Open Access Article
Open Access ArticleThe role of soil components in the sorption of tetracycline and heavy metals in soils†
Zhendong Zhao a,
Tiantian Niea,
Zhenyu Yanga and
Wenjun Zhou
a,
Tiantian Niea,
Zhenyu Yanga and
Wenjun Zhou *ab
*ab
aDepartment of Environmental Science, Zhejiang University, Hangzhou, Zhejiang 310058, China. E-mail: wenjunzhou@zju.edu.cn; Fax: +86-571-88982591; Tel: +86-571-88982591
bZhejiang Provincial Key Laboratory of Organic Pollution Process and Control, Hangzhou, Zhejiang 310058, China
First published on 18th September 2018
Abstract
A natural black soil (BS) was treated to obtain three individual soils referred to as removed organic matter (ROM), removed metal oxide (ROX) and humic acid (HA), and the sorption behaviors of tetracycline (TC) and heavy metals (Cu2+ and Cd2+) on BS and three treated soils were investigated to evaluate the role and contribution of different soil components (organic matter, clay and metal oxide). The three treated soils all showed stronger sorption capacities toward TC than BS, and the sorption amount of ROM, ROX, and HA for TC was greater than that of BS by 1.2 times, 2.3 times, and 3.3 times at an initial TC concentration of 25 mg L−1. Differently, the sorption capacity of BS for Cu2+ and Cd2+ was stronger than that of ROM. The multiple linear regression analysis suggested that soil organic matter made the greatest contribution toward the sorption of TC, whereas, metal oxide was the key component influencing the sorption of Cu2+ and Cd2+. The presence of Cu2+ enhanced the TC sorption of BS, ROM and ROX, but had a suppression effect on HA because of the competition of Cu2+. The presence of Cd2+ did not exert obvious effects on TC sorption of BS, but exhibited a suppression effect on TC sorption of ROM, ROX and HA, which was likely to relate to the surface potentials of the soils. The results in this study are expected to give an insight into the role of different soil components in the sorption and co-sorption of TC and heavy metal through a mathematical model and to reveal the sorption mechanism.
1. Introduction
Veterinary antibiotics are widely used as feed additives to treat livestock diseases and promote the growth of animals around the world.1 Most of the antibiotics with biological activity eventually enter the environment in the form of the original compound and continuously accumulate in soils and sediments, resulting in antibiotic pollution of farmland soils.2–4 Nevertheless, the coexistence of antibiotics and heavy metals in soils is common due to the increasing use of agricultural manure, causing stronger eco-toxicity and a higher abundance of antibiotic resistant genes than a single pollutant.5,6 Specifically, combined contaminants of antibiotics and heavy metals are frequently detected in food and drinking water, posing a potential threat to human health and microbial community through migration and transformation.7 Therefore, it is extremely necessary to understand the environmental behavior and ultimate fate of antibiotics and heavy metals in co-contaminated soils.Sorption process plays a critical role in controlling mobility and bioavailability of contaminations in soil environment.8,9 For different kinds of antibiotics and heavy metals, their structures and functional groups may be the main factors affecting their sorption behaviors.1 Previous studies indicated that antibiotics and heavy metals might be sorbed on soils mainly via the electrostatic interaction between the positive-charged groups and the negative-charged sorption sites, or via the cation exchange between the positive-charged groups and the cations adhering to the surface of soil.9 The –OH and –CONH groups of antibiotics could be sorbed on the negative-charged sorption sites of soils via the cation bridging, and also be sorbed on the surface of metal oxide via the surface complexation with metal ions.6 In addition, the sorption of these pollutions was further dependent on environmental factor (e.g., soil pH, soil texture, and cation exchange capacity).4,6,8 Soil components including such as organic matter, clay mineral and hydrous metal oxide, are important factors affecting the transport and bioavailability of antibiotics and heavy metals in the environment. Some studies demonstrate the difference in antibiotic sorption on diverse clay types,10,11 and the difference can be attributed to different sorption capacities and clay surface area between clay types.12 For hydrous oxides, the formation of surface complexes during antibiotic sorption will promote dissolution of these minerals, but this promotion is related to labile sites for sorbents surface interactions.13 Similarly, the majority of antibiotics existing as cation or zwitterion species, can also complex with humic acid on some specific sites, and hydrogen bonding, ion exchange and cation bridging have been considered as major sorption mechanisms.14,15 However, most of studies were only focused on the sorption of antibiotics and heavy metals on individual soil or component, little knowledge about the sorption of these pollutants on the separated components from natural soil is known. Meanwhile, there is also a lack of study on evaluating the contribution of different soil components toward sorption of antibiotics and heavy metals.
