Open Access Article
Open Access ArticleThe synthesis of HMF-based α-amino phosphonates via one-pot Kabachnik–Fields reaction†
Weigang Fan,
Yves Queneau * and
Florence Popowycz
* and
Florence Popowycz *
*
Université de Lyon, INSA Lyon, ICBMS, UMR 5246, CNRS – Université Lyon 1 – CPE Lyon, Bâtiment Lederer, F-69622 Villeurbanne Cedex, France. E-mail: yves.queneau@insa-lyon.fr; florence.popowycz@insa-lyon.fr
First published on 7th September 2018
Abstract
The first use of biomass-derived HMF in the one-pot Kabachnik–Fields reaction is reported here. A wide range of furan-based α-amino phosphonates were prepared in moderate to excellent yields under mild, effective and environmentally-benign conditions: iodine as a non-metal catalyst, biobased 2-MeTHF as the solvent and room or moderate temperature. The hydroxymethyl group of HMF persists in the Kabachnik–Fields products, widening the scope of further modification and derivatization compared to those arising from furfural. Issues involving the diastereoselectivity and double Kabachnik–Fields condensation were also faced.
Recently, the production of chemicals from renewable biomass has attracted growing interests due to the dwindling reserves of fossil resources and the increasing awareness of environmental concerns.1 5-Hydroxymethylfurfural (HMF), a promising primary biomass-derived platform chemical readily obtained from acid-catalyzed dehydration of six-carbon carbohydrates, displays a strong potential in organic synthesis.2 Besides the well-developed conversions of HMF towards monomers and biofuels via oxidation or reduction reactions,3 some remarkable strategies converting HMF to high value-added fine chemicals have been disclosed.4 Nevertheless, the specific reactivity and reduced stability of HMF, in comparison with the pentose-derived furfural homolog, have limited its use in synthetic applications.5 Furthermore, its commercial availability, though not anymore a barrier nowadays, has limited the number of investigations in the past. In this regard, developing efficient and economic routes to existing or novel fine chemicals from HMF, with its different reactivity compared to simpler aldehydes, is still a challenge.
Multi-component reactions (MCRs) are extremely convenient and efficient strategies to prepare highly functionalized compounds from simple starting materials by one-pot procedures. Since they have many advantages, such as high atom economy, high convergence, time and energy saving, MCRs have gained much attention in modern synthetic organic chemistry.6 Nevertheless, to our knowledge, the direct utilization of HMF in MCR processes has been only rarely explored.
α-Amino phosphonates, due to the structural analogy to natural α-amino acids and their significant biological activities, such as antitumor, antitubercular, cytotoxic activities, and so on,7 have been the subject of considerable interest in the past decades both in synthetic organic and medicinal chemistry.8 Among several methods for preparing α-amino phosphonates, the Kabachnik–Fields reaction, a one-pot condensation of an aldehyde, an amine and a dialkyl phosphite is the most effective and convenient strategy.9 A large number of conditions have been reported for the acid-catalyzed (Lewis/Brønsted)10 and catalyst-free11 Kabachnik–Fields reaction, affording the α-amino phosphonates from various aldehydes. However, none of studies have included HMF as a substrate in their scope although the products from HMF can offer more possibilities for further functionalization, thanks to its CH2OH appendage. The sole synthesis of α-amino phosphonates from HMF was reported by Cottier and Skowroński in a two-step reaction strategy, consisting in pre-formation of the imine which upon isolation reacted with dialkyl phosphites as nucleophilic species at high temperature using trifluoroacetic acid as catalyst.12 Thus, a milder and more direct procedure for the synthesis of α-amino phosphonates from HMF is still to be developed.
As part of our on-going interest on the application of HMF towards fine chemicals and on green and sustainable chemistry,13 we explored the possibility to synthesize furan-based α-amino phosphonates via the one-pot Kabachnik–Fields condensation, directly from HMF.
