on the regioselectivity of the reaction process. Notably, the 1t could be converted to corresponding quaternary ammonium salt 3ta in good yield, whose reduction product bears the key hetero-tetracyclic scaffolds of reserpine.16 The structure of the final product 3ba was characterized by X-ray crystallography (Fig. 2).
The examination of the scope of various alkynes 2 was shown in Table 3. The result revealed that wide substrate tolerance with both internal aryl and alkyl alkynes. For alkynes, both electron-donating and -withdrawing substituents on the phenyl ring proceeded smoothly with 1a to furnish 3ab–e with good yields, though need a longer time than disubstituent alkynes 2a. To our delight, alkyl-substituted alkyne 2f exhibited the similar excellent reactivity as that of aryl-substituted alkyne. Especially, different with other's work,5c,6a,6c,7,8 unsymmetrical alkynes 2g and 2h reacted very fast with 1a to give pure products instead of regioisomers with excellent yields in 10 minutes. The structure of 3ag and 3ah were determined by the NOESY analysis. It needs to point out that the present conditions are specific and highly efficient for the synthesis of tetracylic isoquinolinium salts.
Considering, metal alkenyl intermediates may undergo [3 + 2] annulation to imine group for non-cyclicimines, we have also investigated the scope of non-cyclicimine substrates in Table S2.† Interestingly, we found non-cyclicimines gave N-heterocyclic quaternary ammonium salts of [4 + 2] annulation of imines not of [3 + 2] annulation. Generally, the results of Table 2 and S2† suggested that the dihydroisoquinoline showed much higher activity than non-cyclicimines in the [Cp*RhCl2]2/AgOOCCF3/Cu(OAc)2 catalyst system.
To assess the scalability of this Rh(III)-catalyzed C–H bond activation and annulation process, gram-scale reaction of 1a with 2a and 2f were performed (Table 4). Firstly, 99% yield of 3aa was obtained in 10 minutes while the S/C = 1000 (entry 1). Then the S/C was gradually increased to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and 4.505 g of 3aa was obtained in 99% yield in 22 h (entry 4). What's more, the alkynes 2f could conduct well with 1a at 5000 of S/C ratio and 2.09 g of 3af was obtained in 99% yield (entry 5). These results showed the catalytic system has fairly good catalytic capability and practicality.
000, and 4.505 g of 3aa was obtained in 99% yield in 22 h (entry 4). What's more, the alkynes 2f could conduct well with 1a at 5000 of S/C ratio and 2.09 g of 3af was obtained in 99% yield (entry 5). These results showed the catalytic system has fairly good catalytic capability and practicality.
| Entry | S/C | 3/g | Time | Yield% |
|---|---|---|---|---|
| a Reaction conditions unless otherwise specified: 1a (0.8091 mmol), 1.5 eq. of AgOOCCF3, 15 mL of ethanol.b Isolated yield.c 1a (4.0453 mmol).d 1a (4.0453 mmol), 2 eq. of AgOOCCF3.e 1a (8.0906 mmol), 2 eq. of AgOOCCF3.f 1a (4.0453 mmol), 2 eq. of AgOOCCF3, 15 mL of ethanol. | ||||
| 1 | 1000 | 3aa/0.451 | 10 min | 99 |
| 2c | 5000 | 3aa/2.252 | 24 h | 99 |
| 3d | 5000 | 3aa/2.253 | 2.5 h | 99 |
| 4e | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3aa/4.505 | 22 h | 99 |
| 5f | 5000 | 3af/2.090 | 48 h | 99 |
Conclusions
In summary, we have developed a simple and efficient catalytic method for transforming the imine substrates especially the dihydroisoquinoline compounds to quaternary ammonium salts with the utilization of rhodium catalyzed C–H activation and [4 + 2] annulation in ethanol in a very short time and with remarkable yield under mild reaction conditions. Additionally, with the aid of AgOOCCF3 and Cu(OAc)2, the reaction time and catalytic performance can be greatly enhanced, so that a ratio of S/C up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 could be achieved with gram scale substrate. It provides an efficient strategy to synthesise tetracyclic isoquinolinium salts.
