Open Access Article
Open Access ArticleConversion of dilute nitrous oxide (N2O) in N2 and N2–O2 mixtures by plasma and plasma-catalytic processes†
Xing Fan *a,
Sijing Kanga,
Jian Lia and
Tianle Zhub
*a,
Sijing Kanga,
Jian Lia and
Tianle Zhub
aKey Laboratory of Beijing on Regional Air Pollution Control, College of Environmental and Energy Engineering, Beijing University of Technology, Beijing 100124, China. E-mail: fanxing@bjut.edu.cn
bSchool of Space and Environment, Beihang University, Beijing 100191, China
First published on 30th July 2018
Abstract
A coaxial dielectric barrier discharge (DBD) reactor has been developed for plasma and plasma-catalytic conversion of dilute N2O in N2 and N2–O2 mixtures at both room and high temperature (300 °C). The effects of catalyst introduction, O2 content and inlet N2O concentration on N2O conversion and the mechanism involved in the conversion of N2O have been investigated. The results show that N2O in N2 could be effectively decomposed to N2 and O2 by plasma and plasma-catalytic processes at both room and high temperature, with much higher decomposition efficiency at 300 °C than at room temperature for the same discharge power. Under an N2–O2 atmosphere, however, N2O could be removed only at high temperature, producing not only N2 and O2 but also NO and NO2. Production and conversion of N2O occur simultaneously during the plasma and plasma-catalytic processing of N2O in a N2–O2 mixture, with production and conversion being the dominant processes at room and high temperature, respectively. N2O conversion increases with the increase of discharge power and decreases with the increase of O2 content. Increasing the inlet N2O concentration from 100 to 400 ppm decreases the conversion of N2O under an N2 atmosphere but increases that under an N2–O2 atmosphere. Concentrating N2O in the N2–O2 mixture could alleviate the negative influence of O2 by increasing the involvement of plasma reactive species (e.g., N2(A3Σu+) and O(1D)) in N2O conversion. Packing the discharge zone with a RuO2/Al2O3 catalyst significantly enhances the conversion of N2O and improves the selectivity of N2O decomposition under an N2–O2 atmosphere, revealing the synergy of plasma and catalyst in promoting N2O conversion, especially its decomposition to N2 and O2.
1. Introduction
Nitrous oxide (N2O) emitted from various human activities including agriculture (soil cultivation and the use of nitrogen-fertilizers), biomass burning, fossil fuel combustion, industrial processes (production of adipic and nitric acids), and wastewater treatment is the third most significant anthropogenic greenhouse gas and the largest stratospheric-ozone-depleting substance.1–3 Limiting the formation of N2O is the best solution to reduce N2O emissions from the agricultural sector and uncontrolled biomass burning taking into account the diffuse character of these emissions, while employment of after-treatment technologies is important for control of N2O emissions from combustion and industrial sources.3Technologies developed and adopted so far for abatement of N2O are mainly based on catalysis, including non-selective catalytic reduction (NSCR), selective catalytic reduction (SCR), and direct catalytic decomposition.1,3–8 Among these technologies, the direct catalytic decomposition of N2O to N2 and O2 has received great attention due to simplicity and high efficiency and significant research efforts have been focused on development of novel catalytic materials with satisfactory activity at relatively low temperatures.1,3,5–8 As a promising alternative to develop new catalysts, combination of catalysts with non-thermal plasma has been widely investigated in recent years for treatment of a variety of air contaminants such as volatile organic compounds (VOCs) and nitrogen oxides (NOx).9–16 The synergetic effects between plasma and catalysis include initiating chemical reactions at low temperature and improving products selectivity.9–16 In fact, plasma and plasma-catalysis systems have also been investigated for decomposition of N2O, with nitrogen or argon as the background gas in most cases.17–22 These oxygen-free systems proved to be effective in decomposing N2O even at room temperature.17–22 In real exhaust gases, however, O2 always coexists with N2O and N2 and it is therefore of great significance to investigate the N2O conversion behavior under N2–O2 atmosphere.3