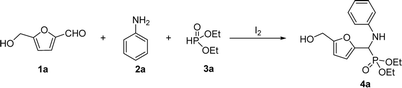
For this study, we selected molecular iodine as a mild and effective Lewis acid catalyst, as often used in multicomponent synthesis because of its operational simplicity, low cost and toxicity and likely to be compatible with HMF sensitivity to acidic conditions.14 Wu and co-workers have confirmed its efficiency in Kabachnik–Fields reactions of simple aldehydes such as benzaldehyde and furfural.15 The primary set of experimental conditions has been fixed as 5 mol% iodine in ethanol [0.5 M] with equimolar stoichiometric ratio of all partners (HMF, aniline, diethyl phosphite). The corresponding Kabachnik–Fields product 4a was obtained in 71% isolated yield after 24 h, together with around 11% of unreacted HMF and 6% of the intermediate imine (Table 1, entry 1).
| Entry | Cat. loading | Solvent [0.5 M] | Temp. | Ratio 1a/2a/3a | Time | Isolated yield |
|---|---|---|---|---|---|---|
| a The reaction was carried out in a sealed tube with HMF, aniline, diethyl phosphite, solvent and iodine, stirred at corresponding temperature for indicated time.b 24 h. | ||||||
| 1 | 5 mol% | EtOH | 25 °C | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 h | 71% |
| 2 | 5 mol% | MeCN | 25 °C | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 h | 60% |
| 3 | 5 mol% | DCM | 25 °C | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 h | 31% |
| 4 | 5 mol% | THF | 25 °C | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 h | 84% |
| 5 | 5 mol% | 2-MeTHF | 25 °C | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 h | 74% |
| 6 | 5 mol% | THF | 25 °C | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
24 h | 90% |
| 7 | 5 mol% | 2-MeTHF | 25 °C | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
8 h | 91% |
| 8 | 2.5 mol% | 2-MeTHF | 25 °C | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
8 h | 77% |
| 9 | 1 mol% | 2-MeTHF | 25 °C | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
8 h | 61% |
| 10 | — | 2-MeTHF | 25 °C | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
8 h | 54% (80%)b |
| 11 | 5 mol% | 2-MeTHF | 50 °C | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
4 h | 83% |
| 12 | 5 mol% | 2-MeTHF | 78 °C | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3 h | 71% |
Based on this preliminary result, the reaction conditions were optimized, first by studying the influence of the solvent. THF was found to provide a better yield than EtOH, MeCN and DCM, affording product 4a in 84% yield (Table 1, entries 1–4). The bio-based 2-methyltetrahydrofuran (2-MeTHF), considered as a greener alternative to THF proved to be also efficient (Table 1 entry 5). Using excess diethyl phosphite (1.5 equivalents) led to better yields both in THF and in 2-MeTHF (Table 1, entries 6 and 7). Considering all the benefits (production from renewable resources, easy degradation, low miscibility with water and enhanced stability),16 we decided to continue the investigation with 2-MeTHF as the solvent.
Decreasing the catalyst loading to 2.5 mol% and 1 mol% led to slightly slower reactions (77% and 61% respectively) (Table 1, entries 8 and 9). It is important to note that the reaction could proceed even without any catalyst, though giving the expected product in a moderate yield (54%) after 8 h, but in a satisfactory 80% yield after 24 h (Table 1, entry 10). Even though the catalyst-free conditions afford good yields in long enough reaction time, the 5 mol% I2 conditions give the best balance between reaction efficiency and reaction duration, and were preserved for the rest of the study. With respect to temperature, performing the reaction at 50 °C led to total conversion of HMF within 4 h but with a slight decrease of the yield (Table 1, entry 11). Prolongation of the reaction time led to lower yields. The same result was observed when the reaction time (equimolar quantities of all reactants) was refluxed in 2-MeTHF (Table 1, entry 12).
