Open Access Article
Open Access ArticleChemical stability and interactions in a new antihypertensive mixture containing indapamide and dihydralazine using FT-IR, HPLC and LC-MS methods
Anna Gumieniczek *a,
Justyna Galezaa,
Anna Bereckaa,
Tomasz Mroczekb,
Krzysztof Wojtanowskib,
Katarzyna Lipskaa and
Joanna Skarbeka
*a,
Justyna Galezaa,
Anna Bereckaa,
Tomasz Mroczekb,
Krzysztof Wojtanowskib,
Katarzyna Lipskaa and
Joanna Skarbeka
aDepartment of Medicinal Chemistry, Medical University of Lublin, Jaczewskiego 4, 20-090 Lublin, Poland. E-mail: anna.gumieniczek@umlub.pl
bDepartment of Pharmacognosy with Medicinal Plant Unit, Medical University of Lublin, Chodźki 1, 20-093 Lublin, Poland
First published on 23rd October 2018
Abstract
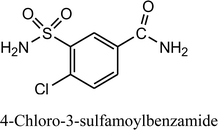
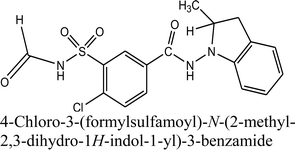
Indapamide and dihydralazine can be combined in fixed-dose formulations because of their complementary actions against hypertension. On the other hand, combined formulations present the problem of chemical interactions between the active ingredients, e.g. accelerated degradation of constituents or generation of quite new degradation products. Therefore, the main goal of the present study was to examine the chemical stability of indapamide and dihydralazine, as individuals and as a mixture, to detect potent interactions between both constituents, using FT-IR, HPLC and LC-MS methods. It was clearly shown that both drugs degraded more when they were in the mixture, i.e. indapamide was degraded more under high temperature/high humidity while dihydralazine was more sensitive to UV/VIS light. In solutions, indapamide was sensitive to strong acidic and strong alkaline conditions while dihydralazine degraded at pH ≥ 7. Generally, the process of degradation of indapamide and dihydralazine followed first order kinetics. The fastest degradation of both indapamide and dihydralazine was found at pH ≥ 10. Several degradation products of indapamide and dihydralazine were detected and identified by our LC-MS method. Interactions between both drugs were confirmed by detection of new degradation products of indapamide, i.e. 4-chloro-3-sulfamoylbenzamide and 4-chloro-3-(formylsulfamoyl)-N-(2-methyl-2,3-dihydro-1H-indol-1-yl)benzamide, only in the presence of dihydralazine.
1. Introduction
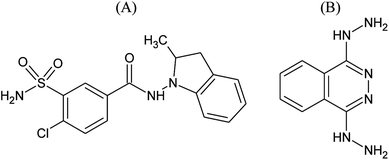
Indapamide is a non thiazide diuretic drug, frequently used for the treatment of hypertension all over the world.1 Dihydralazine is an arterial vasodilator that reduces a resistance in arterial vessels, also used in the treatment of hypertension in Europe, the USA and China (Fig. 1).Owing to their complementary mechanisms of action, these drugs can be combined in fixed-dose antihypertensive formulations. The purpose for using fixed-dose combinations in antihypertensive therapy is to obtain more effective control of blood pressure and enhance compliance by using a single tablet.2 On the other hand, combined tablets present the problem of chemical interactions between the active ingredients with different chemical reactivity, e.g. accelerated degradation of one drug in the presence of another or generation of new degradation products. Therefore, the main goal of the present study was the overall assessment of chemical stability of indapamide and dihydralazine, as individuals and as a mixture, to detect and characterize such interactions, using FT-IR, HPLC and LC-MS methods.
So far, a few HPLC methods were elaborated for determination of indapamide as an individual analyte3,4 or in the presence of other antihypertensive agents like perindopril or lisinopril,5–7 telmisartan8 and amlodipine.9 Next three HPLC methods from the literature were described as stability-indicating procedures, capable to determine of indapamide in the presence of its degradation products.10–12 In addition to them, a stability-indicating LC-MS method was also published.13 Also, a few papers concerning chemical stability of indapamide have been published so far. Above all, stability of indapamide was tested in a solid state at high temperature of 50–80 °C.10–13 As far as photodegradation was concerned, only standard conditions confirming photostability according to ICH Q1B guidelines14 were applied till now.12,13 Stability of indapamide was also studied in 0.1–1 M HCl, 0.1–1 M NaOH and 1–10% H2O2.7,10–12 However, kinetics of degradation of indapamide has not been examined so far. Furthermore, only few HPLC methods have been reported for determination of dihydralazine alone15 or in the presence of other antihypertensive drugs like hydrochlorothiazide,16 triamteren17 and clonidine.18 As far as chemical stability of dihydralazine was concerned, only one report in this area exists in the literature.16
In the present study, indapamide and dihydralazine were degraded at high temperature/high humidity, under UV/VIS light and different pH. Then, a FT-IR method was used for preliminary assessment of the stressed solid samples while a new validated HPLC method was applied for quantitative determination of all stressed samples, for percentage levels of degradation as well as for kinetic measurements. Finally, the degradation products of indapamide and dihydralazine were detected and characterized through our LC-MS method and some degradation pathways were proposed. At the same time, the results obtained for the stressed mixtures of indapamide and dihydralazine were compared with the results obtained for both drugs stressed as individuals, to detect and characterize chemical interactions between the drugs.
2. Experimental
2.1. Materials
Pharmaceutical grade standards of indapamide and dihydralazine sulphate from Sigma-Aldrich (St. Louis, USA), ammonium formate, formic acid, acetonitrile and methanol for LC from Merck (Darmstadt, Germany), acetic acid (CH3COOH), sodium acetate (CH3COONa), hydrochloric acid, sodium chloride (NaCl), sodium tetraborate (Na2B4O7), sulphuric acid, sodium hydrogen phosphate (NaHPO4), sodium hydroxide (NaOH), kalium dihydrogen phosphate (KH2PO4) and kalium hydroxide (KOH) for analysis from POCh (Gliwice, Poland), acetonitrile and water for LC-MS from J. T. Baker (Center Valley, USA), Dihydralazinum® tablets 25 mg from Pabianickie Zakłady Farmaceutyczne (Pabianice, Poland) and Indapen® tablets 2.5 mg from Polpharma (Starogard Gdanski, Poland) were used. All buffers were prepared as described in European Pharmacopoeia19 and have the same ionic strength of 1 M which was attained with 4 M NaCl. The pH measurements were done with a pH-meter HI9024C from Hanna Instruments (Padova, Italy).2.2. FT-IR method
The FT-IR spectra were recorded on a Nicolet 6700 spectrometer (Thermo Scientific, USA), equipped with a Smart iTR accessory. After recording a background spectrum, the samples of approximately 2 mg were placed on the diamond. Then, four scans were recorded for each sample over the range 4000–800 cm−1 with a resolution of 4 cm−1. The FT-IR spectra of stressed individual indapamide and dihydralazine as well as stressed binary mixtures of both drugs were compared with those obtained for the non stressed samples.2.3. HPLC method
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, v/v/v). The flow rate of the mobile phase was 1.4 mL min−1. Chromatography was performed with a model 306 pump with a loop Rheodyne (20 μL) and a model UV170 detector from Gilson (Middleton, USA) set at 228 nm for quantitative measurements, and additively at 254 and 290 nm to detect degradation products. The system was controlled by Omnic software from Gilson.