In a recent study by Jo et al.,23 O2 in N2–O2–N2O mixture was verified to have obviously adverse effects on the plasma-catalytic decomposition of N2O. Besides the negative influence of O2 on the catalytic decomposition of N2O, the intrinsic formation of N2O by discharge in N2–O2 should have also contributed to the decreasing N2O conversion with increasing O2 content, which however was not taken into account in that study.23 From our perspective, a better understanding of both N2O production and conversion processes in the presence of O2 is essential for optimizing the plasma-catalytic decomposition of N2O. On the other hand, Jo et al.23 inferred from the thermodynamic calculations that N2O was mainly decomposed into N2 and O2 in the presence of O2. However, Krawczyk et al.24,25 found that N2O in mixtures with O2 or air was both oxidized to NO and decomposed to N2 and O2 by gliding arc discharge, combined with or without a catalytic bed. Oxidation of N2O into NO and reusing NO for production of nitric acid is a profitable method for reducing concentrated N2O emissions, e.g., in adipic acid plants.24 For removal of dilute N2O from sources such as nitric acid production and fluidized bed combustion, however, decomposition of N2O into N2 and O2 would be more desired.3
The aim of this study is to investigate the conversion behavior and mechanism of dilute N2O in plasma and plasma-catalytic processes, in both the presence and absence of O2 and at both room and high temperature (300 °C). For this purpose, a coaxial dielectric barrier discharge (DBD) reactor was constructed, to generate plasma and to combine plasma with catalysts. RuO2/Al2O3 was chosen as the catalyst besides Al2O3 for plasma-catalytic conversion of N2O due to its reported good performance for catalytic N2O decomposition.26 The effects of catalyst (Al2O3 or RuO2/Al2O3) introduction, O2 content (0–20%, volumetric) and inlet N2O concentration (100–400 ppm, volumetric) on the conversion of N2O were systematically examined. In order to elucidate the mechanism of N2O conversion, the production of N2O by discharge in N2–O2 mixture with and without catalyst was also investigated and products/byproducts generated in these processes were analyzed in detail.
2. Experimental
2.1 Experimental set-up
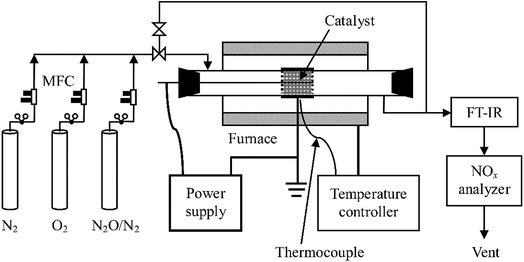
A schematic diagram of the experimental system is shown in Fig. 1. It consists of reaction gas supply, a DBD reactor with an alternating current (AC) high voltage power supply (0–100 kV, 50–500 Hz, sinusoidal wave), and analytical instrumentation. The reaction gas which was fed into the reactor at a total flow rate of 1 L min−1 at ambient temperature and pressure (around 20 °C, 100 kPa) throughout this work was prepared by mixing pure N2, O2, and N2O in N2 (Beijing HaiRui Tongda Gas Technology Co., Ltd., China) whose flow rates were controlled by a set of mass flow controllers (MFC, D07-7, Beijing Sevenstar Electronics Co., Ltd., China). O2 content in the feed gas was adjusted to 0%, 5%, 10% or 20% while inlet concentration of N2O ranged from 0 to 400 ppm.A quartz glass tube reactor (length: 600 mm; inner diameter: 29 mm; thickness: 1.5 mm) was used with a concentric tungsten wire (diameter: 1.4 mm) acting as the discharge electrode and an aluminum foil (50 mm in length) wrapping around the glass tube as the ground electrode. For the plasma-catalytic process, Al2O3 or RuO2/Al2O3 catalyst pellets (20 g, 3–5 mm in diameter) were packed in the space between the discharge electrode and the tube at near maximum packing density, with an apparent volume of ca. 27 mL. The plasma/plasma-catalytic reactor was installed in normal indoor environments or in a temperature-controlled tube furnace to obtain room temperature and high temperature (300 °C) reaction conditions, respectively.
