Open Access Article
Open Access ArticleVersatility of CoPcS in CoPcS/TiO2 for MB degradation: photosensitization, charge separation and oxygen activation
Zhao Gao ,
Hanpei Yang*,
Hongyu Zhu,
Runqiang Guo and
Junmin Wu
,
Hanpei Yang*,
Hongyu Zhu,
Runqiang Guo and
Junmin Wu
Key Laboratory of Integrated Regulation and Resource Development on Shallow Lakes, Ministry of Education, College of Environment, Hohai University, Nanjing 210098, China. E-mail: yanghanpei@hhu.edu.cn; Fax: +86 25 83786090; Tel: +86 25 84968465
First published on 30th October 2018
Abstract
In this report, a composite photocatalyst consisting of cobalt phthalocyanine sulfate (CoPcS) and TiO2 was prepared by a facile synthesis. Careful characterizations and measurements indicate a covalent grafting of CoPcS onto TiO2 through Ti–O–S linkages, acquiring an intimate heterojunction between TiO2 and CoPcS. The obtained composite was evaluated for its photocatalytic activity toward the degradation of methyl blue (MB) under visible light irradiation. The evaluation showed a significantly enhanced degradation rate of MB by CoPcS/TiO2. The improved photocatalytic performance of CoPcS/TiO2 was attributed to the photosensitization of TiO2 by CoPcS, charge separation by electron transfer at the interface of the heterojunction formed between CoPcS and TiO2, and oxygen activation via CoPcS. A synergetic mechanism in improving the photocatalytic performance of TiO2 by CoPcS was investigated.
1. Introduction
Over the past few decades, titanium dioxide (TiO2) has been widely studied in pollution control.1 However, it usually shows inertness under visible light irradiation and low quantum yield in light energy utilization.2 Recent strategies focused on grafting photosensitizers or semiconductors onto TiO2 for expanding their ranges of light absorbance and inhibiting the recombination of photogenerated electron–hole pairs.3,4For an effective photosensitizer of TiO2, two important criteria are required:5 the photoactive compound should have a high extinction coefficient in the visible region, and be capable of being adsorbed on the TiO2 surface via physical/chemical interaction. In constructing heterojunctions, the band gap of the semiconductor used to modify TiO2 should be narrow, and its conduction and valence band positions should be matched with that of TiO2, respectively.4 On these accounts, metal phthalocyanines (MPcs, M = Fe, Co) are benign candidates for the modification of TiO2. More interestingly, the M–N4 structure in MPcs can increase the O–O length of oxygen, which would play an important role in promoting the production of superoxide radical (·O2−) from O2.6 Many efforts have been devoted to coupling of MPcs with TiO2.3,7,8 However, the recognition on the versatility of MPcs in photocatalysis is insufficient, especially, the function of MPcs in activating oxygen in the process of degrading organic pollutants.
In this report, cobalt phthalocyanine sulfate (CoPcS) was composited with TiO2, and the experiments on MB degradation over CoPcS/TiO2 were conducted. The multiple roles of CoPcS in composite were investigated and synergy in photosensitization, charge separation and oxygen activation was proposed.