In addition, the coexistence of antibiotic and heavy metal in soil would alter their individual speciation and consequent environmental behavior. Therefore, studies on co-sorption of antibiotic and heavy metal on soil could facilitate to further improve our understanding of combined pollution risks. In detail, metal ions enhance the sorption of antibiotics in soils through the formation of a soil–metal–antibiotic ternary surface complex or electrostatic attraction;16–19 On the other hand, metal ions suppress the sorption of antibiotics due to competition for sorption sites between positively charged antibiotics and metal ions at low pH.19–21 At the same time, the presence of metal ions also results in changes of the soil properties and structure, such as potential, pore size and content of oxides, then coming into different sorption mechanisms for antibiotics.17,19,22 However, different heavy metal cations are able to exert distinct influence on the sorption of antibiotics, which is likely related to the different binding affinity between metals and antibiotics.23–26 Although the co-sorption of antibiotics and heavy metals in soil has been widely investigated, it is still not clear that the effect of heavy metals on sorption of antibiotics on the separated components from natural soil.9,17,27
In this study, tetracycline (TC), Cu2+ and Cd2+ were chosen as the representation of antibiotics and heavy metals. A natural black soil (BS) was used as a good sorption material as it represents a kind of typical Chinese soil with rich organic matter. The sorption of TC and heavy metals (Cu2+ and Cd2+) on the BS and the corresponding three treated soils (removed organic matter (ROM), removed metal oxide (ROX), humic acid (HA)) was examined. The objectives of this study were to better understand how the different soil components (organic matter, metal oxide and clay) affect the sorption behaviors of antibiotic and heavy metal, and to develop an analytical equation to quantify the contribution of these components toward sorption process. The results from the present study are expected to give an insight into the role of different soil components in the sorption and co-sorption of TC and heavy metal through a mathematical model and to reveal the sorption mechanism.
2. Material and methods
2.1 Chemicals and materials
Tetracycline (TC) was obtained from Sigma-Aldrich Company (USA) with a reported purity of 98%. Copper nitrate (Cu(NO3)2) and cadmium nitrate (Cd(NO3)2) were purchased from Sinopharm Chemical Reagent Co. (China) with analytical grade. Organic solvents used in this study were purchased from Thermo Fisher Scientific Co. Ltd. (USA) with HPLC grade. All other chemicals were of reagent grade. Deionized water was used for all experiments. The molecular structure and some physic-chemical properties of TC are shown in Table S1.† A natural black soil (BS) was collected from Heilongjiang province of China. The soil sample was air-dried, homogenized to pass through a 100 mesh sieve and stored in amber glass bottles at room temperature until use.2.2 Treatment and characterization of soil samples
In order to clearly show the difference in content of organic matter, metal oxide and clay, BS was treated to obtain three individual soils referred to removed organic matter (ROM), removed metal oxide (ROX), and humic acid (HA). In detail, the organic matter of BS was removed by H2O2 oxidation to obtain the ROM and the metal oxide of BS was removed by using the method of citrate-bicarbonate-dithionite (CBD) to obtain the ROX, respectively. HA was obtained by using the method described in the previous study.28 The treatment methods to obtain three individual soils are listed in ESI.† The elemental compositions and zeta potentials of soils were measured using elemental analyzer (Thermo Finnigan Flash EA 1112) and zeta potential analyzer (Zetasizer Nano ZS). The content of organic matter, metal oxide (referred to Fe2O3 and Al2O3) and clay was determined by the methods of Lu (1999).2.3 Sorption experiments
Batch sorption experiments were conducted in triplicates according to the guideline of the Organization for Economic Co-operation and Development (OECD, 2000). A certain amount of BS and three treated soils were respectively weighed into 22 mL glass vials with Teflon-lined screw caps, and 20 mL TC solution of different concentration (2.5–25 mg L−1) was added into each vial in the absence or presence of 4 mg L−1 Cu2+ or Cd2+. The background solution contained 0.01 M NaCl to maintain a constant ionic strength, 100 mg L−1 NaN3 to minimize biodegradation and solution pH was adjusted by addition of 0.1 M HNO3 or NaOH to ensure the desirable pH at sorption equilibrium.The preliminary experiments indicated that 24 h, 48 h, 72 h was sufficient to reach equilibrium for TC sorption on BS, ROM and ROX (48 h) and HA, respectively, and microbial degradation, volatilization or sorption to glass walls were negligible during sorption experiments. The glass vials were kept in dark, shaken for corresponding time at 25 °C and centrifuged at 3500 rpm for 10 min. An appropriate aliquot of the supernatant was then filtered through a 0.45 μm nylon membrane.
Sorption of Cu2+ and Cd2+ on BS and three treated soils were conducted in the same way as the TC sorption. 20 mL background solution containing different concentration (0–30 mg L−1) Cu2+ and Cd2+ as nitrates was added into glass vitals. Preliminary experiments indicated that 24 h was sufficient to reach sorption equilibrium for Cu2+ and Cd2+ sorption on BS and three treated soils. After shaking and centrifuging, an appropriate aliquot of the supernatant was filtered through a 0.45 μm water membrane instead of nylon membrane.
2.4 Analytical methods
The concentration of TC in the supernatants was determined by high performance liquid chromatography (Shimadzu, LC-20 AT, Japan) equipped with an Agilent Eclipse XDB-C18 reversed-phase column (4.6 × 150 mm, 5 μm, Supelco, USA) with column temperature at 30 °C. The mobile phase was 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 (v/v) of acetonitrile and 0.01 M oxalic acid at a flow rate of 1.0 mL min−1. TC was analyzed by a UV detector at 360 nm. The concentration of Cu2+ and Cd2+ in the supernatants was determined using an atomic absorption spectrophotometer (PerkinElmer, AA700, USA).
80 (v/v) of acetonitrile and 0.01 M oxalic acid at a flow rate of 1.0 mL min−1. TC was analyzed by a UV detector at 360 nm. The concentration of Cu2+ and Cd2+ in the supernatants was determined using an atomic absorption spectrophotometer (PerkinElmer, AA700, USA).