30, v/v/v). The flow rate of the mobile phase was 1.4 mL min−1. Chromatography was performed with a model 306 pump with a loop Rheodyne (20 μL) and a model UV170 detector from Gilson (Middleton, USA) set at 228 nm for quantitative measurements, and additively at 254 and 290 nm to detect degradation products. The system was controlled by Omnic software from Gilson.2.4. Degradation in a solid state
Solid mixtures containing indapamide and dihydralazine were prepared by weighing equal amounts of individual substances and mixing them thoroughly in an agate mortar. Then, portions of individual substances and their mixtures were placed in standardized small flat vessels so that the thickness of the layer was approximately 3 mm. The samples were placed in a climate chamber KBF P240 from Binder (Neckarsulm, Germany) set at 70 °C/80% RH for 2 months. This part of experiment was performed according to ICH Q1A(R2) guidelines.20 Similar samples were placed in a Suntest CPS Plus chamber from Atlas (Linsengericht, Germany) and exposed to UV/VIS light in the range 300–800 nm, with energy equal 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 902 kJ m−2, 56
902 kJ m−2, 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 706 kJ m−2 and 113412 kJ m−2. Energy of 18
706 kJ m−2 and 113412 kJ m−2. Energy of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 902 kJ m−2 was equivalent to 1.200.000 lux h and 200 W m−2 that is recommended by ICH Q1B guidelines14 as a dose of light that confirms drug photostability. During whole experiment, temperature in the chamber did not exceed 35 °C.
902 kJ m−2 was equivalent to 1.200.000 lux h and 200 W m−2 that is recommended by ICH Q1B guidelines14 as a dose of light that confirms drug photostability. During whole experiment, temperature in the chamber did not exceed 35 °C.
After irradiation, the amounts of 10 mg of individual substances or 20 mg of the mixtures were weighed and dissolved with methanol to obtain solutions of concentration 1.0 mg mL−1, for both indapamide and dihydralazine. After diluting with methanol to cover the linearity range, the solutions were analyzed using our HPLC method. The procedure was repeated three times for each sample and the concentrations of non degraded (remaining after degradation) indapamide and dihydralazine were calculated from the linear calibration equations. At the same time, percentage levels of degradation of indapamide and dihydralazine were calculated taking into account their starting concentrations.
2.5. Degradation in solutions
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 902 kJ m−2, 56
902 kJ m−2, 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 706 kJ m−2 and 113412 kJ m−2 while temperature in the chamber did not exceed 35 °C. After irradiation, the solutions were diluted with methanol to cover the linearity range and analyzed by means of our HPLC method. The procedure was repeated three times for each sample, and the concentrations of non degraded (remaining after degradation) indapamide or dihydralazine were calculated from the linear calibration equations. At the same time, percentage levels of degradation of indapamide and dihydralazine were calculated taking into account their starting concentrations.
706 kJ m−2 and 113412 kJ m−2 while temperature in the chamber did not exceed 35 °C. After irradiation, the solutions were diluted with methanol to cover the linearity range and analyzed by means of our HPLC method. The procedure was repeated three times for each sample, and the concentrations of non degraded (remaining after degradation) indapamide or dihydralazine were calculated from the linear calibration equations. At the same time, percentage levels of degradation of indapamide and dihydralazine were calculated taking into account their starting concentrations.2.6. LC-MS method
The samples were analyzed with a 6530B accurate-mass-QTOF-MS spectrometer with a dual ESI-Jet Stream ion source, using an Eclipse XDB C18 (150 × 4.6 mm, 3.5 μm) column from Agilent Technologies (Santa Clara, USA). The chromatograph was equipped with a DAD, an autosampler, a binary gradient pump, and a column oven. The mobiles phases were: acetonitrile–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99, v/v) with 10 mM ammonium formate (0.1%) (solvent A) and acetonitrile–water (95
99, v/v) with 10 mM ammonium formate (0.1%) (solvent A) and acetonitrile–water (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) with 10 mM ammonium formate (0.1%) (solvent B). Following elution procedure was used: 0–60 min, 0–95% of solvent B with a stable flow rate 0.4 mL min−1. The injection volume for the samples was 10 μL. The analysis was conducted at 25 °C.
5, v/v) with 10 mM ammonium formate (0.1%) (solvent B). Following elution procedure was used: 0–60 min, 0–95% of solvent B with a stable flow rate 0.4 mL min−1. The injection volume for the samples was 10 μL. The analysis was conducted at 25 °C.
Following parameters of the ion source were applied: a negative ion mode (−ESI), gas (N2) flow rate 12 L min−1, nebulizer pressure 35 psig, vaporizer temperature 350 °C, sheath gas temperature 400 °C, sheath gas (N2) flow 12 L min−1, m/z range 100–1000 mass units with an acquisition mode auto MS/MS, collision induced dissociation (CID) 10 and 40 eV with MS scan rate of 1 spectrum s−1 and 2 spectra per cycle, VCap 4000 V, skimmer 65 V, fragmentor 150 V and Octopole RF Peak 750 V. Additionally, the analysis was made in auto MS/MS with excluded m/z at 966.0007 and 112.9856 for negative ion mode, corresponding to the m/z of reference ions.
Before LC-MS analysis, acetate, phosphate and borate buffers were removed from the stressed samples by the means of Bakerbond SPE C8 disposable extraction columns (3 mL) from J. T. Baker using a UCT Positive Pressure Manifold station (Horsham, USA). The ions were removed from the bed with water while the substances of interest (indapamide, dihydralazine and their degradation products) were eluted with methanol. Respective fractions were pooled, dried under vacuum and finally reconstituted with acetonitrile.