2. Experimental
2.1. Synthesis of samples
All the reagents used in this experiment were received without further purification. The CoPcS/TiO2 composite was fabricated via a hydrothermal route. In a typical preparation, 60 mg CoPcS (optical) and 20 ml absolute ethyl alcohol were mixed and ultrasounded for 30 min to get a homogeneous turbid liquid. Subsequently, another 16 ml absolute ethyl alcohol, 3.2 ml acetic acid and 10 ml Ti(C4H9O)4 were added into the mixture, followed by dropwise addition of 2 ml deionized water under vigorous stirring for 1 h. Then, the compound was loaded into a 100 ml stainless steel autoclave, sealed and moved into an oven and kept at 180 °C for 10 h. After cooling the autoclave to room temperature, the precipitate was washed with ethanol and deionized water thrice, and then dried at 80 °C for 24 h. Finally, the solid was annealed at 300 °C for 2 h in purity N2. For comparison, TiO2 were prepared under same procedure without adding of CoPcS.2.2. Characterizations and measurements
X-ray diffraction (XRD) analysis were performed on a Shimadzu-3A diffractometer at 40 kV and 30 mA with Cu Kα radiation (λ = 0.15418 nm). The morphologies were examined by transmission electron microscopy (TEM, JEM-2100CX, JEOL). Infrared spectra (FT-IR) were acquired with an 8400S spectrometer (Shimadzu) in the transmission mode. X-ray photoelectron spectra (XPS) were obtained by a PHI 5000 Versa Probe spectrometer (ULVAC-PHI) operated at a voltage of 13 kV and an emission current of 28 mA using Al Kα as exciting source (1486.6 eV). The binding energies were referenced to C 1s at 284.5 eV. The UV-vis absorption spectra of samples were obtained from a Shimadzu UV-3600 spectrophotometer equipped with an integrating sphere using BaSO4 as reference. Photoluminescence (PL) spectra were recorded on F-7000 fluorescence spectrophotometer (Hitachi) with a laser excitation of 420 nm. Electron paramagnetic resonance (EPR) signals of paramagnetic species spin-trapped with DMPO were recorded at ambient temperature (298 K) with a Brucker EPR 300E spectrometer, the irradiation source (λ = 532 nm) was a Quanta-Ray Nd:YAG (10 pluses per second) laser system.2.3. Photocatalytic oxidation experiments
The visible-light-driven photocatalytic activity of the as-prepared samples was monitored from the results of the degradation of MB. For each photocatalytic activity measurements, 10 mg of as-prepared catalysts were dispersed into 100 ml of MB solution initialized at 5 mg L−1. The light comes from a 300 W xenon lamp (CEL-HXF-300, Education Au-light Co., Ltd., Beijing, China) equipped with a UV cutoff filter (λ ≥ 400 nm). The photocatalytic reactions took place in the reactor connected to a water bath to main the solution at about 25 °C and the reaction aqueous slurries were magnetic stirred and bubbled with air at a flow rate of 40 ml min−1. The suspension was stirred in the dark for 1 h to obtain adsorption equilibrium of MB before illumination. During the photo-reaction, samples were collected at selected time intervals. The catalyst powders were removed by filtration and the residual concentration of MB was determined by the spectrophotometer. Quenching experiments were conducted under same conditions except the existence of each scavenger in 10 mM of ethylenediamine tetraacetic acid disodium (EDTA-Na2, for ·O2−), tert-butyl alcohol (tBA, for ·OH) and p-benzoquinone (pBQ, for h+).3. Results and discussion
3.1. Morphology and structure
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N at 1638 cm−1.15,16 The peak centered at 917, 1040 and 1232 cm−1 is attributed to Co–N,17 C–N14 and S–O18 in CoPcS, sequentially. The broad peak at 608 cm−1 is induced by Ti–O–Ti and Ti–O–S. This is a strong evidence of covalent attaching of CoPcS on TiO2. The linkage between CoPcS and TiO2 is proposed as Fig. 2B.