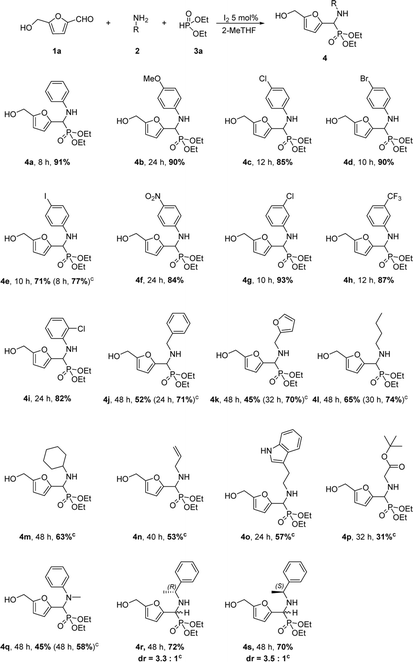
With the optimized conditions in hand (Table 1, entry 7), the scope of the reaction was investigated with respect to the nature of the amine and the phosphite, allowing the access to a library of novel α-amino phosphonates. In Scheme 1 is depicted the scope of amines used in the reaction.
Whatever the electron-donating or electron-withdrawing nature of the para substituent (methoxy-, chloro-, bromo-, iodo- and nitro-) on the aniline, the corresponding α-amino phosphonates 4b–4f were obtained in good to excellent yields (71–90%). An exception was observed for p-iodo-aniline requiring a 50 °C temperature for producing 4e in 77% yield. The same tendency was noticed for meta-substituted anilines, presumed to display low electronic influence on the reactivity (yields of 93% for 4g and 87% for 4h), and in a more unexpected way for 2-chloroaniline (82% for 4i). These results revealed that the substituted group on phenyl ring of aniline has globally a low impact on the reaction. Compared to anilines, aliphatic amines were found consistently as less reactive. In the case of aliphatic amines, elevated temperature (50 °C) was required to promote the reaction. Benzylamine and furfurylamine provided the corresponding α-amino phosphonates 4j and 4k in moderate yields, respectively 71% and 70%. Similar results were obtained for n-butylamine, cyclohexylamine and allylamine (4l–4n). Non-protected tryptamine afforded compound 4o in 57% yield. The product possibly arising from the reaction of the pyrrolic amine of tryptamine was not observed. tert-Butyl glycinate also worked under the conditions but gave a poor yield of 4p (31%). N-Methyl aniline, as an example of secondary amine, was also less reactive than aniline, giving 4q in 58% yield. When the chiral amine (R)-α-methylbenzylamine was used, the mixture of products was obtained 4r in 72% yield, from which the two isomers could not be separated entirely by column chromatography. A moderate diastereoselectivity was observed, with a 3.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of two diastereoisomers observed on the base of 31P NMR spectra. Similarly, (S)-α-methylbenzylamine gave the products 4s as a 3.5
1 ratio of two diastereoisomers observed on the base of 31P NMR spectra. Similarly, (S)-α-methylbenzylamine gave the products 4s as a 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of two diastereoisomers in 70% yield.
1 mixture of two diastereoisomers in 70% yield.
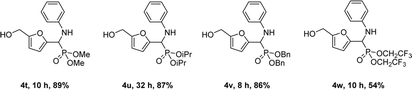
The nature of the dialkyl phosphite was also examined but to a minor extent due to the low diversity of commercially available phosphite reagents (Scheme 2). Dimethyl-, diisopropyl- and dibenzyl-phosphites afforded the corresponding products (4t–4v) in a range of yields of 86–89%. Surprisingly, phosphite with two strongly electron-withdrawing CF3– groups could also be used affording 4w in a modest 54% yield under the optimized conditions.