2.5 Data analysis
The equilibrium sorption amount of TC was calculated according to the mass difference between the initial and equilibrium concentrations in aqueous solutions. The sorption isotherms data obtained in this study were fitted to the Freundlich model as described by eqn (1):| Qe = KfCen | (1) |
3. Results and discussion
3.1 Characterization of natural black soil and three treated soils
The physic-chemical properties of BS and the three treated soils are presented in Table 1. After H2O2 oxidation to remove organic matter (OM), the OM content of ROM showed the significant decrease and was only 16.4% of that in BS, but organic matter has not been completely removed because of being protected by minerals surface. Besides, the OM content of ROX did not exhibit significant change with the treatment of removing metal oxide, suggesting the almost non-existent correlation between the content of OM and metal oxide. Apparently, HA had the highest OM content up to 38.5%, which was consistent with the previous report.28The content of metal oxide (referred to Fe2O3 and Al2O3) of ROM and ROX decreased by 19.2% and 85.7% in comparison with BS, respectively, which was attributed to the fact that metal oxide might be tightly binding to the organic matter, resulting in the decrease in content of metal oxide. The clay content of ROM and ROX increased to 31.53% and 28.92% in comparison with 22.01% of BS, respectively. It was mainly produced at high temperature during the removal of organic matter and metal oxide, which made larger particles in BS (such as silt and sand) well dispersed and fine, indirectly resulting in the slight change in clay content. Remarkably, the content of metal oxide and clay on HA seemed to be neglected because of its unique property.
In order to better show the changes in content of soil components, the interrelations of organic matter, metal oxide and clay of BS and the three treated soils are also illustrated in Fig. S1.† The significantly linear correlations were only embodied between organic matter and clay (R2 = 0.95). Conversely, metal oxide did not show linear dependence on organic matter (R2 = 0.47), as well as clay (R2 = 0.29), which further suggested that there was no obvious correlation between metal oxide and other components.
3.2 Sorption of TC on BS and three treated soils
The sorption isotherms of TC on BS and three treated soils are shown in Fig. 1, and Freundlich model fitting parameters are listed in Table S2.† The experiment data fitted the Freundlich model well as indicated by the correlation coefficients (R2 = 0.983–0.999). The sorbed amounts of TC on BS and three treated soils increased with the increasing TC concentration in equilibrium solution, and the sorption isotherms all showed strong nonlinearity with n values ranging from 0.34 to 0.43. This indicated that pore-filling and some specific interactions with functional groups of soil organic matter or clay minerals played a critical role in TC sorption, in addition to hydrophobic partitioning.19,29 It could be also observed that HA showed the highest sorption affinity toward TC, followed by ROX, ROM and BS in order. For example, the equilibrium sorption amounts of TC on BS, ROM, ROX and HA were 20.84, 46.29, 67.86 and 89.22 mg g−1 at an initial TC concentration of 25 mg L−1, respectively. When the initial TC concentration in the equilibrium solution was low, the sorption capacity of BS and three treated soils for TC still followed the order of HA > ROX > ROM > BS. Moreover, the three treated soils all showed the stronger sorption capacities for TC than BS.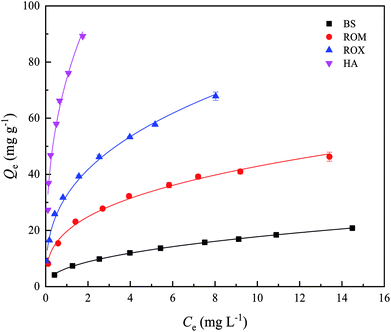 | ||
| Fig. 1 The sorption isotherms of tetracycline (TC) on black soil (BS), removed organic matter (ROM), removed metal oxide (ROX) and humic acid (HA). | ||
Previous studies found that sorption of TC on soils was strongly governed by soil organic matter through complex interaction, cation bridging and H-bonding.30 However, organic matter coating might block the sorption of ionic organic compounds on minerals due to competition for sorption sites.31 Specifically, HA, a subset of natural organic matter, could mainly interact with TC, thus promoting the complex formation of a monoacid with discrete sites.14,32 Differently, the sorption of TC on clay mineral was dependent on TC solution chemistry. Cation exchange was the most important mechanism for cation species, whereas, surface complexation mechanism was important for zwitterion species.10,12 Meanwhile, the sorption of TC should also consider the interaction with soil oxide components in addition to organic matter and clay. The proposed mechanism for TC sorption on metal oxides (e.g., iron oxides, aluminum oxides) would also involved the formation of complexes by surface complexation.13 In order to show the roles of organic matter, metal oxide and clay on sorption behavior of TC on soils, the single-point sorption coefficient (Kd = Qe/Ce) at an initial TC concentration of 25 mg L−1 was used as the representation of sorption capacity of BS and three treated soils, and three-dimensional surface plot of interactive effects of organic matter and metal oxide, organic matter and clay, metal oxide and clay on sorption behavior of TC on soils were illustrated in Fig. 2, which revealed the relationship between soil components and their sorption capacity.