N at 1638 cm−1.15,16 The peak centered at 917, 1040 and 1232 cm−1 is attributed to Co–N,17 C–N14 and S–O18 in CoPcS, sequentially. The broad peak at 608 cm−1 is induced by Ti–O–Ti and Ti–O–S. This is a strong evidence of covalent attaching of CoPcS on TiO2. The linkage between CoPcS and TiO2 is proposed as Fig. 2B.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–C, C–N and C
C, C–C, C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N in CoPcS, respectively.19–22 The O 1s (Fig. 3B) composed of three peaks, the deconvoluted peak observed at 529.3 eV corresponds to the Ti–O–Ti in TiO2. The peak at a binding energy of 530.4 eV is attributed to Ti–O–S,23 indicating a covalent linkage between CoPcS and TiO2. An obvious component with the binding energy at 532.4 eV can be assigned to the oxygen (*O2) coordinated by CoPcS.24
N in CoPcS, respectively.19–22 The O 1s (Fig. 3B) composed of three peaks, the deconvoluted peak observed at 529.3 eV corresponds to the Ti–O–Ti in TiO2. The peak at a binding energy of 530.4 eV is attributed to Ti–O–S,23 indicating a covalent linkage between CoPcS and TiO2. An obvious component with the binding energy at 532.4 eV can be assigned to the oxygen (*O2) coordinated by CoPcS.24
3.2. Photocatalytic activity of samples
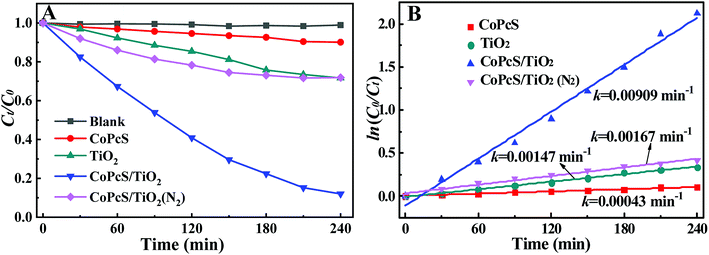
As shown in Fig. 4A, the removal of MB by direct photolysis or photocatalytic degradation on CoPcS was observed negligible. Pure TiO2 exhibited nearly 28.3% of MB degradation mainly attributed to their visible-light-driven activity under self-photosensitization of MB.25 The CoPcS/TiO2 exhibited superior performance on the MB degradation, with the degradation rate of 88% and the pseudo-first-order rate constant of 0.0091 min−1 (almost 6.2 times of that on pure TiO2). Remarkably, obvious decrease in MB degradation was observed on CoPcS/TiO2 under anaerobic conditions (by bubbling N2), suggesting that O2 was crucial in the reaction.3.3. Versatility of CoPcS in CoPcS/TiO2 for MB degradation
 | ||
| Fig. 5 UV-vis diffuse reflectance absorption spectra (A) and their Tauc plot (B) of TiO2 and CoPcS/TiO2. | ||
Under visible light irradiation of CoPcS/TiO2, the singlet excited state (S1) of CoPcS would typically generated from the ground state (S0) and then transformed to triplet excited state (T1) through innersystem crossing.30 The redox potential of S0, S1 and T1 of CoPcS are around 0.46, −1.35 and −0.75 eV (vs. NHE), respectively.31,32 The generation of S1 is normally negligible due to their short lifetime (ns),33 but the excited CoPcS in T1 (ms) can inject charges into the conduction band of TiO2, generating cation radicals of CoPcS (CoPcS+˙). The CoPcS+˙ can participate directly in the degradation of MB33 and contributes to the enhanced activity of CoPcS/TiO2 showed by Fig. 4. Herein, the photosensitization of TiO2 by CoPcS can be expressed as follows:
| CoPcS (S0) + hv → CoPcS (S1) | (1) |
| CoPcS (S1) + hv → CoPcS (T1) | (2) |
| CoPcS (T1) + TiO2 → CoPcS+˙ + TiO2 (eCB−) | (3) |
| CoPcS+˙ + MB → CoPcS (S0) + degradation products | (4) |
Charge separation on the heterojunction was confirmed by PL measurement. As demonstrated by Fig. 6, the remarkable decrease in PL intensity demonstrated that deposition of CoPcS onto TiO2 decreased the carrier recombination rate and improves the separation efficiency of photogenerated electrons and holes, which was favorable to the degradation of MB.39
The presence of ·O2− and ·OH radicals was further confirmed by the electron spin response (ESR) experiments of CoPcS/TiO2 with 5,5-dimethyl-1-pyrroline (DMPO) as a scavenger in a methanol and an aqueous solution. The four characteristic peaks of DMPO-·O2−41 (Fig. 8A) and the system signature (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 signals) of DMPO–·OH radical adducts42 (Fig. 8B) were both observed. In contrast, no DMPO-·O2− and DMPO-·OH signals emerged for bare TiO2 dispersion.