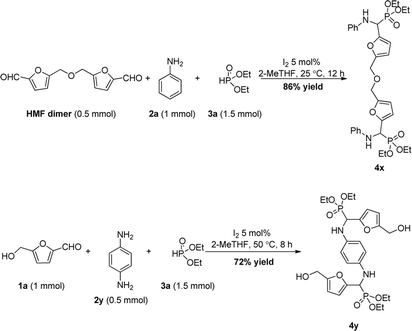
In order to expand the application of HMF, a pre-prepared 5,5′-[oxybis(methylene)]bis-2-furfural via self-etherification of HMF was subjected to the optimized conditions, resulting into the expected double Kabachnik–Fields product 4x in 86% yield. Alternatively, using p-phenylenediamine instead of aniline led to the other type of double Kabachnik–Fields product 4y. The results above undoubtedly indicate the possible application of the strategy towards highly functional polymers via Kabachnik–Fields polycondensation of 5,5′-[oxybis(methylene)]bis-2-furfural and suitable diamines (Scheme 3).17
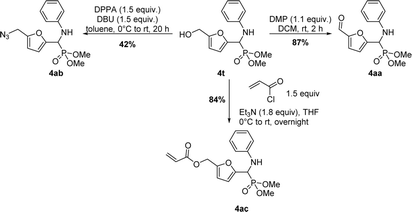
A couple of derivatizations on hydroxyl group of the Kabachnik–Fields product were investigated using 4t as model substrate (Scheme 4). The aldehyde 4aa could be prepared in 87% yield by oxidation of 4t using Dess–Martin periodinane (DMP). The hydroxyl group of 4t could be also converted into an azido group after treatment with diphenylphosphoryl azide in the presence of DBU in 42% yield (compound 4ab). The acrylate 4ac was also easily obtained in a good yield. Diversification and optimization of these reactions are now in progress in the lab for further illustrating the usefulness of the hydroxymethyl appendage and providing a library of new α-amino phosphonates.
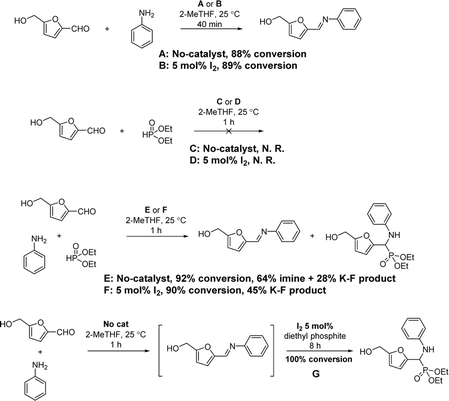
Usually in a multicomponent reaction, the mechanism is not distinct because the reaction may undergo different pathways depending on which reactants react at first step. In order to gain insight into the mechanism of the Kabachnik–Fields reaction in our case, a series of control stepwise experiments were carried out (Scheme 5 and for more details see ESI†). Mixing HMF (1 mmol) and aniline (1 mmol) in 2-MeTHF yielded the imine rapidly with and without iodine, with around 90% conversion observed in the crude NMR after 40 min in both cases (Exp. A and B). Subsequent addition of diethyl phosphite (1.5 mmol) and I2 (5 mol%) to the solution of the in situ formed imine (HMF, aniline, 1 h) afforded cleanly 4a after 8 h as seen by NMR (Exp. G). On the other hand, no reaction occurred when HMF (1 mmol) and diethyl phosphite (1.5 mmol) were mixed, either in the presence or absence of iodine (Exp. C and D). The above results indicate that the reaction likely undergoes the imine pathway, followed by nucleophilic attack by the phosphite to afford the α-amino phosphonate.8e,18 It is also known that iodine, as a Lewis acid, can activate imines in nucleophilic addition reactions.8e,19 This imine pathway was corroborated by the observation of the imine in the crude NMR of the three-component reaction mixture in the absence of iodine (Exp. E). In the presence of iodine, the proton of CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N is shifted from 8.17 ppm to 8.42 ppm which made it difficult to identify, but the imine component was clearly detected in the crude reaction mixture by MS (imine plus H+: 202.0), thus also supporting the imine pathway (Exp. F).
N is shifted from 8.17 ppm to 8.42 ppm which made it difficult to identify, but the imine component was clearly detected in the crude reaction mixture by MS (imine plus H+: 202.0), thus also supporting the imine pathway (Exp. F).