2.2 Experimental methods
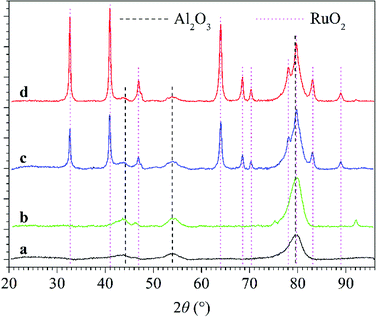
In addition, X-ray diffraction (XRD) patterns of Al2O3 and RuO2/Al2O3 catalysts before and after use in plasma-catalytic conversion of N2O were obtained using a Bruker D8 Discover diffractometer (Co Kα radiation, 35 kV, 30 mA).
The conversion of N2O is calculated based on its inlet (CN2O,in, ppm) and outlet concentrations (CN2O,out, ppm), as shown in eqn (1).
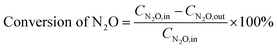 | (1) |
The selectivity of NO, NO2 and NOx (NO + NO2) produced from N2O conversion is calculated based on the N-balance as follows:
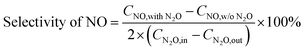 | (2) |
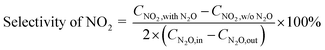 | (3) |
| Selectivity of NOx = selectivity of NO + selectivity of NO2 | (4) |
3. Results and discussion
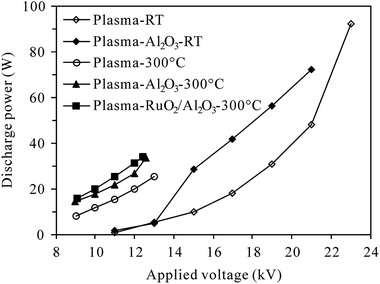
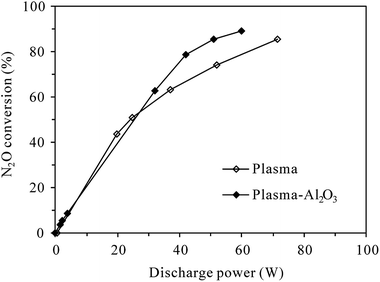
3.1 Conversion of N2O at room temperature
| N2 + e → N2(A3Σu+) + e | (R1) |
| N2(A3Σu+) + N2O → 2N2 + O | (R2) |
| N2(A3Σu+) + O2 → N2O + O | (R3) |
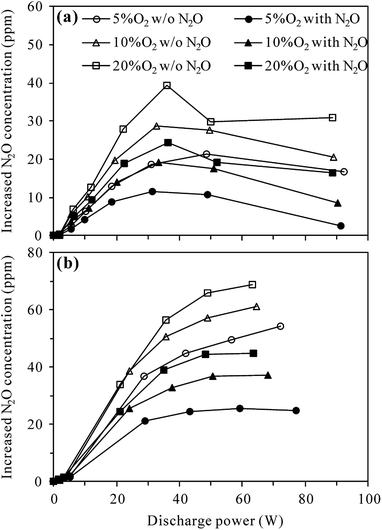
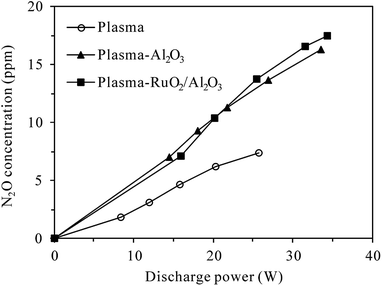
Fig. 4 compares the increased concentration of N2O (CN2O,out − CN2O,in) under different O2 contents with and without 100 ppm-N2O in the N2–O2 mixture. As can be seen from Fig. 4(a), no matter with or without N2O in the inlet gas, the increased concentration of N2O in the plasma reactor first increased and then decreased with the increase of discharge power, attaining a maximum at ca. 35 W. The reason for this may be that the increase of discharge power promotes not only the formation of N2O via (R3), but also N2O loss by (R2) and/or (R4)–(R6).27,29–31 Higher discharge power means that more energy could be used to excite/dissociate N2 and O2 molecules, producing more reactive species such as N2(A3Σu+) and O(1D). At relatively low discharge power, the concentration of N2O increased with increasing discharge power due to the enhanced production of N2(A3Σu+) species for N2O formation (R3). With the increase of N2O concentration and increasing production of reactive species (N2(A3Σu+) and O(1D)), the probability of N2O loss reactions ((R2), (R5) and (R6)) raised, explaining the observed decrease of N2O concentration at higher discharge power. In the whole discharge power range tested, however, the production of N2O surpassed the loss since the increased concentration of N2O was always positive.