1 signals) of DMPO–·OH radical adducts42 (Fig. 8B) were both observed. In contrast, no DMPO-·O2− and DMPO-·OH signals emerged for bare TiO2 dispersion.
 | ||
| Fig. 8 DMPO spin-trapping ESR spectra recorded with as-prepared samples in (A) methanol dispersion (for DMPO–·O2−) and (B) aqueous dispersion (for DMPO–·OH) under visible light irradiation. | ||
According to the above results, we consider that oxygen in the reaction is activated by the Co–N4 structure in CoPcS. The electronic configuration of 3d orbital of free Co2+ (in spherical field) is diagrammatically presented as Fig. 10 (a). In a square-planar crystal field offered by CoPcS in Fig. 2B, the degenerate energy level of 3d-orbitals split into four levels as sketched as (b).43 Coordination of dioxygen (as a fifth ligand44) to Co2+ surrounded by the macrocyclic ligand as CoPcS cause a further rearranging of energy into two levels with eg and t2g symmetry as (c) in Fig. 9.45 However, this octahedral symmetric configuration in a non-liner molecular is instable due to the non-full occupation in 3d orbital of Co2+,46 the configuration will be distorted as (d) by a Jahn–Teller effect.47
In such a configuration, most of the interpretations of experimental and theoretical investigation coincided in the conclusion that the 3dz2 orbital is half filled.48 A σ-rich orbital of O2 donates electron density to 3dz2 of Co2+, forming a σ-type bond, while a π interaction is produced between the dπ (dxz, dyz) orbitals and π* orbitals of dioxygen, with charge transfer from metal to O2.49 This electrons rearranging get oxygen activated and increase the O–O bond length from the usual 1.21 to ∼1.30 Å.6 According to literature, the redox potential of oxygen in ground state E (3O2/·O2−) is around −0.048 eV.50 However, with the activation, the potential value can increase to ∼0.77 eV,6 which is more positive than that of ECB in TiO2.
Based on the above results, the synergy of photosensitization, charge separation and oxygen activation on CoPcS/TiO2 was proposed as Fig. 10. The electrons generated by photosensitization and charge separation on the conduction band of TiO2 can be more easily trapped by the activated oxygen (*O2), derivating more ·O2− species participating in degrading MB. Some of the ·O2− reacts with H+, followed by producing ·OH of a redox potential of 2.4 eV. The redox potentials of generated CoPcS+˙ and holes were 1.2 and 1.05 eV, respectively.37,38 Thus, the MB was oxidized by ·OH, CoPcS+˙ and holes. By this way, the photocatalytic activity of CoPcS/TiO2 in degrading MB was enhanced.
4. Conclusion
In this work, TiO2 was composited with CoPcS via the Ti–O–S linkage. The photosensitization of TiO2 by CoPcS and charge separation on the heterojunction were promoted. At the same time, the oxygen was activated by CoPcS. Due to the versatility of CoPcS on TiO2, the degradation rate of MB over CoPcS/TiO2 reached 88% under visible light in 4 h. This synergy is of great potential for design of high-photoreactive catalysts using CoPcS as a component.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The project was financially supported by the Foundation of National Key Scientific Instrument and Equipment Development Project of China (No. 2014YQ060773), the Priority Academic Program Development of Jiangsu Higher Education Institutions.References
- G. Y. Rao, Q. Y. Zhang, H. L. Zhao, J. T. Chen and Y. Li, Novel titanium dioxide/iron (III) oxide/graphene oxide photocatalytic membrane for enhanced humic acid removal from water, Chem. Eng. J., 2016, 302, 633–640 CrossRef CAS.
- I. C. Kang, Q. Zhang, S. Yin, T. Sato and F. Saito, Improvement in photocatalytic activity of TiO2 under visible irradiation through addition of N-TiO2, Environ. Sci. Technol., 2008, 42(10), 3622–3626 CrossRef CAS PubMed.
- Q. Sun and Y. M. Xu, Sensitization of TiO2 with aluminum phthalocyanine: factors influencing the efficiency for chlorophenol degradation in water under visible light, J. Phys. Chem. C, 2009, 113, 12387–12394 CrossRef CAS.
- Q. C. Xu, D. V. Wellia, Y. H. Ng, R. Amal and T. T. Y. Tan, Synthesis of porous and visible-light absorbing Bi2WO6/TiO2 heterojunction films with improved photoelectrochemical and photocatalytic performances, J. Phys. Chem. C, 2011, 115, 7419–7428 CrossRef CAS.
- P. V. Kamat, Photoelectrochemistry in particulate systems. 9. Photosensitized reduction in a colloidal titania system using anthracene-9-carboxylate as the sensitizer, J. Phys. Chem., 1989, 93, 859–864 CrossRef CAS.