Conclusion
To summarize, we have reported the first application of biomass-derived HMF in the one-pot Kabachnik–Fields reaction, leading to hydroxymethylated heterocyclic α-amino phosphonates. The conditions are simple to settle, effective and environmentally benign. The hydroxymethylfuran moiety in the targeted Kabachnik–Fields products provides additional opportunities for further modification widening the scope of possibly reachable α-amino phosphonates by this route. Moreover, a new possibility for the synthesis of functional polymers via Kabachnik–Fields polycondensation from the 5,5′-[oxybis(methylene)]bis-2-furfural was proposed.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from Université Lyon 1, CNRS, INSA Lyon, CPE Lyon is gratefully acknowledged. We also thank the China Scholarship Council for a PhD grant to W. Fan.Notes and references
- (a) L. T. Mika, E. Csefalvay and A. Nemeth, Chem. Rev., 2018, 118, 505 CrossRef PubMed; (b) Z. Zhang, J. Song and B. Han, Chem. Rev., 2017, 117, 6834 CrossRef PubMed; (c) S. S. Chen, T. Maneerung, D. C. W. Tsang, Y. S. Ok and C.-H. Wang, Chem. Eng. J., 2017, 328, 246 CrossRef; (d) S. Shylesh, A. A. Gokhale, C. R. Ho and A. T. Bell, Acc. Chem. Res., 2017, 50, 2589 CrossRef PubMed; (e) J. G. de Vries, Chem. Rec., 2016, 16, 2787 CrossRef PubMed; (f) L. Wu, T. Moteki, A. A. Gokhale, D. W. Flaherty and F. D. Toste, Chem, 2016, 1, 32 CrossRef; (g) M. Besson, P. Gallezot and C. Pinel, Chem. Rev., 2014, 114, 1827 CrossRef PubMed; (h) P. Gallezot, Chem. Soc. Rev., 2012, 41, 1538 RSC.
- (a) J. G. de Vries, in Adv. Heterocycl. Chem., ed. E. F. V. Scriven and C. A. Ramsden, Academic Press, 2017, vol. 121, p. 247 Search PubMed; (b) P. Domínguez de María and N. Guajardo, ChemSusChem, 2017, 10, 4123 CrossRef PubMed; (c) L. Hu, L. Lin, Z. Wu, S. Zhou and S. Liu, Renewable Sustainable Energy Rev., 2017, 74, 230 CrossRef; (d) R.-J. van Putten, J. C. van der Waal, E. de Jong, C. B. Rasrendra, H. J. Heeres and J. G. de Vries, Chem. Rev., 2013, 113, 1499 CrossRef PubMed; (e) A. A. Rosatella, S. P. Simeonov, R. F. M. Frade and C. A. M. Afonso, Green Chem., 2011, 13, 754 RSC; (f) F. A. Kucherov, L. V. Romashov, K. I. Galkin and V. P. Ananikov, ACS Sustainable Chem. Eng., 2018, 6, 8064 CrossRef.
- (a) S. Biswas, B. Dutta, A. Mannodi-Kanakkithodi, R. Clarke, W. Song, R. Ramprasad and S. L. Suib, Chem. Commun., 2017, 53, 11751 RSC; (b) W. Gong, K. Zheng and P. Ji, RSC Adv., 2017, 7, 34776 RSC; (c) Z. Gui, S. Saravanamurugan, W. Cao, L. Schill, L. Chen, Z. Qi and A. Riisager, ChemistrySelect, 2017, 2, 6632 CrossRef; (d) G. Li, Z. Sun, Y. Yan, Y. Zhang and Y. Tang, ChemSusChem, 2017, 10, 494 CrossRef PubMed; (e) J. Li, G. Lv, B. Lu, Y. Wang, T. Deng, X. Hou and Y. Yang, Energy Technol., 2017, 5, 1429 CrossRef; (f) Y.-M. Li, X.-Y. Zhang, N. Li, P. Xu, W.-Y. Lou and M.-H. Zong, ChemSusChem, 2017, 10, 304 CrossRef; (g) S. M. McKenna, P. Mines, P. Law, K. Kovacs-Schreiner, W. R. Birmingham, N. J. Turner, S. Leimkuhler and A. J. Carnell, Green Chem., 2017, 19, 4660 RSC; (h) D. K. Mishra, H. J. Lee, J. Kim, H.-S. Lee, J. K. Cho, Y.-W. Suh, Y. Yi and Y. J. Kim, Green Chem., 2017, 19, 1619 RSC; (i) Q. Wang, W. Hou, S. Li, J. Xie, J. Li, Y. Zhou and J. Wang, Green Chem., 2017, 19, 3820 RSC; (j) S. Xu, P. Zhou, Z. Zhang, C. Yang, B. Zhang, K. Deng, S. Bottle and H. Zhu, J. Am. Chem. Soc., 2017, 139, 14775 CrossRef PubMed; (k) H. Zhang, Q. Wu, C. Guo, Y. Wu and T. Wu, ACS Sustainable Chem. Eng., 2017, 5, 3517 CrossRef; (l) J. Li, J.-l. Liu, H.-y. Liu, G.-y. Xu, J.-j. Zhang, J.-x. Liu, G.-l. Zhou, Q. Li, Z.-h. Xu and Y. Fu, ChemSusChem, 2017, 10, 1436 CrossRef PubMed.
- (a) O. G. Mohamed, Z. G. Khalil and R. J. Capon, Org. Lett., 2018, 20, 377 CrossRef PubMed; (b) A. J. Kumalaputri, C. Randolph, E. Otten, H. J. Heeres and P. J. Deuss, ACS Sustainable Chem. Eng., 2018, 6, 3419 CrossRef PubMed; (c) M.-M. Zhu, L. Tao, Q. Zhang, J. Dong, Y.-M. Liu, H.-Y. He and Y. Cao, Green Chem., 2017, 19, 3880 RSC; (d) F. A. Kucherov, K. I. Galkin, E. G. Gordeev and V. P. Ananikov, Green Chem., 2017, 19, 4858 RSC; (e) K. Galkin, F. Kucherov, O. Markov, K. Egorova, A. Posvyatenko and V. Ananikov, Molecules, 2017, 22, 2210 CrossRef PubMed; (f) S. Tšupova, F. Rominger, M. Rudolph and A. S. K. Hashmi, Green Chem., 2016, 18, 5800 RSC; (g) H. Sugimura, M. Kikuchi, S. Kato, W. Sekita and I. Sasaki, Tetrahedron, 2016, 72, 7638 CrossRef; (h) L. V. Romashov and V. P. Ananikov, Org. Biomol. Chem., 2016, 14, 10593 RSC; (i) S. Sowmiah, L. F. Veiros, J. M. Esperanca, L. P. Rebelo and C. A. Afonso, Org. Lett., 2015, 17, 5244 CrossRef PubMed; (j) P. F. Koh and T. P. Loh, Green Chem., 2015, 17, 3746 RSC; (k) J. P. M. Antonio, R. F. M. Frade, F. M. F. Santos, J. A. S. Coelho, C. A. M. Afonso, P. M. P. Gois and A. F. Trindade, RSC Adv., 2014, 4, 29352 RSC.
- K. I. Galkin, E. A. Krivodaeva, L. V. Romashov, S. S. Zalesskiy, V. V. Kachala, J. V. Burykina and V. P. Ananikov, Angew. Chem., Int. Ed., 2016, 55, 8338 CrossRef PubMed.
- (a) H. G. O. Alvim, J. R. Correa, J. A. F. Assumpção, W. A. da Silva, M. O. Rodrigues, J. L. de Macedo, M. Fioramonte, F. C. Gozzo, C. C. Gatto and B. A. D. Neto, J. Org. Chem., 2018, 83, 4044 CrossRef PubMed; (b) G. Mari, M. Verboni, L. De Crescentini, G. Favi, S. Santeusanio and F. Mantellini, Org. Chem. Front., 2018, 5, 2108 RSC; (c) X. Chang, X. Zhang and Z. Chen, Org. Biomol. Chem., 2018, 16, 4279 RSC; (d) R. Mishra, A. Jana, A. K. Panday and L. H. Choudhury, Org. Biomol. Chem., 2018, 16, 3289 RSC; (e) G.-L. Wu and Q.-P. Wu, Adv. Synth. Catal., 2018, 360, 1949 CrossRef.
- (a) S. A. R. Mulla, M. Y. Pathan, S. S. Chavan, S. P. Gample and D. Sarkar, RSC Adv., 2014, 4, 7666 RSC; (b) X.-C. Huang, M. Wang, Y.-M. Pan, G.-Y. Yao, H.-S. Wang, X.-Y. Tian, J.-K. Qin and Y. Zhang, Eur. J. Med. Chem., 2013, 69, 508 CrossRef PubMed; (c) Ł. Winiarski, J. Oleksyszyn and M. Sieńczyk, J. Med. Chem., 2012, 55, 6541 CrossRef PubMed; (d) Z. Rezaei, H. Firouzabadi, N. Iranpoor, A. Ghaderi, M. R. Jafari, A. A. Jafari and H. R. Zare, Eur. J. Med. Chem., 2009, 44, 4266 CrossRef PubMed.
- (a) R. M. Abdel-Rahman, T. E. Ali and S. M. Abdel-Kariem, ARKIVOC, 2016, 2016, 183 Search PubMed; (b) T. E. Ali and S. M. Abdel-Kariem, ARKIVOC, 2015, 2015, 246 Search PubMed; (c) M. Ordóñez, J. L. Viveros-Ceballos, C. Cativiela and F. J. Sayago, Tetrahedron, 2015, 71, 1745 CrossRef; (d) K. Ramakrishna, J. M. Thomas and C. Sivasankar, J. Org. Chem., 2016, 81, 9826 CrossRef PubMed; (e) Y.-Q. Yu, Synthesis, 2013, 2545 CrossRef; (f) Y. Gao, Z. Huang, R. Zhuang, J. Xu, P. Zhang, G. Tang and Y. Zhao, Org. Lett., 2013, 15, 4214 CrossRef PubMed; (g) M. Ordóñez, H. Rojas-Cabrera and C. Cativiela, Tetrahedron, 2009, 65, 17 CrossRef PubMed; (h) K. C. Kumara Swamy, S. Kumaraswamy, K. Senthil Kumar and C. Muthiah, Tetrahedron Lett., 2005, 46, 3347 CrossRef.
- (a) M. Haji, Beilstein J. Org. Chem., 2016, 12, 1269 CrossRef PubMed; (b) G. Keglevich and E. Bálint, Molecules, 2012, 17, 12821 CrossRef PubMed; (c) N. S. Zefirov and E. D. Matveeva, ARKIVOC, 2008, 2008, 1 Search PubMed; (d) V. I. G. R. A Cherkasov, Russ. Chem. Rev., 1998, 67, 857 CrossRef; (e) E. K. Fields, J. Am. Chem. Soc., 1952, 74, 1528 CrossRef; (f) M. I. Kabachnik and T. Y. Medved, Dokl. Akad. Nauk SSSR, 1952, 83, 689 Search PubMed.
- (a) J. Kim, Y. Heo, Y. Jung, J. Lee and I. Kim, Tetrahedron, 2017, 73, 5759 CrossRef; (b) C. K. Khatri, V. B. Satalkar and G. U. Chaturbhuj, Tetrahedron Lett., 2017, 58, 694 CrossRef; (c) E. Idris and T. Soufiane, Curr. Org. Synth., 2017, 14, 272 CrossRef; (d) X.-C. Li, S.-S. Gong, D.-Y. Zeng, Y.-H. You and Q. Sun, Tetrahedron Lett., 2016, 57, 1782 CrossRef; (e) N. Domingues, A. de Oliveira, R. Katla, M. Rocha, T. Albuquerque, C. da Silva, V. Kupfer and A. Rinaldi, Synthesis, 2016, 4489 CrossRef; (f) H. Eshghi, M. Mirzaei, M. Hasanpour and M. Mokaber-Esfahani, Phosphorus, Sulfur Silicon Relat. Elem., 2015, 190, 1606 CrossRef; (g) M. Nazish, S. Saravanan, N.-u. H. Khan, P. Kumari, R. I. Kureshy, S. H. R. Abdi and H. C. Bajaj, ChemPlusChem, 2014, 79, 1753 Search PubMed; (h) P. S. Reddy, P. V. G. Reddy and S. M. Reddy, Tetrahedron Lett., 2014, 55, 3336 CrossRef; (i) M. Shen, S. Shang, Z. Song, D. Wang, X. Rao, H. Gao and J. Wang, Synth. Commun., 2014, 44, 361 CrossRef; (j) M. Varalakshmi, D. Srinivasulu, D. Rajasekhar, C. N. Raju and S. Sreevani, Phosphorus, Sulfur Silicon Relat. Elem., 2014, 189, 106 CrossRef; (k) N. Louaisil, N. Rabasso and A. Fadel, Synthesis, 2007, 289 Search PubMed.