| O2 + e → O(3P) + O(1D) + e | (R4) |
| O(1D) + N2O → NO + NO | (R5) |
| O(1D) + N2O → N2 + O2 | (R6) |
Fig. 4(a) also shows that in both the presence and absence of N2O in the inlet gas, more N2O was produced in the plasma reactor under higher O2 contents for a given discharge power, indicating the important role of O2 in N2O production.27,29 Compared with the case without N2O in the inlet gas, introduction of 100 ppm-N2O significantly reduced the production of N2O under all O2 contents. In the presence of initial N2O, more N2(A3Σu+) species would be consumed in N2O decomposition (reaction (R2)), reducing the amount of N2(A3Σu+) species for N2O production (reaction (R3)) as a result.
Compared to plasma alone, more N2O was produced in the plasma-Al2O3 reactor (Fig. 4(b)) under otherwise similar conditions, indicating the promotion effects of Al2O3 catalyst on N2O formation by discharge. In a study focusing on N2O formation by DBD in N2–O2 mixtures, Tang et al. also reported similar increase of N2O production by packing Al2O3 in the discharge zone and surface oxygen species (Al2O3–O*) brought by Al2O3 into the plasma chemical process (reaction (R7)) was considered as the main reason.29
| N2(A3Σu+) + Al2O3–O* → N2O + Al2O3 | (R7) |
In the presence of Al2O3 catalyst (Fig. 4(b)), the increased concentration of N2O first increased and then tended to reach equilibrium values with the increase of discharge power, demonstrating that N2O production was counterbalanced by N2O loss at high discharge power, especially when N2O was introduced into the inlet gas. As in the plasma case, introduction of 100 ppm-N2O into the N2–O2 mixture also resulted in less production of N2O in the plasma-Al2O3 reactor (Fig. 4(b)).
In addition, it is worth mentioning that although the presence of 100 ppm-N2O in the N2–O2 mixture significantly affected the production of N2O by discharge, it did not induce significant changes to the formation behavior of NO and NO2 in both plasma (Fig. S1†) and plasma-Al2O3 reactors (Fig. S2†) at room temperature.
3.2 Conversion of N2O at high temperature
In order to decompose N2O in the O2-containing atmosphere, the reaction temperature was raised to 300 °C in this section and RuO2/Al2O3 was also investigated besides Al2O3 for plasma-catalytic decomposition of N2O.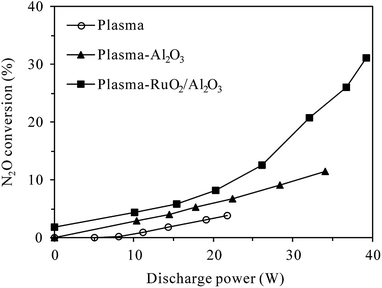 | ||
| Fig. 5 Dependence of N2O conversion on the discharge power in plasma and plasma-catalytic reactors at 300 °C (inlet N2O: 400 ppm; O2 content: 5%). | ||
On the other hand, Fig. 6 shows the production of N2O in the plasma and plasma-catalytic processes at 300 °C without N2O in the inlet gas (inlet gas composition: 5% O2 + N2). It can be seen that for both plasma and plasma-catalytic processes, N2O concentration increased almost linearly with discharge power in the range tested. Packing catalyst in the discharge zone greatly enhanced the production of N2O, but no significant difference was observed in N2O production between the plasma-Al2O3 and plasma-RuO2/Al2O3 systems. Compared to the room-temperature case (Fig. 4), much less N2O was produced at 300 °C for the same O2 content (5%) and discharge power. Considering the low conversion of N2O obtained, especially in the plasma and plasma-Al2O3 processes (Fig. 5), the observed less production of N2O (Fig. 6) should be mainly due to the low effectiveness of N2O formation reactions (e.g., (R3)) at high temperature. In other words, high reaction temperature is favorable not only for conversion of N2O but also for reduction of N2O formation by discharge in N2–O2 mixture. At high temperature (300 °C), introducing catalyst, especially RuO2/Al2O3 into the discharge zone significantly enhances the conversion of N2O, overbalancing its promoting effects on N2O production, which finally results in effective removal of N2O from the N2–O2 mixture (Fig. 5).
| N2(A3Σu+) + O2 → N2 + O + O | (R8) |
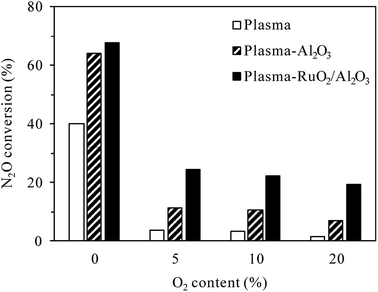
As also shown in Fig. 7, for the same discharge power of 34 W, N2O conversion in the plasma-Al2O3 process decreased from 64.3% for 0% O2 content to 11.4% for 5% O2 content, while that in the plasma-RuO2/Al2O3 process decreased from 67.9% to 24.4%. The superiority of RuO2/Al2O3 over Al2O3 was more pronounced in the O2-containing cases. Further increase of the O2 content from 5% to 10% and 20% caused further decrease of the N2O conversion, but the extent of decrease was less prominent.
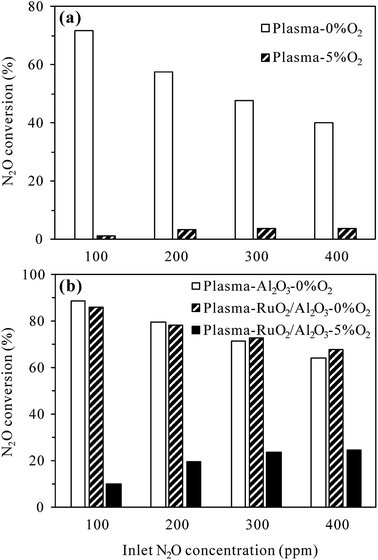
For the plasma-catalytic process (Fig. 8(b)), at a given discharge power of 34 W, the conversion of N2O without O2 also decreased with increasing inlet N2O concentration, but the decrease was less significant compared to that in the plasma process, proving the higher capacity of plasma-catalytic process in decomposing N2O. Besides, it was noticed that the difference in the N2O conversion between the plasma-Al2O3 and plasma-RuO2/Al2O3 processes was insignificant in the absence of O2, indicating the minor role of RuO2 in promoting N2O decomposition under N2 atmosphere although RuO2 greatly improved the N2O conversion under N2–O2 atmosphere (Fig. 5 and 7).
As in the plasma process (Fig. 8(a)), the conversion of N2O in the plasma-RuO2/Al2O3 process also increased with the increase of inlet N2O concentration in the presence of 5% O2, especially from 100 to 200 and 300 ppm (Fig. 8(b)). Further increasing the inlet N2O concentration, e.g., to 400 ppm, showed limited effects in enhancing the N2O conversion, probably due to the limited amount of reactive species (N2(A3Σu+) and O(1D)) produced under a given discharge power for N2O conversion. From the point of fully utilizing the generated reactive species and reducing the negative influence of O2 on N2O conversion, dilute N2O in N2–O2 mixture should be concentrated, e.g., by adsorption–desorption process before being converted by the plasma-catalytic process.20
In addition, it is noteworthy that for the same inlet N2O concentration of 100 ppm and background gas of N2, N2O conversion in the plasma process increased from 47.1% at room temperature (Fig. 3) to 71.6% at 300 °C (Fig. 8(a)) for the same discharge power of 22 W. Similarly, N2O conversion in the plasma-Al2O3 process increased from 66.2% at room temperature (Fig. 3) to 88.7% at 300 °C (Fig. 8(b)) for the same discharge power of 34 W. These results suggest that N2O in N2 could be decomposed more efficiently by plasma and plasma-catalytic processes at higher reaction temperature.
3.3 Mechanism of N2O conversion
On the other hand, as stated in Section 3.2, N2O in N2–O2 mixture could be removed by plasma and plasma-catalytic processes only at high temperature. Fig. S4† shows typical FT-IR spectra of the effluents of plasma and plasma-catalytic reactors with and without 400 ppm-N2O in the inlet gas (O2 content 5%) and before and after discharge at 300 °C. Clearly, discharge in N2–O2 mixture at 300 °C produced N2O, NO and NO2 as byproducts no matter the catalyst was present or not. When N2O was introduced into the N2–O2 mixture, NO and NO2 were also detected besides the residual N2O. In order to clarify whether N2O was converted to NO and NO2 under N2–O2 atmosphere, the outlet concentrations of NO and NO2 detected with and without 400 ppm-N2O in the inlet gas were compared in Fig. 9.
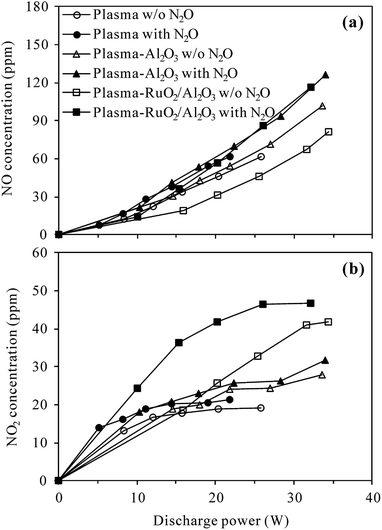 | ||
| Fig. 9 Concentrations of (a) NO and (b) NO2 formed with and without 400 ppm N2O in the inlet gas of plasma and plasma-catalytic reactors at 300 °C (O2 content: 5%). | ||
Overall, NO and NO2 concentrations increased with the increase of discharge power and the presence of 400 ppm-N2O in the N2–O2 mixture did not obviously change the variation trends of NO or NO2 concentrations. For a given discharge power, however, higher concentrations of NO and NO2 were always detected when N2O was introduced, especially in the plasma-RuO2/Al2O3 process. This result revealed that N2O was partially transformed into NO and NO2 during the O2-containing conversion processes, being in agreement with the observations of Krawczyk et al.24,25 Table 1 lists the selectivity of NO, NO2 and NOx (NO + NO2) at typical discharge power in the plasma and plasma-catalytic processes. As seen, the selectivity of NOx ranged from 28.7% of the plasma-Al2O3 process at 34.0 W to 79.5% of the plasma process at 14.4 W. Other removed N2O should have been degraded to benign N2 and O2 since no N-containing byproducts other than NO and NO2 were observed.
| Process | Discharge power (W) | Selectivity of NO (%) | Selectivity of NO2 (%) | Selectivity of NOx (%) |
|---|---|---|---|---|
| Plasma | 14.4 | 59.2 | 20.3 | 79.5 |
| 21.8 | 37.1 | 7.6 | 44.7 | |
| Plasma-Al2O3 | 14.4 | 34.0 | 6.5 | 40.5 |
| 34.0 | 24.7 | 4.0 | 28.7 | |
| Plasma-RuO2/Al2O3 | 15.4 | 36.9 | 38.7 | 75.6 |
| 32.1 | 28.4 | 3.4 | 31.8 |
Table 1 also shows that compared to the plasma process, the selectivity of NOx was relatively low in the plasma-catalytic process, indicating that catalyst packed in the discharge zone promoted N2O decomposition to N2 and O2 to a larger extent than oxidation to NO and NO2. For all processes, the selectivity of NO and NO2 decreased with the increase of discharge power, probably due to the substantial increase of NO and NO2 formation from plasma-induced reactions between N2 and O2 which competitively consumed oxidative species, as shown in reactions (R9)–(R12).27,29,32,37 Besides, the selectivity of NO was much higher than that of NO2 except at low discharge power of the plasma-RuO2/Al2O3 process. This can be easily ascribed to the step-wise oxidation of N2O (first (R5) and then (R11) and (R12)) under oxidative plasma atmosphere.27,32,37
| N2(A3Σu+) + O → NO + N(2D) | (R9) |
| N(2D) + O2→ NO + O | (R10) |
| NO + O + M → NO2 + M (M = N2, O2, NO, NO2, N2O) | (R11) |
| NO + O3 → NO2 + O2 | (R12) |
Compared to Al2O3 catalyst, RuO2/Al2O3 catalyst significantly enhanced the selectivity of NO2 at low discharge power. At higher discharge power, however, the difference in the selectivity of NO and NO2 between the two plasma-catalytic processes became insignificant. At the relatively high discharge power tested, more than 40% and ca. 30% of the removed nitrogen in N2O was transformed into NOx in the plasma and plasma-catalytic process, respectively.
According to literatures, the decomposition of N2O over metal oxide catalysts can be expressed as a Langmuir–Hinshelwood mechanism:
| N2O + * → N2 + O* | (R13) |
| O* + O* → O2 + 2* | (R14) |
| O + O* → O2 + * | (R15) |
4. Conclusions
In the present work, conversion of dilute N2O in N2 and N2–O2 mixtures by plasma and plasma-catalytic processes was investigated at both room and high temperature (300 °C). It is found that N2O in N2 can be effectively decomposed to N2 and O2 by plasma and plasma-catalytic processes at both room and high temperature, with much higher decomposition efficiency at 300 °C than at room temperature for the same discharge power. However, N2O in N2–O2 mixture can be removed only at high temperature, producing not only N2 and O2 but also NO and NO2. Production and conversion of N2O occur simultaneously during the plasma and plasma-catalytic processing of N2O in N2–O2 mixture, with production and conversion being the dominant process at room and high temperature, respectively.N2O conversion increases with the increase of discharge power and decreases with the increase of O2 content. The negative influence of O2 on N2O conversion could be suppressed to some extent by concentrating N2O in N2–O2 mixture which increases the involvement of plasma reactive species (e.g., N2(A3Σu+) and O(1D)) in N2O conversion. Introducing catalyst, especially RuO2/Al2O3 into the discharge zone significantly enhances the conversion of N2O and improves the selectivity of N2O decomposition under N2–O2 atmosphere, revealing the synergy of plasma and catalyst in promoting N2O conversion, especially its decomposition to N2 and O2. The combined plasma-catalytic processing may be an efficient way for reducing N2O emissions from combustion and industrial sources.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant numbers 21707004, 51638001) and the Natural Science Foundation of Beijing Municipality (grant number 8152011).References
- J. Pérez-RamíRez, F. Kapteijn, K. Schöffel and J. A. Moulijn, Appl. Catal., B, 2003, 44, 117–151 CrossRef.
- K. R. Sistani, M. Jn-Baptiste, N. Lovanh and K. L. Cook, J. Environ. Qual., 2011, 40, 1797–1805 CrossRef PubMed.
- M. Konsolakis, ACS Catal., 2015, 5, 6397–6421 CrossRef.
- A. Ates, A. Reitzmann, C. Hardacre and H. Yalcin, Appl. Catal., A, 2011, 407, 67–75 CrossRef.
- F. Zhang, X. Wang, X. Zhang, M. Turxun, H. Yu and J. Zhao, Chem. Eng. J., 2014, 256, 365–371 CrossRef.
- M. Konsolakis, F. Aligizou, G. Goula and I. V. Yentekakis, Chem. Eng. J., 2013, 230, 286–295 CrossRef.
- S. S. Kim, S. J. Lee and S. C. Hong, Chem. Eng. J., 2011, 169, 173–179 CrossRef.
- Z. Liu, C. He, B. Chen and H. Liu, Catal. Today, 2017, 297, 78–83 CrossRef.
- H. L. Chen, H. M. Lee, S. H. Chen, M. B. Chang, S. J. Yu and S. N. Li, Environ. Sci. Technol., 2009, 43, 2216–2227 CrossRef PubMed.
- J. V. Durme, J. Dewulf, C. Leys and H. V. Langenhove, Appl. Catal., B, 2008, 78, 324–333 CrossRef.
- X. Fan, T. L. Zhu, Y. F. Sun and X. Yan, J. Hazard. Mater., 2011, 196, 380–385 CrossRef PubMed.
- Y. J. Wan, X. Fan and T. L. Zhu, Chem. Eng. J., 2011, 171, 314–319 CrossRef.
- X. Fan, T. L. Zhu, Y. J. Wan and X. Yan, J. Hazard. Mater., 2010, 180, 616–621 CrossRef PubMed.
- X. Fan, T. L. Zhu, M. Y. Wang and X. M. Li, Chemosphere, 2009, 75, 1301–1306 CrossRef PubMed.
- Q. Yu, Y. Gao, X. Tang, H. Yi, R. Zhang, S. Zhao, F. Gao and Y. Zhou, Catal. Commun., 2018, 110, 18–22 CrossRef.
- A. Mizuno, Catal. Today, 2013, 211, 2–8 CrossRef.
- X. Hu, J. Nicholas, J. Zhang, T. M. Linjewile, P. D. Filippis and P. K. Agarwal, Fuel, 2002, 81, 1259–1268 CrossRef.
- G. B. Zhao, X. D. Hu, M. D. Argyle and M. Radosz, Ind. Eng. Chem. Res., 2004, 43, 5077–5088 CrossRef.
- S. Mahammadunnisa, E. L. Reddy, P. R. M. K. Reddy and C. Subrahmanyam, Plasma Processes Polym., 2013, 10, 444–450 CrossRef.
- Q. Trinh, S. H. Kim and Y. S. Mok, Chem. Eng. J., 2016, 302, 12–22 CrossRef.
- D. H. Lee and T. Kim, N2O decomposition by catalyst-assisted cold plasma, 20th Int. Symp. Plasma Chem., Philadelphia, USA, 2012 Search PubMed.
- H. Hu, H. Huang, J. Xu, Q. Yang and G. Tao, Plasma Sci. Technol., 2015, 17, 1043–1047 CrossRef.
- J. Jo, Q. H. Trinh, S. H. Kim and Y. S. Mok, Catal. Today, 2018, 310, 42–48 CrossRef.
- K. Krawczyk and M. Młotek, Appl. Catal., B, 2001, 30, 233–245 CrossRef.
- K. Schmidt-Szałowski, K. Krawczyk and M. Młotek, J. Adv. Oxid. Technol., 2007, 10, 330–336 Search PubMed.
- G. Pekridis, C. Athanasiou, M. Konsolakis, I. V. Yentekakis and G. E. Marnellos, Top. Catal., 2009, 52, 1880–1887 CrossRef.
- I. A. Kossyi, A. Y. Kostinsky, A. A. Matveyev and V. P. Silakov, Plasma Sources Sci. Technol., 1992, 1, 207–220 CrossRef.
- M. P. Iannuzzi, J. B. Jeffries and F. Kaufman, Chem. Phys. Lett., 1982, 87, 570–574 CrossRef.
- X. Tang, J. Wang, H. Yi, S. Zhao, F. Gao, Y. Huang, R. Zhang and Z. Yang, Energy Fuels, 2017, 31, 13901–13908 CrossRef.
- J. T. Herron and D. S. Green, Plasma Chem. Plasma Process., 2001, 21, 459–481 CrossRef.
- K. Krawczyk, IEEE Trans. Plasma Sci., 2009, 37, 884–889 Search PubMed.
- G. B. Zhao, S. Garikipati, X. D. Hu, M. D. Argyle and M. Radosz, AIChE J., 2005, 51, 1800–1812 CrossRef.
- G. Sathiamoorthy, S. Kalyana, W. C. Finney, R. J. Clark and B. R. Locke, Ind. Eng. Chem. Res., 1999, 38, 1844–1855 CrossRef.
- S. Kanazawa, J. S. Chang, G. F. Round, G. Sheng, T. Ohkubo, Y. Nomoto and T. Adachi, J. Electrost., 1997, 40–41, 651–656 CrossRef.
- Y. S. Mok, J. H. Kim, I. S. Nam and S. W. Ham, Ind. Eng. Chem. Res., 2000, 39, 3938–3944 CrossRef.
- M. S. Bak, W. Kim and M. A. Cappelli, Appl. Phys. Lett., 2011, 98, 011502 CrossRef.
- Y. Zhang, X. Tang, H. Yi, Q. Yu, J. Wang, F. Gao, Y. Gao, D. Li and Y. Cao, RSC Adv., 2016, 6, 63946–63953 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra05607b |
| This journal is © The Royal Society of Chemistry 2018 |