000 could be achieved with gram scale substrate. It provides an efficient strategy to synthesise tetracyclic isoquinolinium salts.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was co-supported by the research project of the Fund of Science and Technology Agency of Chengdu (2015-HM01-00311-SF), the Administration of Traditional Chinese Medicine of Sichuan (2016Q037), the fund of Antibiotics Research and Re-evaluation Key Laboratory of Sichuan Province, Sichuan Industrial Institute of Antibiotics, Chengdu University (ARRLKF17-10) and the Fundamental Research Funds for the Central Universities (2682017CX091).Notes and references
- (a) L. Grycová, J. Dostál and R. Marek, Phytochemistry, 2007, 68, 150 CrossRef PubMed; (b) J. Jayakumar and C.-H. Cheng, Chem.–Eur. J., 2016, 22, 1800 CrossRef PubMed.
- (a) T. Lipińska, Tetrahedron Lett., 2002, 43, 9565 CrossRef; (b) A. Nunez, A. M. Cuadro, J. Alvarez-Builla and J. J. Vaquero, Org. Lett., 2007, 9, 2977 CrossRef PubMed.
- (a) K. Bhadra and G. S. Kumar, Med. Res. Rev., 2011, 31, 821 CrossRef PubMed; (b) Y.-X. Wang, Y.-P. Wang, H. Zhang, W.-J. Kong, Y.-H. Li, F. Liu, R.-M. Gao, T. Liu, J.-D. Jiang and D.-Q. Song, Bioorg. Med. Chem. Lett., 2009, 19, 6004 CrossRef PubMed; (c) H. Tanabe, H. Suzuki, A. Nagatsu, H. Mizukami, Y. Ogihara and M. Inoue, Phytomedicine, 2006, 13, 334 CrossRef PubMed; (d) K. Ingkaninan, P. Phengpa, S. Yuenyongsawad and N. Khorana, J. Pharm. Pharmacol., 2006, 58, 695 CrossRef PubMed; (e) T. Yokozawa, A. Satoh, E. J. Cho, Y. Kashiwada and Y. Ikeshiro, J. Pharm. Pharmacol., 2005, 57, 367 CrossRef PubMed; (f) R. M. Suarez, P. Bosch, D. Sucunza, A. M. Cuadro, A. Domingo, F. Mendicuti and J. J. Vaquero, Org. Biomol. Chem., 2015, 13, 527 RSC; (g) M. Schulze, O. Siol, M. Decker and J. Lehmann, Bioorg. Med. Chem. Lett., 2010, 20, 2946 CrossRef PubMed; (h) S. Oloff, R. B. Mailman and A. Tropsha, J. Med. Chem., 2005, 48, 7322 CrossRef PubMed.
- (a) D. Sucunza, A. M. Cuadro, J. Alvarez-Builla and J. J. Vaquero, J. Org. Chem., 2016, 81, 10126 CrossRef PubMed; (b) J. R. Hummel, J. A. Boerth and J. A. Ellman, Chem. Rev., 2016, 117, 9163 CrossRef PubMed; (c) D. A. Colby, R. G. Bergman and J. A. Ellman, Chem. Rev., 2010, 110, 624 CrossRef PubMed; (d) V. P. Boyarskiy, D. S. Ryabukhin, N. A. Bokach and A. V. Vasilyev, Chem. Rev., 2016, 116, 5894 CrossRef PubMed; (e) L. Ackermann, Chem. Rev., 2011, 111, 1315 CrossRef PubMed.
- (a) L. Li, W. W. Brennessel and W. D. Jones, J. Am. Chem. Soc., 2008, 130, 12414 CrossRef PubMed; (b) K. Parthasarathy, N. Senthilkumar, J. Jayakumar and C.-H. Cheng, Org. Lett., 2012, 14, 3478 CrossRef PubMed; (c) G. Zhang, L. Yang, Y. Wang, Y. Xie and H. Huang, J. Am. Chem. Soc., 2013, 135, 8850 CrossRef PubMed.
- (a) N. Senthilkumar, P. Gandeepan, J. Jayakumar and C.-H. Cheng, Chem. Commun., 2014, 45, 3106 RSC; (b) S. Prakash, K. Muralirajan and C.-H. Cheng, Angew. Chem., Int. Ed., 2016, 55, 1844 CrossRef PubMed; (c) C.-Z. Luo, P. Gandeepan, J. Jayakumar, K. Parthasarathy, Y.-W. Chang and C.-H. Cheng, Chem.–Eur. J., 2013, 19, 14181 CrossRef PubMed; (d) J. Jayakumar, K. Parthasarathy and C.-H. Cheng, Angew. Chem., 2012, 124, 197 CrossRef PubMed; (e) C. Z. Luo, P. Gandeepan, Y. C. Wu, C. H. Tsai and C.-H. Cheng, ACS Catal., 2015, 5, 4837 CrossRef; (f) C. Z. Luo, P. Gandeepan and C.-H. Cheng, Chem. Commun., 2013, 49, 8528 RSC.
- W. Zhang, C.-Q. Wang, H. Lin, L. Dong and Y.-J. Xu, Chem.–Eur. J., 2016, 22, 907 CrossRef PubMed.
- J. Tang, S. Li, Z. Liu, Y. Zhao, Z. She, V. D. Kadam, G. Gao, J. Lan and J. You, Org. Lett., 2017, 19, 604 CrossRef PubMed.
- N. Guimond and K. Fagnou, J. Am. Chem. Soc., 2009, 131, 12050 CrossRef PubMed.
- A. B. Pawar, D. Agarwal and D. M. Lade, J. Org. Chem., 2016, 81, 11409 CrossRef PubMed.
- Q. R. Zhang, J. R. Huang, W. Zhang and L. Dong, Org. Lett., 2014, 16, 1684 CrossRef PubMed.
- C. T. Pei, Y. F. Wang and S. Chiba, Org. Lett., 2010, 12, 5688 CrossRef PubMed.
- S. C. Chuang, P. Gandeepan and C.-H. Cheng, Org. Lett., 2013, 15, 5750 CrossRef PubMed.
- R. S. Manan and P. Zhao, Nat. Commun., 2016, 7, 11506 CrossRef PubMed.
- (a) S. E. Winterton and J. M. Ready, Org. Lett., 2016, 18, 2608 CrossRef PubMed; (b) M. V. Pham and N. Cramer, Chem.–Eur. J., 2016, 22, 2270 CrossRef PubMed; (c) H. Liu, J. Li, M. Xiong, J. Jiang and J. Wang, J. Org. Chem., 2016, 81, 6093 CrossRef PubMed; (d) S.-S. Li, L. Wu, L. Qin, Y.-Q. Zhu, F. Su, L. Dong, S.-S. Li and Y.-J. Xu, Org. Lett., 2016, 18, 4214 CrossRef PubMed; (e) H. Lin, S.-S. Li and L. Dong, Org. Biomol. Chem., 2015, 13, 11228 RSC; (f) J. Zhang, A. Ugrinov and P. Zhao, Angew. Chem., Int. Ed., 2013, 52, 6681 CrossRef PubMed; (g) N. Tran Duc and N. Cramer, Angew. Chem., Int. Ed., 2013, 52, 10630 CrossRef PubMed; (h) L. Dong, C.-H. Qu, J.-R. Huang, W. Zhang, Q.-R. Zhang and J.-G. Deng, Chem.–Eur. J., 2013, 19, 16537 CrossRef PubMed; (i) D. N. Tran and N. Cramer, Angew. Chem., Int. Ed., 2011, 50, 11098 CrossRef PubMed; (j) Z.-M. Sun, S.-P. Chen and P. Zhao, Chem.–Eur. J., 2010, 16, 2619 CrossRef PubMed; (k) T. Fukutani, N. Umeda, K. Hirano, T. Satoh and M. Miura, Cheminform, 2010, 41, 5141 Search PubMed.
- G. Varchi, A. Battaglia, C. Samorì, E. Baldelli, B. Danieli, G. Fontana, A. Guerrini and E. Bombardelli, J. Nat. Prod., 2005, 68, 1629 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1828400. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8ra05443f |
| This journal is © The Royal Society of Chemistry 2018 |