- (a) Z. Long, M. Liu, R. Jiang, G. Zeng, Q. Wan, H. Huang, F. Deng, Y. Wan, X. Zhang and Y. Wei, Ultrason. Sonochem., 2017, 35, 319 CrossRef PubMed; (b) R. M. N. Kalla, J. Bae and I. Kim, New J. Chem., 2017, 41, 6653 RSC; (c) J. Lewkowski and M. Rodriguez Moya, Phosphorus, Sulfur Silicon Relat. Elem., 2017, 192, 713 CrossRef; (d) E. Bálint, Á. Tajti, D. Kalocsai, B. Mátravölgyi, K. Karaghiosoff, M. Czugler and G. Keglevich, Tetrahedron, 2017, 73, 5659 CrossRef; (e) E. Mollashahi, H. Gholami, M. Kangani, M. Lashkari and M. T. Maghsoodlou, Heteroat. Chem., 2015, 26, 322 CrossRef; (f) K. Azizi, M. Karimi and A. Heydari, Tetrahedron Lett., 2014, 55, 7236 CrossRef; (g) R. Karpowicz, J. Lewkowski, EwaMiękoś and M. Zieliński, Heteroat. Chem., 2014, 25, 163 CrossRef.
- L. Cottier, G. Descotes, J. Lewkowski and R. Skowroński, Phosphorus, Sulfur Silicon Relat. Elem., 1996, 116, 93 CrossRef.
- (a) C. Verrier, S. Moebs-Sanchez, Y. Queneau and F. Popowycz, Org. Biomol. Chem., 2018, 16, 676 RSC; (b) W. Fan, Y. Queneau and F. Popowycz, Green Chem., 2018, 20, 485 RSC; (c) J.-N. Tan, M. Ahmar and Y. Queneau, RSC Adv., 2015, 5, 69238 RSC; (d) J.-N. Tan, M. Ahmar and Y. Queneau, RSC Adv., 2013, 3, 17649 RSC.
- Y.-M. Ren, C. Cai and R.-C. Yang, RSC Adv., 2013, 3, 7182 RSC.
- J. Wu, W. Sun, H.-G. Xia and X. Sun, Org. Biomol. Chem., 2006, 4, 1663 RSC.
- V. Pace, P. Hoyos, L. Castoldi, P. Domínguez de María and A. R. Alcántara, ChemSusChem, 2012, 5, 1369 CrossRef PubMed.
- (a) F. Moldenhauer, R. Kakuchi and P. Theato, ACS Macro Lett., 2016, 5, 10 CrossRef; (b) J.-J. Qiu, Q. Xue, Y.-Y. Liu, M. Pan and C.-M. Liu, Phosphorus, Sulfur Silicon Relat. Elem., 2014, 189, 361 CrossRef.
- (a) E. D. Matveeva and N. S. Zefirov, Dokl. Chem., 2008, 420, 137 CrossRef; (b) R. Gallardo-Macias and K. Nakayama, Synthesis, 2010, 57 Search PubMed.
- (a) B. S. Lee, S. Mahajan and K. D. Janda, Synlett, 2005, 1325 Search PubMed; (b) X.-F. Lin, S.-L. Cui and Y.-G. Wang, Tetrahedron Lett., 2006, 47, 3127 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra05983g |
| This journal is © The Royal Society of Chemistry 2018 |