- S. Kattel, P. Atanassov and B. Kiefer, Catalytic activity of Co-Nx/C electrocatalysts for oxygen reduction reaction: a density functional theory study, Phys. Chem. Chem. Phys., 2013, 15, 148–153 RSC.
- Z. C. Guo, B. Chen, J. B. Mu and M. Y. Zhang, Iron phthalocyanine/TiO2 nanofiber heterostructures with enhanced visible photocatalytic activity assisted with H2O2, J. Hazard. Mater., 2012, 219–220, 156–163 CrossRef CAS PubMed.
- A. Ebrahimian, M. A. Zanjanchi, H. Noei, M. Arvand and Y. Wang, TiO2 nanoparticles containing sulphonated cobalt phthalocyanine: Preparation, characterization and photocatalytic performance, J. Environ. Chem. Eng., 2014, 2, 484–494 CrossRef CAS.
- K. Wang, J. J. Xu and K. S. Tang, Solid-contact potentiometric sensor for ascorbic acid based on cobalt phthalocyanine nanoparticles as ionophore, Talanta, 2005, 67, 798–805 CrossRef CAS PubMed.
- Z. C. Guo, B. Chen, J. B. Mu and M. Y. Zhang, Iron phthalocyanine/TiO2 nanofiber heterostructures with enhanced visible photocatalytic activity assisted with H2O2, J. Hazard. Mater., 2012, 219–220, 156–163 CrossRef CAS PubMed.
- B. Z. Zhao, X. D. Niu, X. R. Hao, Y. Y. Li, Q. Fu and Y. G. Chen, Research in the energy band structures for transition metalphthalocyanine compounds, J. Northeast Norm. Univ., 2004, 36(3), 66–69 CAS.
- S. Yurdakul and S. Badoğlu, FT-IR spectra, vibrational assignments, and density functional calculations of imidazo[1,2-a]pyridine molecule and its Zn(II) halide complexes, Struct. Chem., 2009, 20(3), 423–434 CrossRef CAS.
- S. X. Liu, X. Y. Chen and X. A. Chen, TiO2/AC composite photocatalyst with high activity and easy separation prepared by a hydrothermal method, J. Hazard. Mater., 2007, 143(1), 257–263 CrossRef CAS PubMed.
- R. Seoudi, G. S. El-Bahy and Z. A. E. Sayed, FTIR, TGA and DC electrical conductivity studies of phthalocyanine and its complexes, J. Mol. Struct., 2005, 753, 119–126 CrossRef CAS.
- T. C. Canevari, J. Arguello, M. S. P. Franciso and Y. Gushikem, Cobalt phthalocyanine prepared in situ on a sol–gel derived SiO2/SnO2 mixed oxide: Application in electrocatalytic oxidation of oxalic acid, J. Electroanal. Chem., 2007, 609(2), 61–67 CrossRef CAS.
- N. Sundaraganesan, S. Kalaichelvan, C. Meganathan, B. D. Joshua and J. Cornard, FT-IR, FT-Raman spectra and ab initio HF and DFT calculations of 4-N,N'-dimethylamino pyridine, Spectrochim. Acta, Part A, 2008, 71(3), 898–906 CrossRef CAS PubMed.
- Z. Xu, H. Li, G. Cao, Q. L. Zhang, K. Z. Li and X. N. Zhao, Electrochemical performance of carbon nanotube-supported cobalt phthalocyanine and its nitrogen-rich derivatives for oxygen reduction, J. Mol. Catal. A: Chem., 2011, 335(1), 89–96 CrossRef CAS.
- L. I. Nan, S. S. Dong, L. V. Wangyang, S. Q. Huang, H. X. Chen, Y. Y. Yao and W. X. Chen, Enhanced electrocatalytic oxidation of dyes in aqueous solution using cobalt phthalocyanine modified activated carbon fiber anode, Sci. China: Chem., 2013, 56(12), 1757–1764 CrossRef.
- M. H. Baek, W. C. Jung, J. W. Yoon, J. S. Hong, Y. S. Lee and J. K. Suh, Preparation, characterization and photocatalytic activity evaluation of micro- and mesoporous TiO2/spherical activated carbon, J. Ind. Eng. Chem., 2013, 19, 469–477 CrossRef CAS.