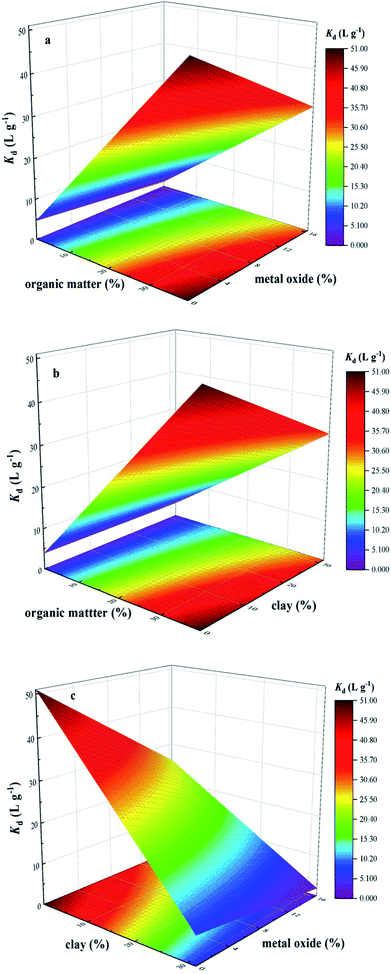 | ||
| Fig. 2 Three-dimensional surface plot of interactive effects of organic matter and metal oxide (a), organic matter and clay (b), metal oxide and clay (c) on sorption capacity for tetracycline (TC). | ||
It was clearly observed from Fig. 2 that sorption capacity of TC increased with the increasing content of organic matter, whereas the increasing content of clay or metal oxide caused a slight decrease in sorption capacity of TC. When the content of clay and metal oxide increased simultaneously, the sorption capacity of TC showed the obvious decrease (Fig. 2c), which indicated the strong competition interaction for the sorption sites of TC between clay and metal oxide, as well as the significant effect of organic matter as a dominant contribution on sorption behavior of TC. In order to study the contribution of all investigated soil components to TC sorption, a first order polynomial equation was obtained by using multiple linear regression analysis (eqn (2)), which quantified the relationship between equilibrium sorption capacity of soils for TC and the content of investigated soil components.
| YTC = −27.714 + 2.045 × A + 0.938 × B + 0.017 × C | (2) |
3.3 Sorption of Cu2+ and Cd2+ on BS and three treated soils
The sorption isotherms of Cu2+ and Cd2+ on BS and three treated soils are shown in Fig. 3, and Freundlich model fitting parameters are listed in Table S3.† The experiment data fitted the Freundlich model well as indicated by the correlation coefficients (R2 = 0.990–0.996), which suggested that the sorption occurred on heterogeneous surface by multilayer sorption. It assumed that soil surface had the exponential distribution of sites and their energies.33 When the metal concentration was relatively low in equilibrium solution, the sorbed amounts of Cu2+ and Cd2+ on BS and three treated soils increased sharply with the increasing metal concentrations, but the slope of the sorption isotherms was going to be relatively stable as Cu2+ and Cd2+ concentrations gradually increased to attain a high value. The equilibrium sorption amounts of Cu2+ and Cd2+ on BS, ROM, ROX and HA were 8.94, 7.36, 16.82, 20.05 mg g−1 and 7.67, 6.01, 13.97, 17.66 mg g−1 at an initial Cu2+ and Cd2+ concentrations of 20 mg L−1, respectively. Therefore, both HA and ROX showed the stronger sorption capacities for Cu2+ and Cd2+ than BS, whereas the sorption capacity of ROM for Cu2+ and Cd2+ was weaker than BS.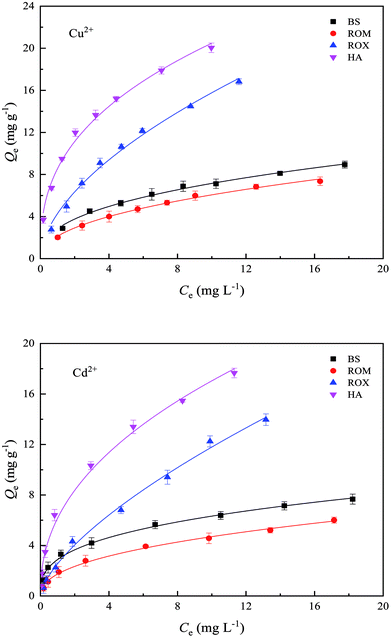 | ||
| Fig. 3 Sorption isotherms of Cu2+ and Cd2+ on black soil (BS), removed organic matter (ROM), removed metal oxide (ROX) and humic acid (HA). | ||
It was reported from previous studies that the increase in sorption amounts of Cu2+ and Cd2+ with the increasing metal concentration could be attributed to the change of sorption sites.34 Heavy metals can be sorbed on soil surface through specific sorption mechanism and nonspecific sorption mechanism. Specific sorption is a relatively strong sorption process, and can form inner-sphere complexes. In turn, nonspecific sorption is a relatively weak sorption process, and mainly forms outer-sphere complexes through electrostatic interaction. Most natural soils are heterogeneous media which provide a wide range of sorption sites with different bonding properties. Sorption affinity varies across the surface due to the inhomogeneity of soil surface.33,35 The high affinity sorption site has high bonding energy and heavy metal ions (Cu2+ and Cd2+) are sorbed through specific sorption mechanisms such as co-precipitation between heavy metal ions and Fe–Mn oxides surface.35 The low affinity sorption site has rather low bonding energy, therefore, heavy metal ions (Cu2+ and Cd2+) are sorbed through the nonspecific sorption that is a relatively weak sorption process including electrostatic attraction.36 When Cu2+ and Cd2+ concentrations in equilibrium solution were low, the high binding energy sites had stronger affinity, thus preferentially sorbing Cu2+ and Cd2+ through specific sorption. As Cu2+ and Cd2+ concentrations increased, high binding energy sites were gradually replaced by low binding energy sites, so nonspecific sorption was the main sorption mechanism. In addition, the amounts of Cu2+ sorbed on BS and three treated soils were clearly higher than Cd2+ due to lower hydrolysis constant of first step (pK1) and solubility product of hydroxide precipitate than Cd2+.37 In particular, hydrolysis constant of first step (pK1) significantly affected the sorption of heavy metal ions, and sorption affinity of metal ions decreased with the increase of pK1. The pK1 of Cu2+ and Cd2+ were 7.8 and 10.1, respectively, thus, sorption affinity of Cu2+ on soil was higher than that of Cd2+. On the other side, this hydrolysis might be accompanied by the precipitation of metal hydroxides and carbonates. The solubility product of Cu2+ hydroxides and carbonates was far lower than that of Cd2+, thus, surface precipitation was more likely to occur between Cu2+ and soil/soil components, consequently, resulting in the greater sorption of Cu2+ than Cd2+.