3. Results and discussion
3.1. FT-IR analysis of the stressed samples
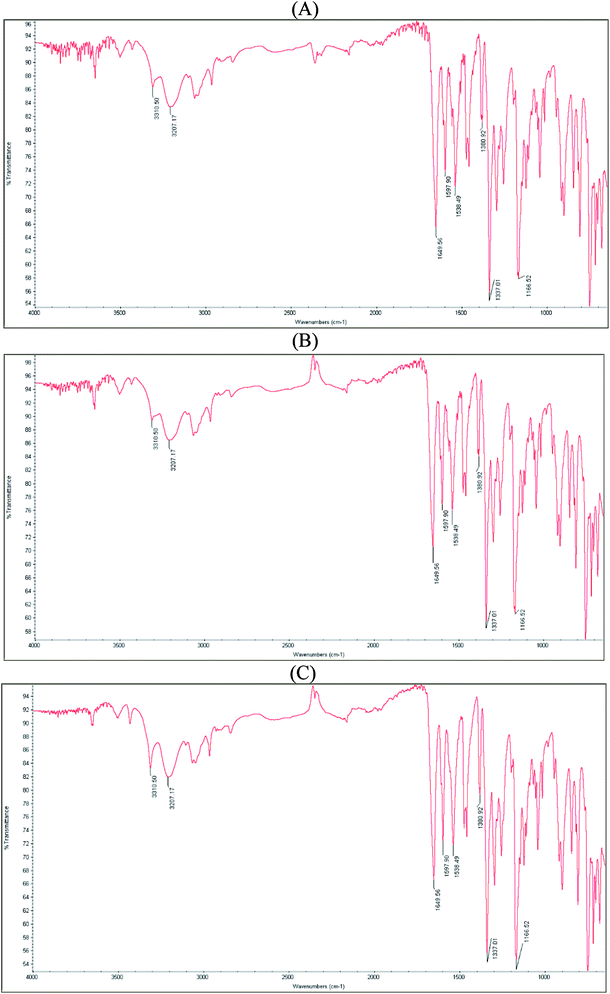
FT-IR spectrum of pure indapamide showed significant bands at 3310 and 3207 cm−1 due to N–H stretching, at 1649 cm−1 due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, at 1597 cm−1 due to aromatic C–H stretching, and at 1380 and 1166 cm−1 due to SO2 stretching vibrations. In addition, characteristic bands at 1538 and 1337 cm−1 were clearly seen (Fig. 2A).
O stretching, at 1597 cm−1 due to aromatic C–H stretching, and at 1380 and 1166 cm−1 due to SO2 stretching vibrations. In addition, characteristic bands at 1538 and 1337 cm−1 were clearly seen (Fig. 2A).
 | ||
| Fig. 2 FT-IR spectra of pure indapamide (A); indapamide stressed at high temperature/high humidity (B); indapamide stressed under UV/VIS light (C). | ||
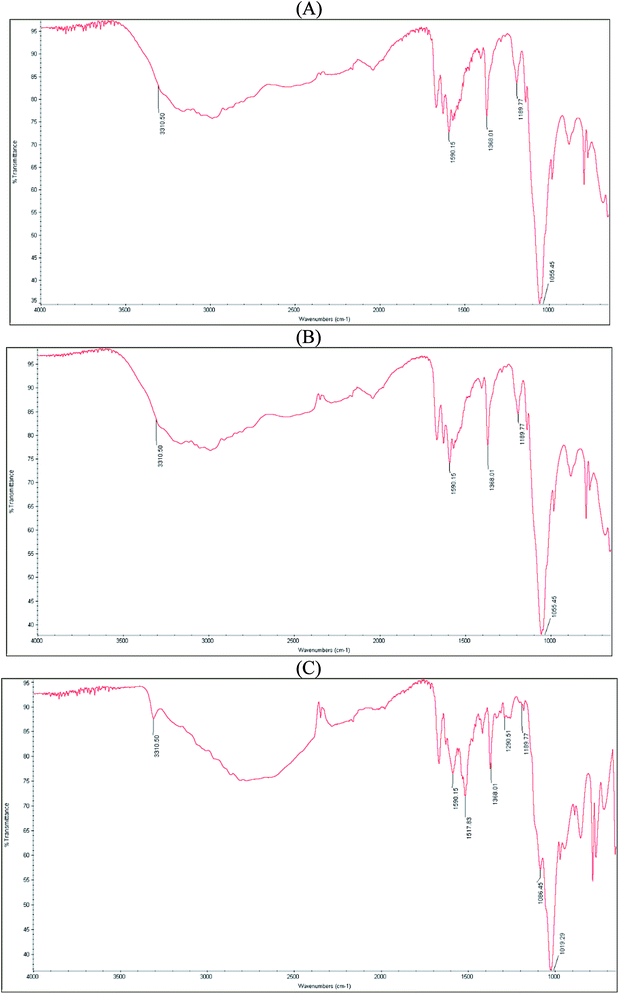
FT-IR spectrum of pure dihydralazine showed significant bands at 3310 cm−1 corresponding to N–H stretching and at 1590 cm−1 due to aromatic C–H stretching vibrations. In addition, characteristic bands at 1368, 1189, and 1055 cm−1 were clearly seen (Fig. 3A).
 | ||
| Fig. 3 FT-IR spectra of pure dihydralazine (A); dihydralazine stressed at high temperature/high humidity (B); dihydralazine stressed under UV/VIS light (C). | ||
The spectrum of individual indapamide did not change after degradation at high temperature/high humidity (Fig. 2B) and under UV/VIS light (Fig. 2C), similarly as the spectrum of individual dihydralazine affected by high temperature/high humidity (Fig. 3B). However, the spectrum of individual dihydralazine affected by UV/VIS light showed visible changes as compared to the standard spectrum, i.e. broadening the band at 1590 cm−1 together with appearing of new bands at 1517, 1290 and 1086 cm−1. At the same time, a broad band at 1055 cm−1 deteriorated to 1019 cm−1 (Fig. 3C). In addition, the same sample analyzed by means of our LC-MS method showed the presence of new degradation products of dihydralazine formed by loss of hydrazine and breaking phenazine ring (Table 1).
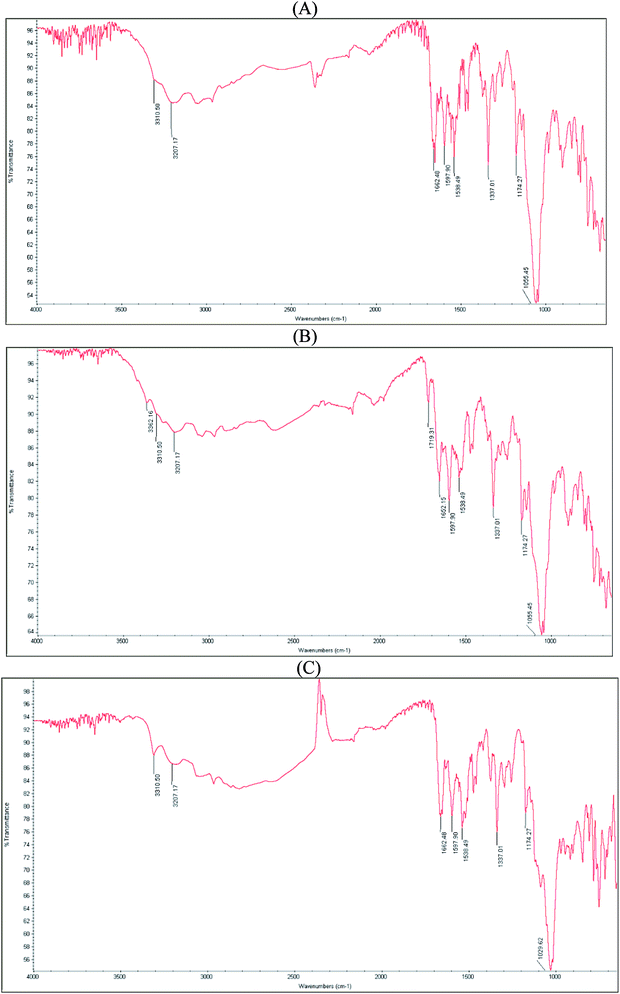
The spectrum of the mixture of indapamide and dihydralazine showed overlapping of their bands at 3310 cm−1 due to N–H stretching vibrations, overlapping a band of indapamide at 1597 cm−1 with that of dihydralazine at 1590 cm−1 due to aromatic C–H stretching vibrations, overlapping a band of indapamide at 1166 cm−1 with that of dihydralazine at 1189 cm−1 to form a new band at 1174 cm−1, as well as decreasing a band of indapamide at 3207 cm−1 and that of dihydralazine at 1368 cm−1. In addition, a band of indapamide at 1649 cm−1 due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations deteriorated to 1662 cm−1 (Fig. 4A).
O stretching vibrations deteriorated to 1662 cm−1 (Fig. 4A).
 | ||
| Fig. 4 FT-IR spectra of non stressed mixture of indapamide with dihydralazine (A); the mixture stressed at high temperature/high humidity (B); the mixture stressed under UV/VIS light (C). | ||
Even more visible changes were detected in respective spectra after stress degradation. At the spectrum of the mixture stressed by high temperature/high humidity, a new band at 1719 cm−1 appeared while a band at 1662 cm−1 deteriorated to 1652 cm−1 (Fig. 4B). However, our LC-MS experiments for this sample did not show new degradation products of indapamide or dihydralazine (Table 1). When the mixture was affected by UV/VIS light, the most significant changes were observed as disappearing a band at 1174 cm−1 and changing the shape of a broad band in the range 1100–950 cm−1 (Fig. 4C). Therefore, we supposed that photo-induced interactions between indapamide and dihydralazine were different than those caused by high temperature/high humidity. At the same time, our LC-MS experiments for these samples resulted as identification of new degradation product of indapamide formed by loss of 2-methyl-1H-indole (Table 1).
3.2. Identification of degradation products by LC-MS method
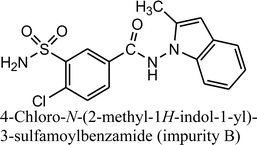
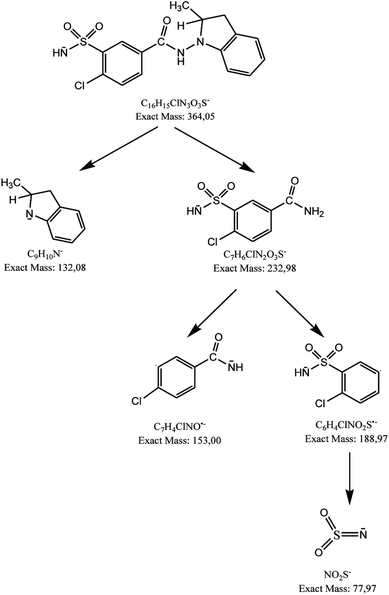
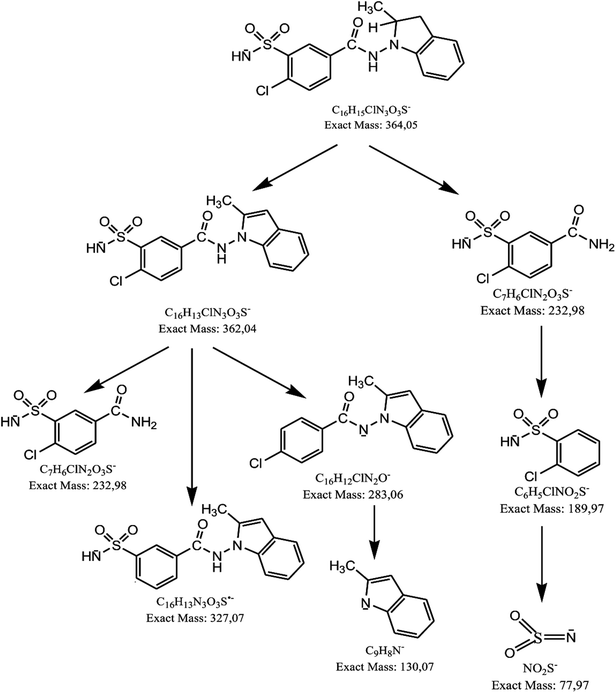
Using negative ionization mode (ESI) to indapamide standard, a deprotonated molecule [M − H]− of m/z 364.05 was obtained which followed a parallel fragmentation pathway. Firstly, after losing 2-methyl-2,3-dihydro-1H-indole, an ion of m/z 232.98 was formed. Its further fragmentation led to formation of an ion of m/z 153.00 (loss of sulfonamide group) or ions of m/z 188.97 (loss of amide group) and 77.97 (loss of chlorobenzene). Secondly, from a deprotonated molecule of indapamide of m/z 364.05, an ion of m/z 132.08 was formed, after losing 4-chloro-3-sulfamoylbenzamide (Fig. 5). These results are consistent with previous results from the literature concerning fragmentation of indapamide with negative ESI.13The monograph of indapamide in European Pharmacopoeia19 lists two impurities, i.e. 2-methyl-1-nitroso-2,3-dihydro-1H-indole (impurity A) and 4-chloro-N-(2-methyl-1H-indol-1-yl)-3-sulfamoylbenzamide (impurity B). In the present study, the main degradation product of indapamide was detected as a fragment ion of m/z 362.04 and identified as impurity B. It was detected in all stressed samples (Table 1). Thus, our results confirmed those obtained previously by El-Gindy et al.10 and Jogia et al.12 who detected impurity B as a result of acid, base and photo-induced degradation of indapamide.
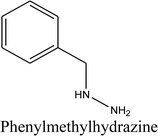
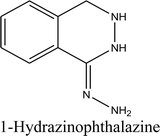
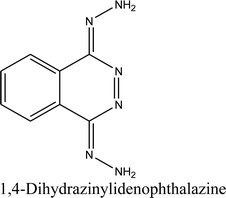
When dihydralazine standard was fragmented using negative ESI mode, a deprotonated molecule [M − H]− of m/z 189.09 was formed. Then, fragment ions of m/z 161.08 and 118.07 were produced. So, we concluded for a second time that dihydralazine fragmented by loss of hydrazine and breaking phenazine ring.16 In our LC-MS studies, products of m/z 121.03 and 161.04 were detected after degradation in buffer of pH 10 and under UV/VIS light (in a solid state and in methanolic solutions). They were identified as phenylmethylhydrazine and 1-hydrazinophthalazine. Furthermore, next product of degradation of dihydralazine in 1 M NaOH of m/z 187.04 was detected and identified as 1,4-dihydrazinylidenophthalazine (Table 1). Two of these compounds, i.e. phenylmethylhydrazine and 4-dihydrazinylidenophthalazine were described for the first time in our last paper.16
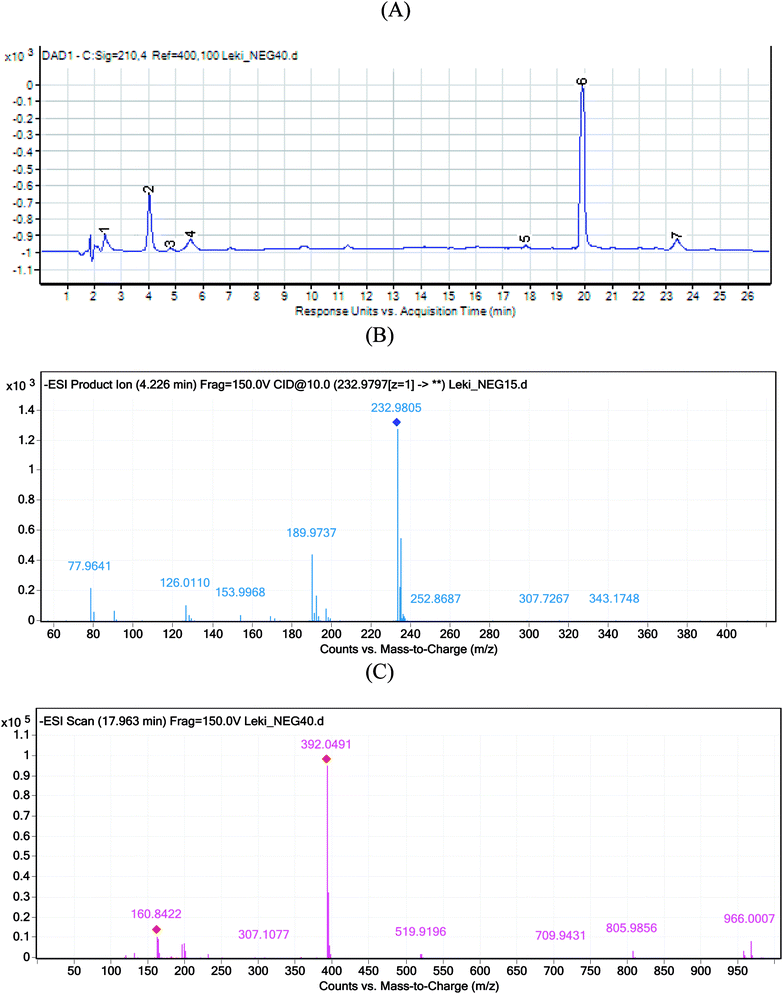
When the stressed mixtures of indapamide and dihydralazine were examined, fragment ions of m/z 121.03, 161.04 and 187.04 from dihydralazine (after degradation in borate buffer of pH 10, 1 M NaOH and under UV/VIS light), as well as of m/z 362.04 from indapamide (after degradation in all conditions) were detected. In addition, quite new product of degradation of indapamide was detected as a fragment ion of m/z 232.98, as a result of degradation in 1 M HCl, borate buffer of pH 10 and under UV/VIS light (in solid state and in methanolic solution). This compound was identified as 4-chloro-3-sulfamoylbenzamide (Table 1). Respective LC-DAD chromatogram and mass spectrum were presented in Fig. 6A and B. The proposed degradation pathway including this new degradation product was shown in Fig. 7.
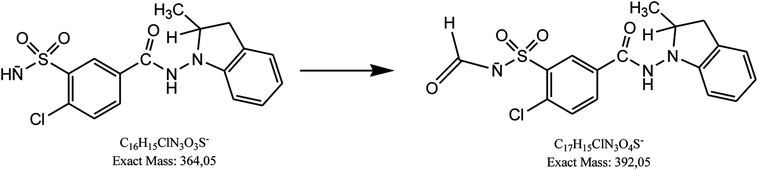
Besides, next degradation product of indapamide was detected as an ion of m/z 392.05 and identified as 4-chloro-3-(formylsulfamoyl)-N-(2-methyl-2,3-dihydro-1H-indol-1-yl)benzamide (Table 1). Respective LC-DAD chromatogram and mass spectrum were presented in Fig. 6A and C. It was solely detected in the mixture of indapamide with dihydralazine after stressing with borate buffer of pH 10. At the same time, this formate adduct was not observed in other samples from our experiments. Therefore, it was not formed due to the presence of formate ions in the mobile phase used for our LC separation. Thus, a new way of indapamide degradation could be proposed (Fig. 8).
3.3. Validation of HPLC method
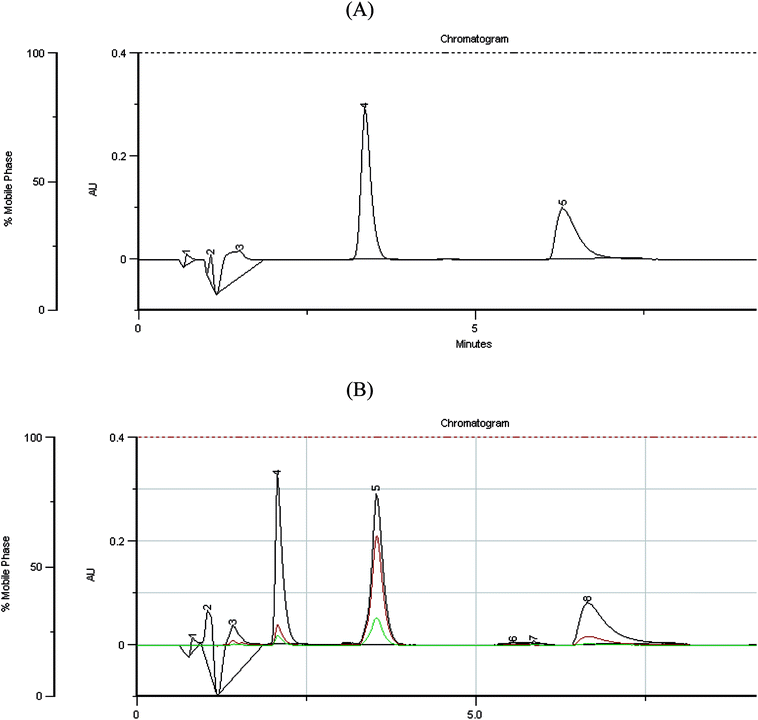
A simple, isocratic HPLC method was developed for simultaneous determination of indapamide and dihydralazine in the presence of their degradation products with satisfactory retention times and peak shapes (Fig. 9A). It is worth mentioning that any similar report has not been described in the literature so far. Respective chromatograms showed that the peaks of indapamide and dihydralazine were free from interferences of the degradation products, confirming selectivity of the method (Fig. 9B).Resistance of the method to small changes in analytical parameters, i.e. acetonitrile content (30 ± 2%), flow rate (1.4 ± 0.2 mL min−1), detection wavelength (228 ± 3 nm) and column temperature (22 ± 2 °C) was examined. Uniformity of the obtained peak areas confirmed the robustness of the method. However, the calculated values of peak symmetry of indapamide indicated sensitivity of the method to changes of detection wavelength. As far as dihydralazine was concerned, the method was sensitive to changes of acetonitrile content in the mobile phase and flow rate of the mobile phase.
The quantitative method was found to be linear over the concentration range of 10–60 μg mL−1 for both drugs, with average R2 of 0.9992 for indapamide and 0.9990 for dihydralazine. The calculated LOD and LOQ were 1.81 μg mL−1 and 5.50 μg mL−1 for indapamide, and 1.69 μg mL−1 and 5.13 μg mL−1 for dihydralazine. The RSD values in the range 0.25–1.52% for indapamide and 0.61–1.32% for dihydralazine (the one-day precision), and 0.85–1.96% for indapamide and 0.82–1.93% for dihydralazine (the inter-day precision) were obtained. Accuracy of the method was confirmed by determining the both drugs in the powdered tablets. Recovery values were obtained in the range 99.66–100.72% for indapamide and 98.72–101.13% for dihydralazine (Table 2).
| Parameter | Indapamide | Dihydralazine |
|---|---|---|
| Linearity range (μg mL−1) | 10–60 | 10–60 |
| Slope | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 739 739 |
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 036 036 |
| SD of slope | 6986 | 3097 |
| Intercept | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 333 333 |
−21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 66 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 667 667 |
| SD for intercept | 471404 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 678 678 |
| R2 | 0.9992 | 0.9990 |
| SD of R2 | 0.00047 | 0.00037 |
| LOD (μg mL−1) | 1.81 | 1.69 |
| LOQ (μg mL−1) | 5.50 | 5.13 |
| Accuracy (% recovery) | 99.66–100.72 | 98.72–101.13 |
| Precision (RSD) | ||
| Intra-day | 0.25–1.52 | 0.61–1.32 |
| Inter-day | 0.85–1.96 | 0.82–1.93 |
| Retention time (min) | 3.58 | 6.58 |
| Asymmetry factor | 1.33 | 1.75 |
The chromatograms obtained for the samples of powdered tablets showed that the peaks of interest were free from interferences of excipients, confirming selectivity of the method once more.
3.4. Percentage levels of degradation at high temperature/high humidity
According to the literature, the highest degradation of indapamide (ca. 5%) occurred at 50 °C/80% RH after 1 month.13 In the present study, indapamide showed lower degradation equal 1.05% at 70 °C/80% RH after 2 months. However, in the mixture with dihydralazine, an increase of indapamide degradation to 14.96% was observed (Table 3). Thus, combining indapamide with dihydralazine in fixed-dose formulations seemed to be unfavorable from the point of its chemical stability. At the same time, the present study showed that dihydralazine as an individual was sensitive to high temperature/high humidity (21.68% of degradation). However, mixing it with indapamide did not affect its stability (Table 3). Therefore, the combined formulations of indapamide and dihydralazine could be manufactured, but high protection from high temperature and high humidity should be provided.| Conditions | Level of degradation [%] | |||
|---|---|---|---|---|
| Indapamide | Dihydralazine | |||
| Individual | Mixture | Individual | Mixture | |
a 1ICH = 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 902 kJ m−2; 3ICH = 56 902 kJ m−2; 3ICH = 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 706 kJ m−2; 6ICH = 113412 kJ m−2. 706 kJ m−2; 6ICH = 113412 kJ m−2. |
||||
| Degradation in a solid state | ||||
| 70 °C/80% RH | 1.05 | 14.69 | 21.68 | 26.15 |
| 1ICHa | 1.76 | 1.87 | 1.45 | 5.98 |
| 3ICHa | 3.86 | 3.95 | 8.62 | 23.14 |
| 6ICHa | 7.15 | 8.23 | 25.78 | 48.76 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Degradation in solutions | ||||
| 1ICHa | 17.52 | 17.97 | 2.78 | 21.08 |
| 3ICHa | 32.38 | 34.15 | 12.98 | 41.15 |
| 6ICHa | 67.16 | 69.88 | 100.0 | 100.0 |
3.5. Percentage levels of photodegradation
According to the literature, the lack of photodegradation of indapamide in a solid state7,12 or 100% of degradation of indapamide in methanolic solution10 were reported. Present experiment showed that indapamide in a solid state was rather resistant to the light impact. After irradiation with energy equal 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 902 kJ m−2, the level of degradation of indapamide was determined as 1.76%. When higher doses of light were used, degradation of indapamide at the levels 3.86 and 7.15% was shown (Table 3).
902 kJ m−2, the level of degradation of indapamide was determined as 1.76%. When higher doses of light were used, degradation of indapamide at the levels 3.86 and 7.15% was shown (Table 3).