- M. S. Nahar, J. Zhang, K. Hasegawa, S. Kagaya and S. Kuroda, Phase transformation of anatase–rutile crystals in doped and undoped TiO2 particles obtained by the oxidation of polycrystalline sulfide, Mater. Sci. Semicond. Process., 2009, 12, 168–174 CrossRef CAS.
- B. Qiu, Y. Zhou, Y. F. Ma, X. L. Yang, W. Q. Sheng, M. Y. Xing and J. L. Zhang, Facile synthesis of the Ti3+ self-doped TiO2-graphene nanosheet composites with enhanced photocatalysis, Sci. Rep., 2015, 5, 8591 CrossRef CAS PubMed.
- M. Y. Xing, F. Shen, B. Qiu and J. L. Zhang, Highly-dispersed boron-doped graphene nanosheets loaded with TiO2 nanoparticles for enhancing CO2 photoreduction, Sci. Rep., 2014, 5, 6341 Search PubMed.
- K. J. Antony Raj, R. Shanmugam, R. mahalakshmi and B. Viswanathan, XPS and IR spectral studies on the structure of phosphate and sulphate modified titania-A combined DFT and experimental study, Indian J. Chem., 2010, 49, 9–17 Search PubMed.
- J. Liu, J. Meeprasert, S. Namuangruk, K. Zha, H. R. Li, L. Huang, P. Maitarad, L. Y. Shi and D. S. Zhang, Facet–activity relationship of TiO2 in Fe2O3/TiO2 nanocatalysts for selective catalytic reduction of NO with NH3: in situ DRIFTs and DFT studies, J. Phys. Chem. C, 2017, 121, 4970–4979 CrossRef CAS.
- A. Hosseinnia, M. Keyanpour-Rad and M. Pazouki, Photo-catalytic Degradation of Organic Dyes with Different Chromophores by Synthesized Nanosize TiO2 Particles, World Appl. Sci. J., 2010, 8, 1327–1332 CAS.
- Z. Xu, G. Zhang, Z. Cao, J. S. Zhao and H. J. Li, Effect of N atoms in the backbone of metal phthalocyanine derivatives on their catalytic activity to lithium battery, J. Mol. Catal. A: Chem., 2010, 318, 101–105 CrossRef CAS.
- L. Edwards and M. Gouterman, Porphyrins: XV. Vapor absorption spectra and stability: phthalocyanines, J. Mol. Spectrosc., 1970, 33(2), 292–310 CrossRef CAS.
- Z. H. Zhao, J. M. Fan, M. M Xie and Z. Z. Wang, Photo-catalytic reduction of carbon dioxide with in situ synthesized CoPc/TiO2 under visible light irradiation, J. Cleaner Prod., 2009, 17(11), 1025–1029 CrossRef CAS.
- T. Ma, K. Inoue, H. Noma, K. Yao and E. Abe, Effect of functional group on photochemical properties and photosensitization of TiO2, electrode sensitized by porphyrin derivatives, J. Photochem. Photobiol., A, 2002, 152, 207–212 CrossRef CAS.
- R. Bonnett, Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy, Chem, 1995, 26, 19–33 Search PubMed.
- T. Ohno and S. Kato, Electron-transfer reactions of excited phthalocyanines: spin restriction on reaction rate of electron transfer and energy transfer to cobalt compounds, J. Phys. Chem., 2002, 88, 1670–1674 CrossRef.
- V. Iliev, Phthalocyanine-modified titania—catalyst for photooxidation of phenols by irradiation with visible light, J. Photochem. Photobiol., A, 2002, 151(1), 195–199 CrossRef CAS.
- C. E. Diaz-Uribe, M. C. Daza and F. Maetinez, Visible light superoxide radical anion generation by tetra(4-carboxyphenyl)porphyrin/TiO2: EPR characterization, J. Photochem. Photobiol., A, 2010, 215, 172–178 CrossRef CAS.
- Z. H. Zhao, J. M. Fan, M. M. Xie and Z. Z. Wang, Photo-catalytic reduction of carbon dioxide with in situ synthesized CoPc/TiO2 under visible light irradiation, J. Cleaner Prod., 2009, 17(11), 1025–1029 CrossRef CAS.