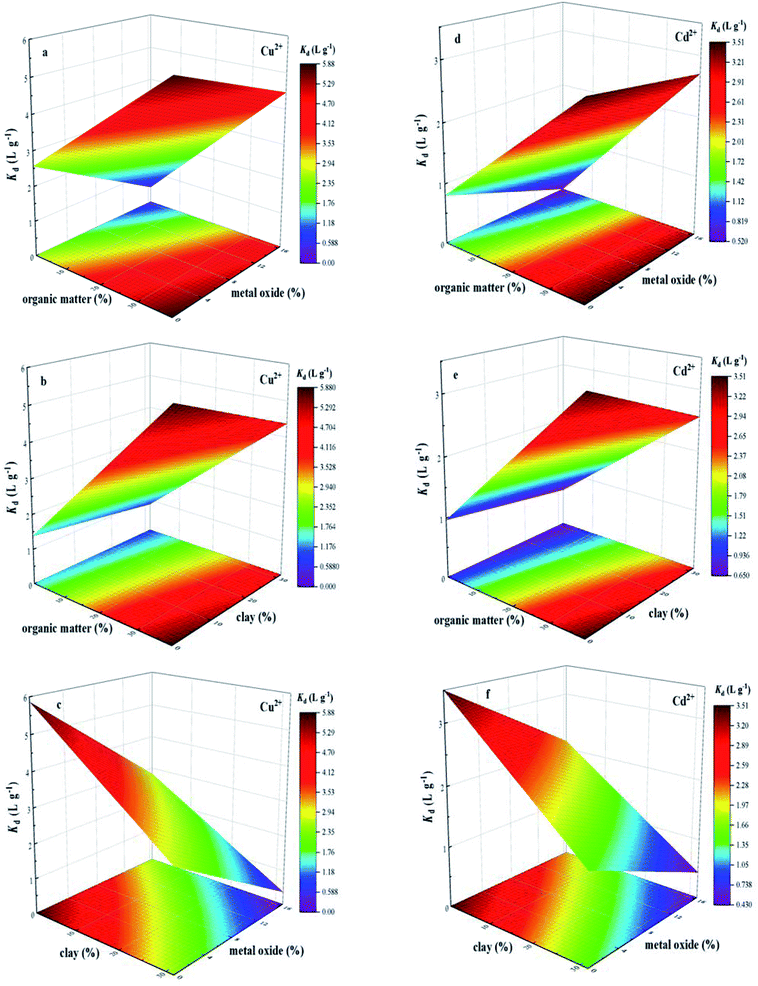
In order to show the effects of organic matter, metal oxide and clay on sorption behavior of Cu2+ and Cd2+, the single-point sorption coefficient (Kd) at an initial Cu2+ and Cd2+ concentration of 8 mg L−1 was used as the representation of sorption capacity of BS and three treated soils, and three-dimensional surface plot of interactive effects of organic matter and metal oxide, organic matter and clay, metal oxide and clay on the sorption behavior of Cu2+ and Cd2+ on soils were illustrated in Fig. 4, which revealed relationship between soil components and their sorption capacity for Cu2+ and Cd2+. As the content of organic matter increased, sorption amounts of Cu2+ and Cd2+ gradually increased. For one thing, multi-oxygen containing functional groups, such as hydroxyl and carboxyl, could enhance Cu2+ and Cd2+ sorption through electrostatic interaction.38 Besides, the formation of organic matter–metal–soil ternary surface complex also resulted in the increasing amounts of Cu2+ and Cd2+ sorbed on soil.21,34 The three-dimensional surface plot of interactive effects of organic matter and metal oxide (Fig. 4a and d), organic matter and clay (Fig. 4b and e) on the sorption behavior of Cu2+ and Cd2+ on soils suggested that the sorption amounts of Cu2+ and Cd2+ exhibited a slight decrease with the single increase in content of metal oxide and clay in the presence of organic matter, respectively. Meanwhile, Fig. 4c and f illustrated the significant decrease in sorption amounts of Cu2+ and Cd2+ with the simultaneously increasing content of clay and metal oxide, also suggesting the competition interaction for the sorption sites of Cu2+ and Cd2+ between clay and metal oxide. In order to study the contribution of all investigated soil components to Cu2+ and Cd2+ sorption, a first order polynomial equation was obtained by using multiple linear regression analysis (eqn (3) and (4)), which quantified the relation between equilibrium sorption capacity of soils for Cu2+ and Cd2+ and the content of investigated soil components.
| YCu = +3.755 + 0.055 × A − 0.037 × B − 0.135 × C | (3) |
| YCd = +2.301 + 0.031 × A − 0.030 × B − 0.056 × C | (4) |
3.4 Effects of heavy metal on TC sorption on soils
The sorption isotherms of TC on BS and three treated soils in the presence of Cu2+ and Cd2+ are showed in Fig. 5, and the equilibrium sorption amounts of TC on the four soils at an initial TC concentration of 25 mg L−1 with and without the presence of Cu2+ and Cd2+ are compared in Table 2. It could be found from Fig. 5 and Table 2 that the presence of Cu2+ significantly enhanced the sorption of TC on BS, ROM and ROX, and the equilibrium sorption amounts of TC on BS, ROM and ROX at an initial TC concentration of 25 mg L−1 increased from 20.84, 46.29 and 67.86 mg g−1 to 33.44, 67.45 and 77.9 mg g−1 in the presence of 4 mg L−1 Cu2+, respectively. The promotion rate of TC sorption on BS and three treated soils in the presence of Cu2+ followed the order of BS > ROM > ROX. However, the presence of Cu2+ showed the suppression effect on TC sorption on HA, and the equilibrium sorption amount of TC on HA decreased from 89.22 mg g−1 to 80.76 mg g−1 at an initial TC concentration of 25 mg L−1 in the presence of 4 mg L−1 Cu2+.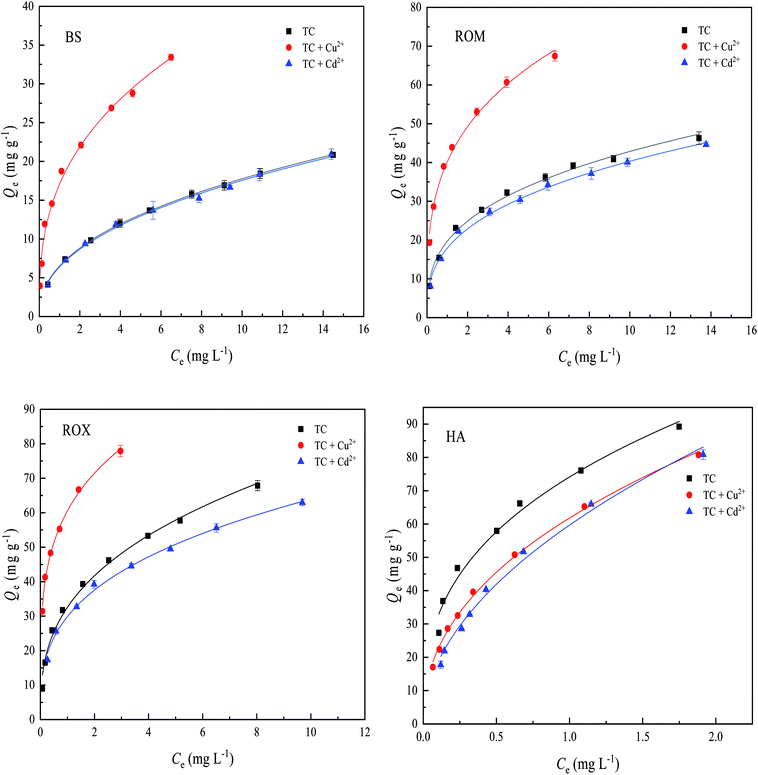 | ||
| Fig. 5 Effects of Cu2+ and Cd2+ on the sorption of tetracycline (TC) on black soil (BS), removed organic matter (ROM), removed metal oxide (ROX) and humic acid (HA). | ||
| Sorbents | No metal | Added 4 mg L−1 Cu2+ | Added 4 mg L−1 Cd2+ | ||
|---|---|---|---|---|---|
| TC sorption amount (mg g−1) | TC sorption amount (mg g−1) | Increase percent (%) | TC sorption amount (mg g−1) | Increase percent (%) | |
| BS | 20.84 | 33.44 | 60.46 | 20.94 | 0.48 |
| ROM | 46.29 | 67.45 | 45.71 | 44.64 | −3.56 |
| ROX | 67.86 | 77.90 | 14.80 | 62.98 | −7.19 |
| HA | 89.22 | 80.76 | −9.48 | 80.85 | −9.37 |
The different effects of Cu2+ on TC sorption on BS and three treated soils were likely to relate to properties of soil components. It was reported that strong complexes (CuHTC+ or CuTC0) could be formed between Cu2+ and TC,39 these predominant complex species were easily sorbed onto soil surfaces.16 Besides, TC could be sorbed on the sites where Cu2+ was specifically sorbed, and acted as a bridge between TC and soil particles.25 Both mechanisms were able to explain the promotion effect of Cu2+ on TC sorption. On the other hand, the fact that the promotion effect of Cu2+ on TC sorption on BS was stronger than ROM and ROX might be attributed to the role of soil components. It was both organic matter and metal oxide that possessed the additional sorption sites, resulting in the production of ternary surface complexes which had a strong sorption affinity toward TC. Therefore, it could be also proposed from the promotion rate of Cu2+ on TC sorption in Table 2 that (1) the TC–Cu complex had a higher affinity to BS than ROM and ROX, (2) the role of Cu2+ bridging enhancing TC sorption on BS was stronger than that of ROM and ROX. However, the suppression effect of Cu2+ on TC sorption on HA could be ascribed to (1) the competition of Cu2+ for the same sorption sites on HA, which was unfavorable for the sorption of TC+, (2) the formation of surface complexes (CuHTC+ or CuTC0) made the surface of HA less negatively charged, thus resulting in lower affinity for the sorption of TC+ through electrostatic attraction, which was also observed from similar reports.19,21
Different from the effect of Cu2+ on TC sorption, the presence of Cd2+ did not increase the sorption of TC on BS, suggesting that complexation affinity of metal cations was one of key factors affecting the sorption of TC. Previous studies found that the Cd2+ exhibited a far weaker binding affinity with TC compared with Cu2+, thus having no obvious effect on the TC sorption on BS.25,40 In addition, the equilibrium sorption amounts of TC on ROM, ROX and HA at an initial TC concentration of 25 mg L−1 decreased from 46.29, 67.86 and 89.22 mg g−1 to 44.64, 62.98 and 80.85 mg g−1 in the presence of 4 mg L−1 Cd2+, indicating the suppression effect of Cd2+ on TC sorption on ROM, ROX and HA, and the suppression rate of Cd2+ followed a soil order of HA > ROX > ROM, which could be explained through the competition of Cd2+ for sorption sites on ROM, ROX and HA.
In order to account for the suppression effect of Cd2+ on TC sorption on ROM, ROX and HA, the surface potentials of BS and three treated soils in the examined pH range were investigated, as showed in Fig. S2.† There was a significant difference in terms of their respective zeta potentials when pH was approximately 4.5 in equilibrium solution. The zeta potential of BS got close to its isoelectric point (IEP) at pH 4.5, whereas ROM, ROX and HA carried a considerable amount of negative charges, which was mainly due to the decrease in content of metal oxide. Therefore, Cd2+ could be sorbed on ROM, ROX and HA through electrostatic attraction, leading to the competition for sorption sites with TC. It was clearly observed from Fig. S2† that HA possessed more negatively charges compared to ROM and ROX, thus being easier to promote the competition of Cd2+ for sorption sites on HA through electrostatic interaction.
4. Conclusions
The present study indicates that sorption behavior of tetracycline (TC) and heavy metals (Cu2+ and Cd2+) on soil is a process together decided by different soil components. HA showed the strongest sorption capacity toward TC, followed by ROX, ROM and BS, but BS showed stronger sorption capacity toward Cu2+ and Cd2+ than ROM. The three-dimensional surface plot about the interactive effects of soil components on sorption behavior illustrated that sorption capacity of TC, Cu2+ and Cd2+ increased with the increasing content of organic matter, but slightly decreased with the increasing content of clay and metal oxide. Besides, the simultaneous increase of clay and metal oxide resulted in the obvious decrease in sorption capacity for TC, which suggested the strong competition interaction between clay and metal oxide. The multiple linear regression analysis suggested that soil organic matter made the greatest contribution toward the sorption of TC, followed by clay and metal oxide. In contrast, clay and metal oxide made a negative contribution toward Cu2+ and Cd2+ sorption in comparison with soil organic matter. However, metal oxide was the most important component influencing the sorption of Cu2+ and Cd2+, followed by organic matter and clay. The presence of Cu2+ had obvious suppression effect on TC sorption on HA because of the competition of Cu2+, but significantly promoted TC sorption on BS, ROM and ROX through surface complexation and cation bridging, and the promotion rate corresponded to the following order of BS, ROM and ROX, implying that (1) the TC–Cu complex had a higher affinity to BS than ROM and ROX, (2) the role of Cu2+ bridging enhancing TC sorption on BS was stronger than that of ROM and ROX. The presence of Cd2+ had almost no effect on TC sorption on BS because of the weak complexation ability between TC and Cd2+, but exhibited a suppression effect on TC sorption on ROM, ROX and HA, and the suppression rate corresponded to the following order of HA, ROX and ROM, which was likely to relate to surface potentials of soils.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (21577124) and the National Key Research and Development Program of China (2017YFA0207002).References
- J. Tolls, Environ. Sci. Technol., 2001, 35, 3397–3406 CrossRef PubMed.
- A. B. A. Boxall, D. W. Kolpin, B. Halling-Sorensen and J. Tolls, Environ. Sci. Technol., 2003, 37, 286A–294A CrossRef PubMed.
- A. B. A. Boxall, L. A. Fogg, P. A. Blackwell, P. Kay, E. J. Pemberton and A. Croxford, Rev. Environ. Contam. Toxicol., 2004, 180, 1–91 CrossRef.
- Y. Y. Zhou, X. C. Liu, Y. J. Xiang, P. Wang, J. C. Zhang, F. F. Zhang, J. H. Wei, L. Luo, M. Lei and L. Tang, Bioresour. Technol., 2017, 245, 266–273 CrossRef PubMed.
- Y. G. Zhu, T. A. Johnson, J. Q. Su, M. Qiao, G. X. Guo, R. D. Stedtfeld, S. A. Hashsham and J. M. Tiedje, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 3435–3440 CrossRef PubMed.
- Y. Y. Zhou, Y. Z. He, Y. J. Xiang, S. J. Meng, X. C. Liu, J. F. Yu, J. Yang, J. C. Zhang, P. F. Qin and L. Luo, Sci. Total Environ., 2019, 646, 29–36 CrossRef PubMed.
- R. Z. Hao, R. T. Zhao, S. F. Qiu, L. G. Wang and H. B. Song, Science, 2015, 348, 1100 CrossRef PubMed.
- Y. Y. Zhou, X. C. Liu, L. Tang, F. F. Zhang, G. M. Zeng, X. Q. Peng, L. Luo, Y. C. Deng, Y. Pang and J. C. Zhang, J. Hazard. Mater., 2017, 333, 80–87 CrossRef PubMed.
- M. Graouer-Bacart, S. Sayen and E. Guillon, Ecotoxicol. Environ. Saf., 2015, 122, 470–476 CrossRef PubMed.
- M. E. Essington, J. Lee and Y. Seo, Soil Sci. Soc. Am. J., 2010, 74, 1577–1588 CrossRef.
- Q. Zhang, C. Yang, W. L. Huang, Z. Dang and X. H. Shu, Chemosphere, 2013, 93, 2180–2186 CrossRef PubMed.
- R. A. Figueroa, A. Leonard and A. A. Mackay, Environ. Sci. Technol., 2004, 38, 476–483 CrossRef PubMed.
- C. Gu and K. G. Karthikeyan, Environ. Sci. Technol., 2005, 39, 2660–2667 CrossRef PubMed.
- C. Gu, K. G. Karthikeyan, S. D. Sibley and J. A. Pedersen, Chemosphere, 2007, 66, 1494–1501 CrossRef PubMed.
- A. A. Mackay and B. Canterbury, J. Environ. Qual., 2005, 34, 1964 CrossRef PubMed.
- Y. J. Wang, D. A. Jia, R. J. Sun, H. W. Zhu and D. M. Zhou, Environ. Sci. Technol., 2008, 42, 3254–3259 CrossRef PubMed.
- Z. G. Pei, X. Q. Shan, S. Z. Zhang, J. J. Kong, B. Wen, J. Zhang, L. R. Zheng, Y. N. Xie and K. Janssens, J. Hazard. Mater., 2011, 186, 842–848 CrossRef PubMed.
- Y. G. Xu, W. T. Yu, Q. Ma and H. Zhou, Chemosphere, 2015, 138, 701–707 CrossRef PubMed.
- Z. G. Pei, S. Yang, L. Y. Li, C. M. Li, S. Z. Zhang, X. Q. Shan, B. Wen and B. Y. Guo, Environ. Pollut., 2014, 184, 579–585 CrossRef PubMed.
- Y. Y. Bao, Y. Wan, Q. X. Zhou, W. M. Li and Y. X. Liu, J. Soils Sediments, 2013, 13, 364–374 CrossRef.
- D. A. Jia, D. M. Zhou, Y. J. Wang, H. W. Zhu and J. L. Chen, Geoderma, 2008, 146, 224–230 CrossRef.
- S. A. Sassman and L. S. Lee, Environ. Sci. Technol., 2005, 39, 7452–7459 CrossRef PubMed.
- B. Pan, M. Y. Qiu, M. Wu, D. Zhang, H. B. Peng, D. Wu and B. S. Xing, Environ. Pollut., 2012, 161, 76–82 CrossRef PubMed.
- Y. P. Zhao, X. Y. Gu, S. X. Gao, J. J. Geng and X. R. Wang, Geoderma, 2012, 183, 12–18 CrossRef.
- Y. P. Zhao, Y. Y. Tan, Y. Guo, X. Y. Gu, X. R. Wang and Y. Zhang, Environ. Pollut., 2013, 180, 206–213 CrossRef PubMed.
- Q. Zhang, X. H. Shu, X. T. Guo, D. Q. Mo, S. G. Wei and C. Yang, RSC Adv., 2016, 6, 53175–53181 RSC.
- T. Tang, C. Yang, L. Wang, X. Y. Jiang, Z. Dang and W. L. Huang, Environ. Sci. Pollut. Res., 2018, 25, 11576–11583 CrossRef PubMed.
- K. Yang, G. F. Miao, W. H. Wu, D. H. Lin, B. Pan, F. C. Wu and B. S. Xing, Chemosphere, 2015, 138, 657–663 CrossRef PubMed.
- S. Thiele-Bruhn, T. Seibicke, H. R. Schulten and P. Leinweber, J. Environ. Qual., 2004, 33, 1331–1342 CrossRef PubMed.
- Y. Y. Lou, Z. L. Ye, S. H. Chen, X. Ye, Y. J. Deng and J. Q. Zhang, J. Environ. Sci., 2018, 65, 144–152 CrossRef PubMed.
- D. Wu, H. Li, S. H. Liao, X. L. Sun, H. B. Peng, D. Zhang and B. Pan, Sci. Total Environ., 2014, 481, 209–216 CrossRef PubMed.
- I. Christl, M. Ruiz, J. R. Schmidt and J. A. Pedersen, Environ. Sci. Technol., 2016, 50, 9933–9942 CrossRef PubMed.
- X. R. Xu and X. Y. Li, Chemosphere, 2010, 78, 430–436 CrossRef PubMed.
- P. Li, M. Lang, X. X. Wang and T. L. Zhang, Clean: Soil, Air, Water, 2016, 44, 1547–1556 Search PubMed.
- B. J. Wilkins, N. Brummel and J. Loch, Water, Air, Soil Pollut., 1998, 101, 349–362 CrossRef.
- R. G. Mclaren, R. S. Swift and J. G. Williams, J. Soil Sci., 1981, 32, 247–256 CrossRef.
- E. F. Covelo, F. A. Vega and M. L. Andrade, J. Hazard. Mater., 2008, 159, 342–347 CrossRef PubMed.
- E. Roth, V. Mancier and B. Fabre, Geoderma, 2012, 189–190, 133–143 CrossRef.
- Y. Ma, Q. Zhou, S. C. Zhou, W. Wang, J. Jin, J. W. Xie, A. M. Li and C. D. Shuang, Chem. Eng. J., 2014, 258, 26–33 CrossRef.
- M. Y. Jia, F. Wang, Y. R. Bian, X. Jin, Y. Song, F. O. Kengara, R. K. Xu and X. Jiang, Bioresour. Technol., 2013, 136, 87–93 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra06631k |
| This journal is © The Royal Society of Chemistry 2018 |