In addition, the FT-IR spectrum of the stressed indapamide did not show significant changes in comparison with the spectrum of the non stressed substance (Fig. 2C). However, when methanolic solution of indapamide was irradiated with energy equal 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 902 kJ m−2, 17.52% degradation of indapamide was observed. When higher doses of light were used, degradation of indapamide at the levels 32.38 and 67.16% occurred (Table 3). Bearing in mind the percentage levels of degradation, dihydralazine did not significantly increase photo-sensitivity of indapamide.
902 kJ m−2, 17.52% degradation of indapamide was observed. When higher doses of light were used, degradation of indapamide at the levels 32.38 and 67.16% occurred (Table 3). Bearing in mind the percentage levels of degradation, dihydralazine did not significantly increase photo-sensitivity of indapamide.
Of the two, individual dihydralazine was shown to be more sensitive to the light impact than indapamide with degradation in a solid state of 1.45%, 8.62% and 25.78% after irradiation with energy equal 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 902 kJ m−2, 56
902 kJ m−2, 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 706 kJ m−2 and 113412 kJ m−2, respectively. In addition, the FT-IR spectrum of the stressed dihydralazine showed significant changes in comparison with the spectra of the non stressed drug (Fig. 3C). In methanolic solutions, respective levels of degradation were determined as 2.78%, 12.98% and 100%. Moreover, dihydralazine in presence of indapamide underwent higher degradation than as an individual substance, even under lower doses of light. In a solid state the levels of degradation increased to 5.98 vs. 1.45%, 23.14 vs. 8.62% and 48.76 vs. 25.78% while in solutions to 21.08 vs. 2.78% and 41.15 vs. 12.98% of degradation, under energy equal 18
706 kJ m−2 and 113412 kJ m−2, respectively. In addition, the FT-IR spectrum of the stressed dihydralazine showed significant changes in comparison with the spectra of the non stressed drug (Fig. 3C). In methanolic solutions, respective levels of degradation were determined as 2.78%, 12.98% and 100%. Moreover, dihydralazine in presence of indapamide underwent higher degradation than as an individual substance, even under lower doses of light. In a solid state the levels of degradation increased to 5.98 vs. 1.45%, 23.14 vs. 8.62% and 48.76 vs. 25.78% while in solutions to 21.08 vs. 2.78% and 41.15 vs. 12.98% of degradation, under energy equal 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 902 kJ m−2 and 56
902 kJ m−2 and 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 706 kJ m−2, respectively. These results allowed the conclusion that indapamide increased sensitivity of dihydralazine to light (Table 3). Thus, the combined formulations of indapamide and dihydralazine could be manufactured only when high protection from UV/VIS light is provided.
706 kJ m−2, respectively. These results allowed the conclusion that indapamide increased sensitivity of dihydralazine to light (Table 3). Thus, the combined formulations of indapamide and dihydralazine could be manufactured only when high protection from UV/VIS light is provided.
3.6. Percentage levels of degradation in different pH
According to the literature, the highest degradation of indapamide occurred in 1 M HCl and 1 M NaOH (20.80% and 17.50%, respectively).7 At the same time, there is not any report concerning degradation of indapamide in buffers of different pH values. Our experiments showed that indapamide was prone to degradation in 1 M HCl (11.08%), in borate buffer of pH 10 (21.27%) and in 1 M NaOH (16.18%). On the other hand, degradation of indapamide did not increase extensively in the presence of dihydralazine (Table 4).| Stress conditions | Level of degradation [%] | Linear equation y = ax + b | R2 | k [s−1] | t0.5 [h] |
|---|---|---|---|---|---|
| Indapamide | |||||
| 1 M HCl | 11.08 | y = −0.0003x + 4.7884 | 0.9395 | 1.15 × 10−5 | 16.74 |
| Buffer pH 4 | 2.15 | — | — | — | — |
| Buffer pH 7 | 2.15 | — | — | — | — |
| Buffer pH 10 | 21.27 | y = −0.0005x + 4.7965 | 0.7207 | 1.92 × 10−5 | 10.03 |
| 1 M NaOH | 16.18 | y = −0.0005x + 4.4774 | 0.9526 | 1.92 × 10−5 | 10.03 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Indapamide in the mixture with dihydralazine | |||||
| 1 M HCl | 12.42 | y = −0.0003x + 4.0893 | 0.7872 | 1.15 × 10−5 | 16.74 |
| Buffer pH 4 | 2.87 | — | — | — | — |
| Buffer pH 7 | 2.86 | — | — | — | — |
| Buffer pH 10 | 23.31 | y = −0.0011x + 4.1307 | 0.9388 | 4.22 × 10−5 | 4.56 |
| 1 M NaOH | 17.08 | y = −0.0007x + 4.1016 | 0.9314 | 2.69 × 10−5 | 7.16 |
As far as dihydralazine was concerned, the present study showed its stability in acidic environment (1.32% of degradation in 1 M HCl and 4.98% of degradation in buffer of pH 4). At the same time, high sensitivity of dihydralazine to 1 M NaOH and to buffers of pH 7 and 10 was confirmed (100%, 38.55% and 74.06% of degradation, respectively). Quite new conclusion was that dihydralazine in the presence of indapamide degraded to a greater extent in 1 M HCl (3.49%), in buffer of pH 4 (7.76%) and in buffer of pH 10 (89.24%). At pH 7, the impact of indapamide on degradation of dihydralazine was not observed (Table 5).
| Stress conditions | Levelof degradation [%] | Linear equation y = ax + b | R2 | k [s−1] | t0.5 [h] |
|---|---|---|---|---|---|
| Dihydralazine | |||||
| 1 M HCl | 1.32 | — | — | — | — |
| Buffer pH 4 | 4.98 | — | — | — | — |
| Buffer pH 7 | 38.55 | y = −0.0018x + 4.7378 | 0.9073 | 6.91 × 10−5 | 2.78 |
| Buffer pH 10 | 74.06 | y = −0.0045x + 4.7966 | 0.9842 | 1.73 × 10−4 | 1.11 |
| 1 M NaOH | 100.0 | y = −0.0118x + 3.9392 | 0.9811 | 4.53 × 10−4 | 0.42 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Dihydralazine in the mixture with indapamide | |||||
| 1 M HCl | 3.49 | — | — | — | — |
| Buffer pH 4 | 7.76 | — | — | — | — |
| Buffer pH 7 | 38.55 | y = −0.0018x + 4.1759 | 0.9637 | 6.91 × 10−5 | 2.78 |
| Buffer pH 10 | 89.24 | y = −0.0078x + 4.0678 | 0.9806 | 2.99 × 10−4 | 0.64 |
| 1 M NaOH | 100.0 | y = −0.0142x + 3.8147 | 0.8184 | 5.45 × 10−4 | 0.35 |
3.7. Kinetics
At some conditions, the level of degradation of indapamide and dihydralazine was too low to obtain reliable kinetic data. Thus, kinetic parameters were calculated only when the levels of degradation of the drugs were at least 10% after 300 min. The concentrations of drugs remaining after each time point were calculated from respective linear regression equations and represented in terms of mean after triplicate analysis. Then, the concentrations (C) or ln(C) were plotted against time to determine the reaction order of degradation. For the both drugs, stronger correlations (higher R2 values) were obtained for semi-logarithmic plots, indicating that degradation of indapamide and dihydralazine followed the first order kinetics. From the slopes of these semi-logarithmic plots, the observed rate constant (k) and degradation time of 50% substance (t0.5) were calculated.It was shown that indapamide degraded in different pH with k values at the levels of 10−5 s−1. The shortest t0.5 (ca. 10 h) was obtained in 1 M NaOH and in buffer of pH 10. However, in the presence of dihydralazine, degradation of indapamide was observed as much faster reaction with t0.5 of 4.56–7.16 h (Table 4). As was described above, in the literature there is not any report involving kinetic parameters for indapamide degradation. Thus, the results presented here supplemented the literary resources in this area. As far as dihydralazine was concerned, the k values of degradation processes were obtained at the levels of 10−5 s−1 to 10−4 s−1. The degradation rate increased with the pH increase when the pH of the solution was in basic part. These results indicated specific basic catalysed effects on the degradation of dihydralazine. The shortest t0.5 (0.42 h) was calculated for degradation in 1 M NaOH. At the same time, the presence of indapamide significantly shortened the t0.5 value for degradation of dihydralazine in borate buffer of pH 10 (Table 5).
4. Conclusions
Indapamide in a solid state is resistant to high temperature/high humidity but sensitive to UV/VIS light while dihydralazine is sensitive to both stressors. In solutions, indapamide is sensitive to strong acidic and strong basic conditions while dihydralazine degrades intensively at pH ≥ 7. Generally, percentage degradation of drugs in their mixtures is greater than that of individual drugs under the same stress conditions.Increased sensitivity of the both active substances to stressors and potent chemical interactions between them were confirmed by detection of new degradation products of indapamide, i.e. 4-chloro-3-sulfamoylbenzamide and 4-chloro-3-(formylsulfamoyl)-N-(2-methyl-2,3-dihydro-1H-indol-1-yl)benzamide. Such degradation products of indapamide were not described in the literature so far.
Thus, the current knowledge about degradation of indapamide as well as of dihydralazine was significantly improved. Such results may be the starting point for new studies on toxicity of new degradation products and then, for qualifying them as new related substances in pharmacopoeial monographs. These data may also serve as a starting point for designing new antihypertensive formulations containing indapamide and dihydralazine. These two active substances can be combined to fixed-dose formulations because of their complementary actions against hypertension. However, possibility of chemical interactions between these drugs, i.e. their accelerated degradation and generation of new degradation products in the mixture, was clearly shown. Thus, the necessity of highly protecting indapamide and dihydralazine from high temperature, high humidity and UV/VIS light was proved.
Conflicts of interest
There are no conflicts of interest to declare.References
- M. Inaba, Y. Noguchi, T. Yamamoto, T. Imai, M. Hatano, S. Yagi and S. Katayama, Hypertens. Res., 2004, 27, 141–145 CrossRef CAS PubMed.
- N. S. Skolnik, J. D. Beck and M. Clark, Am. Fam. Physician, 2000, 61, 3049–3056 CAS.
- H. K. H. Pannu, M. P. Mahajan and S. D. Sawant, Der Pharma Chem., 2012, 4, 996–1002 CAS.
- J. B. Pai, K. S. A. Sathish, B. Gopinath and G. Chenna, Int. J. PharmTech Res., 2011, 3, 1482–1487 CAS.
- J. Joseph, B. Philip and M. Sundarapandian, Int. J. Pharmacol. Pharm. Sci., 2011, 3, 288–293 CAS.
- R. Tiwari, A. Jain, D. Maliwal and E. Toppo, Asian J. Pharm. Clin. Res., 2012, 5, 50–53 CAS.
- A. Fernández-Carballido, E. Barcia, D. Córdoba-Díaz, M. Córdoba-Díaz and S. Negro, Curr. Pharm. Anal., 2014, 10, 10–19 CrossRef.
- C. S. Satpute, P. Pagare, V. M. Jadhav and V. J. Kadam, Res. J. Pharm. Technol., 2013, 6, 1217–1224 Search PubMed.
- T. M. Kalyankar, P. K. Khadkutkar and R. B. Kakde, Int. J. Res. Ayurveda Pharm., 2012, 3, 729–732 CrossRef CAS.
- A. El-Gindy, M. W. Nassar, K. A. S. Attia, H. H. Abu-Seada and M. El-Ghandour, J. Liq. Chromatogr. Relat. Technol., 2014, 37, 696–712 CrossRef CAS.
- G. Rashmita, T. Ramesh and M. Ramesh, Der Pharma Chem., 2013, 5, 347–352 Search PubMed.
- H. Jogia, U. Khandelwal, T. Gandhi, S. Singh and D. Modi, J. AOAC Int., 2010, 93, 108–115 CAS.
- K. A. M. Attia, M. W. I. Nassar, M. M. K. El-Din, M. K. Sharaf, A. A. Mohamad and M. M. Y. Kaddah, Anal. Methods, 2016, 8, 1836–1851 RSC.
- ICH Topic Q1B, Photostability Testing of New Active Substances and Medicinal Products, 2006 Search PubMed.
- S. K. Raul, B. V. Ravi Kumar, A. K. Pattnaik and N. N. Rao, Int. J. Pharm., 2013, 3, 116–121 CAS.
- A. Gumieniczek, J. Galeza, T. Mroczek, K. Wojtanowski, K. Lipska and R. Pietras, Chromatographia, 2018, 81, 1147–1162 CrossRef CAS PubMed.
- B. Q. Che, Acta Pharmacol. Sin., 2004, 39, 618–620 CAS.
- P. F. Jin, Y. T. Kuang, D. Zou, X. Hu, W. Q. Jiang and X. J. Wu, Chin. Pharm. J., 2011, 46, 152–155 CAS.
- A. Gumieniczek, J. Galeza, A. Berecka, T. Mroczek, K. Wojtanowski, K. Lipska and J. Skarbek, European Pharmacopeia, Council of Europe, Strasbourg, 9th edn, 2016 Search PubMed.
- ICH Topic Q1A(R2), Stability Testing of New Drug Substances and Products, 2003 Search PubMed.
| This journal is © The Royal Society of Chemistry 2018 |