- D. L. Li, X. D. Jiang, Y. P. Zhang and B. Zhang, A novel route to ZnO/TiO2 heterojunction composite fibers, J. Mater. Res., 2013, 28(3), 507–512 CrossRef CAS.
- M. Jakob, H. Levanon and P. V. Kamat, Charge distribution between UV-irradiated TiO2 and Gold nanoparticles:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) determination of shift in the fermi level, Nano Lett., 2016, 3(3), 353–358 CrossRef.
determination of shift in the fermi level, Nano Lett., 2016, 3(3), 353–358 CrossRef. - M. G. Betti, P. Gargiani, R. Frisenda, R. Biagi, A. Cossaro, A. Verdini, L. Floreano and C. Mariani, Localized and dispersive electronic states at ordered FePc and CoPc chains on Au(110), J. Phys. Chem. C, 2010, 114(49), 21638–21644 CrossRef CAS.
- W. Wu, N. M. Harrison and A. J. Fisher, Electronic structure and exchange interactions in cobalt-phthalocyanine chains, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 88, 244261–244269 Search PubMed.
- Y. H. Ao, J. Q. Bao, P. F. Wang, C. Wang and J. Hou, Bismuth oxychloride modified titanium phosphate nanoplates: a new p–n heterostructured photocatalysts with high activity for the degradation of different kinds of organic pollutants, J. Colloid Interface Sci., 2016, 476, 71–78 CrossRef CAS PubMed.
- C. H. Wang, X. T. Zhang and Y. C. Liu, Promotion of multi-electron transfer for enhanced photocatalysis: a review focused on oxygen reduction reaction, Appl. Surf. Sci., 2015, 358, 28–45 CrossRef CAS.
- M. Li, S. Yin, T. Wu, J. Di, M. X. Ji and B. Wang, Controlled preparation of MoS2/PbBiO2I hybrid microspheres with enhanced visible-light photocatalytic behavior, Adv. Colloid Interface Sci., 2018, 517, 278 CrossRef CAS PubMed.
- L. Khachatryan, E. Vejerano, S. Lomnicki and B. Dellinger, Environmentally persistent free radicals (EPFRs). 1. Generation of reactive oxygen species in aqueous solutions, Environ. Sci. Technol., 2011, 45(19), 8559–8566 CrossRef CAS PubMed.
- T. Kobayashi, The far spectra of phthalocyanine and its metal derivatives, Spectrochim. Acta, 1970, 26, 1313–1322 CrossRef CAS.
- J. H. Zagal, Metallophthalocyanines as catalysts in electrochemical reactions, Coord. Chem. Rev., 1992, 119, 89–136 CrossRef CAS.
- T. Kroll, V. Y. Aristov, O. V. Molodtsova, Y. A. Ossipyan, D. V. Vyalikh, B. Biichner and M. Knupfer, Spin and orbital ground state of Co in cobalt phthalocyanine, J. Phys. Chem. A, 2009, 113, 8917–8922 CrossRef CAS PubMed.
- J. P. Graham and G. Brown, Molecular orbital studies of nitrosyl metalloporphyrin complexes, J. Arkansas Acad. Sci., 2011, 55, 31–42 Search PubMed.
- B. M. Hoffman and M. A. Ratner, Jahn-Teller effects in metalloporphyrins and other four-fold symmetric systems, Mol. Phys., 2015, 35(4), 901–925 CrossRef.
- R. Othman, A. L. Dicks and Z. G. Zhu, Non precious metal catalysts for the PEM fuel cell cathode, Int. J. Hydrogen Energy, 2012, 37, 357–372 CrossRef CAS.
- J. H. Zagal, S. Griveau, F. Silva, T. Nyokong and F. Bedioui, Metallophthalocyanine-based molecular materials as catalysts for electrochemical reactions, Coord. Chem. Rev., 2010, 254, 2755–2791 CrossRef CAS.
- Y. He, L. Zhang, B. Teng and M. H. Fan, New application of Z-scheme Ag3PO4/g-C3N4 composite in converting CO2 to fuel, Environ. Sci. Technol., 2015, 49(1), 649–656 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |