Open Access Article
Open Access ArticlePhosphorus pentoxide/metal chloride mediated efficient and facile catalytic conversion of fructose into 5-hydroxymethylfurfural†
Songyan Jia *a,
Xinjun Hea,
Jiao Maa,
Zhanwei Xub,
Kangjun Wanga and
Z. Conrad Zhang
*a,
Xinjun Hea,
Jiao Maa,
Zhanwei Xub,
Kangjun Wanga and
Z. Conrad Zhang *b
*b
aCollege of Chemical Engineering, Shenyang University of Chemical Technology, Shenyang, Liaoning 110142, China. E-mail: jiasongyan@126.com; Tel: +86-24-89386342
bState Key Laboratory of Catalysis, Dalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning 116023, China. E-mail: zczhang@yahoo.com; Tel: +86-411-84379462
First published on 19th September 2018
Abstract
Phosphorus pentoxide (P2O5)/metal chloride mixtures could significantly improve 5-HMF yield and selectivity for the catalytic conversion of fructose under mild conditions, whereas neither P2O5 nor tested metal chloride alone gave reasonable performances. A maximum 5-HMF yield of 75% with ∼85% selectivity could be achieved within 30 min at 80 °C.
Utilization of alternative energy sources has emerged as an important worldwide strategy to compensate for depleting fossil fuels. Renewable biomass has attracted extensive attention for the production of biobased chemicals over the last decades.1–4 5-Hydroxymethylfurfural (5-HMF) is a representative platform compound derived from carbohydrates.5–7 5-HMF can be converted into high value-added chemicals by a variety of processes, with the potential of sustainable development.8–11
Currently, 5-HMF is primarily produced through conversion of carbohydrates such as fructose, glucose and oligosaccharides. Among these carbohydrates, fructose is the most reactive feedstock and extensively investigated, although glucose is more abundant in nature.5–9 Indeed, the conversion of glucose into 5-HMF is considered as a two-step tandem reaction including “isomerization of glucose into fructose” and “dehydration of fructose into 5-HMF”.12–14 Therefore, efficient conversion of fructose is an essential step for 5-HMF production.
Pioneering works have demonstrated that fructose can be effectively converted into 5-HMF with mineral acids, metal salts, zeolites and other functionalized acidic materials.15–19 A number of reaction media such as polar organic solvents, ionic liquids and water—organic solvent biphasic solvents have been shown to be benign to the conversion of fructose.20–22 However, most reported technologies still face some questions as follows: (1) high temperatures at 150–180 °C are necessary for biphasic systems; (2) reaction temperature can be decreased to around 120 °C in polar organic solvents, while reaction time still remains 1–3 h; (3) the viscosity of ionic liquid limits its application at low temperatures; (4) cost and time for the preparation of some functionalized materials is an issue. Therefore, efficient and facile conversion of fructose under mild conditions remains an important research subject.
The conversion of fructose into 5-HMF at low temperatures (25–50 °C) had been mainly achieved in ionic liquid based media, in which 5-HMF yields of ∼40–80% were available. However, relatively long reaction time (e.g. 4–12 h) was mandatory for a reasonable 5-HMF yield.23–26 In addition, a common ionic liquid, 1-butyl-3-methylimidazolium chloride, was used as a solvent in the works above. This ionic liquid has a melting point at ∼70 °C,25 indicating that additional operations are necessary to lower the viscosity at low temperatures. A solvent with low viscosity is more desirable for fructose conversion under mild conditions.
In this work, we investigated approaches to achieve the effective conversion of fructose into 5-HMF under mild conditions. Dimethyl sulfoxide (DMSO) was used as a solvent due to its ability of suppressing side reactions.27 A variety of metal chlorides were preliminarily employed as the catalysts because they could efficiently catalyze the production of 5-HMF from fructose in an ionic liquid at moderate temperatures as previously reported.12
The conversion of fructose was first evaluated over different metal chloride catalysts in DMSO at 80 °C for 30 min. As shown in Fig. 1, the reaction with SnCl4·5H2O showed a maximum conversion of 43%, while the 5-HMF yield and selectivity reached only 14% and 33%, respectively. Although CrCl3·6H2O and AlCl3·6H2O resulted in a little amount of 5-HMF, most catalysts did not actually work at all, implying that more severe conditions were necessary for 5-HMF synthesis with the metal chlorides alone. According to literatures, fructose undergoes acid catalyzed dehydration to yield 5-HMF.28–30 The three metal salts, SnCl4·5H2O, CrCl3·6H2O and AlCl3·6H2O, may be more acidic than other tested metal salts in solutions,31 which lead to the formation of 5-HMF.
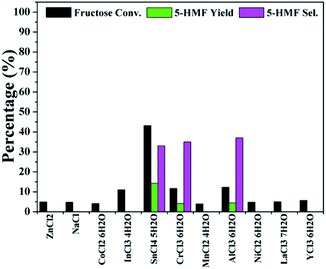 | ||
| Fig. 1 Screening tests on the conversion of fructose into 5-HMF with metal chlorides as catalysts (reaction conditions: fructose 60 mg; metal chloride 10 mol% to fructose; DMSO 1 mL; 80 °C; 30 min). | ||
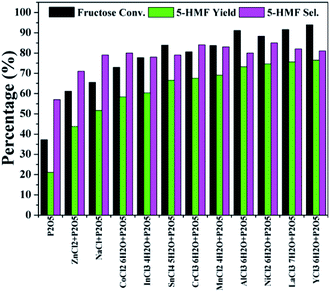
As discussed above, the production of 5-HMF undergoes acid catalyzed dehydration of fructose.28–30 Efforts should be put on acid catalysis as well as water removal. Phosphorus pentoxide (P2O5) as an anhydride is acidic and hygroscopic, which is potentially appropriate for the dehydration of fructose. Based on that, P2O5 was also investigated as a catalyst in this work. As seen in Fig. 2, P2O5 showed a superior activity to SnCl4·5H2O alone with 5-HMF yield and selectivity of 21% and 57%, respectively, possibly because P2O5 could provide acidity and/or absorb the in situ formed water.32,33 However, higher P2O5 loadings did not increase the 5-HMF yield much (Fig. S1†). The slightly decreased 5-HMF selectivity demonstrated that more side reactions occurred. Unexpectedly, both 5-HMF yield and selectivity were significantly improved when using mixtures of P2O5 and tested metal chloride as catalysts (Fig. 2). The 5-HMF selectivity stayed at about 80% in the presence of P2O5 accompanied by most metal chlorides except ZnCl2. Analysis of products showed that no or only trace amount of rehydration products such as levulinic acid were produced in the above reactions (Fig. S2†). In consideration of reaction performance and catalyst cost, NiCl2·6H2O was selected as a representative chloride for subsequent investigations.
Fig. 3 and 4 showed the effect of catalyst compositions on the conversion of fructose. The loading of either NiCl2·6H2O or P2O5 was set to be constant for examination. A slightly decreased 5-HMF yield was observed when 5 mol% P2O5 was added. The reaction performance remained basically unchanged with increasing P2O5 loading. In addition, tuning the loading of NiCl2·6H2O did not obviously affect the conversion. The above results demonstrated the importance of synergistic effect between P2O5 and metal chlorides. However, the reactivity could not be simply correlated with the catalyst compositions. The loadings of P2O5 and NiCl2·6H2O were set to be 10 mol% (to fructose) for further studies as a better 5-HMF yield was obtained at this catalyst composition.
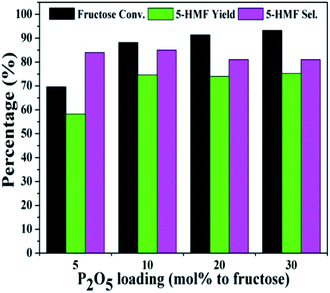 | ||
| Fig. 3 Effect of P2O5 loadings on the conversion of fructose with a constant loading of NiCl2·6H2O (reaction conditions: fructose 60 mg; NiCl2·6H2O 10 mol% to fructose; DMSO 1 mL; 80 °C; 30 min). | ||
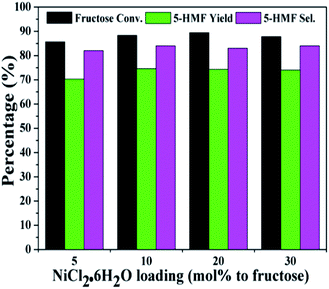 | ||
| Fig. 4 Effect of NiCl2·6H2O loadings on the conversion of fructose with a constant loading of P2O5 (reaction conditions: fructose 60 mg; P2O5 10 mol% to fructose; DMSO 1 mL; 80 °C; 30 min). | ||
The effect of anions of nickel salts on the conversion of fructose was then investigated. As seen in Table 1 (entries 2–4), the reactions with NiCl2·6H2O, Ni(NO3)2·6H2O and NiSO4·6H2O exhibited different performances although they all showed improved 5-HMF yield and selectivity compared to the one with P2O5 alone. As reported, the production of 5-HMF from fructose underwent a 2-deoxy-2-halo intermediate, which was through a oxocarbenium precursor followed by the nucleophilic attack by a halide ion.34 It is proposed that fructose underwent such a mechanism as well in this work, accounting for the performance in the presence of NiCl2·6H2O. Ni(NO3)2·6H2O and NiSO4·6H2O showed decreased activities probably because of the higher steric hindrance of nitrate and sulfate anions. Moreover, the reactions with different chromium salts further demonstrated the similar effect of the anions (Table S1†).
| Entry | Catalyst | Fructose conv. (%) | 5-HMF yield (%) | 5-HMF sel. (%) |
|---|---|---|---|---|
| a Reaction conditions: fructose 60 mg; P2O5 10 mol% to fructose; Ni fraction 10 mol% to fructose; DMSO 1 mL; 80 °C; 30 min. | ||||
| 1 | P2O5 | 37 | 21 | 57 |
| 2 | P2O5 + NiCl2·6H2O | 88 | 75 | 85 |
| 3 | P2O5 + Ni(NO3)2·6H2O | 65 | 46 | 71 |
| 4 | P2O5 + NiSO4·6H2O | 47 | 33 | 70 |
Highly concentrated conversion process is more desirable, since using dilute solutions for biomass refinery may limit the efficacy. Fig. S3† illustrated the effect of initial fructose loading on the reaction performance. When a less amount of fructose (40 mg) was added, slightly higher 5-HMF yield (77%) and selectivity (88%) was obtained than that with 60 mg of fructose, which was attributed to the stabilization of 5-HMF by sufficient solvation effect of DMSO.35 However, increasing fructose loading may result in more byproducts and humins. These compounds could be formed by 5-HMF self-condensation or coupling with other compounds,36,37 leading to decreased 5-HMF yield and selectivity.
To get an optimized 5-HMF yield, the conversion of fructose was examined as a function of time at different temperatures (Fig. 5). The reaction could proceed steadily at 70 °C, while a relatively long time up to 90 min was required to obtain a reasonable 5-HMF yield. Since the production of 5-HMF from fructose is mainly endothermic,38,39 it is expected that elevating temperatures lead to fast reaction rate and improved reaction performance. The conversion was almost completed within 30 min at 80 °C, and the time could be shortened to 15 min at 90 °C. A maximum 5-HMF yield of ∼80% was achievable under such mild conditions.
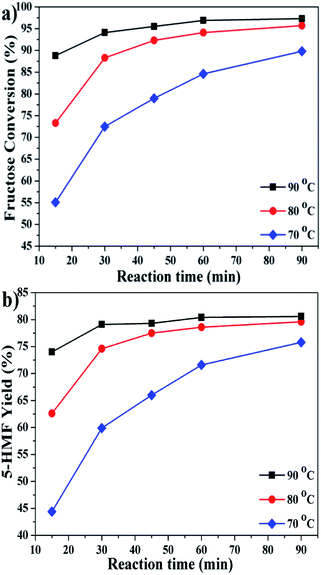 | ||
| Fig. 5 Effect of reaction temperature and time on the conversion of fructose (reaction conditions: fructose 60 mg; P2O5 10 mol% to fructose; NiCl2·6H2O 10 mol% to fructose; DMSO 1 mL). | ||
Preliminary scaling-up tests were also carried out. After 30 min at 80 °C, a 5-HMF yield of 74% with about 90% fructose conversion was achieved from 5-fold and 10-fold scaling-up tests, respectively, demonstrating the potential for a large scale production.
Inulin is a kind of fructan material, which can be obtained from natural biomass such as Jerusalem artichoke and chicory. Table S2† presented the conversion of inulin in the studied system. 5-HMF with a yield of ∼45% was facilely produced at 80 °C, and elevating reaction temperature can accelerate the formation of 5-HMF as discussed above.
Glucose is of high abundance in nature, and therefore has a great potential for 5-HMF synthesis.5–9 It is generally difficult to convert glucose into 5-HMF because the stability of six membered pyran ring of glucose limits its conversion.12 It is accepted that glucose should be first isomerized into fructose prior to the production of 5-HMF.12–14 In order to get a reasonable yield of 5-HMF, the conversion of glucose was investigated at 100 °C. However, as seen in Table S3,† the studied system did not work for the production of 5-HMF although the conversion could be improved with mixtures of P2O5 and metal chlorides. The tiny yield of 5-HMF indicated that metal chlorides such as CrCl3·6H2O and AlCl3·6H2O did not enable the isomerization of glucose into fructose under tested conditions.
As known, DMSO has a good capacity for dissolving 5-HMF, which may be an issue for the isolation of 5-HMF. Tong et al. gave a strategy for separating 5-HMF from DMSO system.40 DMSO can be first collected by distillation under reduced pressure. Then, 5-HMF can be isolated by extracting the residue with an appropriate low boiling organic solvent followed by the subsequent distillation of organic solvent.
Finally, a few control experiments were conducted to gain some preliminary mechanistic insights. P2O5 as an anhydride is expected to produce phosphoric acid (H3PO4) by reacting with water during the dehydration of fructose. To confirm whether H3PO4 worked for the conversion, water was used as an additive to promote the formation of H3PO4. As seen in Table 2, a small amount of water indeed suppressed the conversion of fructose (entries1, 3 and 4), indicating that H3PO4 should not be the actual catalyst. Then, H3PO4 was employed to instead of P2O5. The results further demonstrated that H3PO4 did not work as well as P2O5 (entries1, 2, 5 and 6). On the other hand, a phosphate could be formed if P2O5 was converted into H3PO4 followed by interacting with metal salts in the studied system. However, as shown in entries 7–10, the tested phosphates gave rather inferior performance to that with both P2O5 and NaCl. Although the mechanism remains unknown, the above investigations demonstrate a synergy effect between P2O5 and metal salts in the conversion of fructose, which needs further study to elaborate in the future.
| Entry | Catalyst | Fructose conv. (%) | 5-HMF yield (%) |
|---|---|---|---|
| a Reaction conditions: fructose 60 mg; P2O5 10 mol% to fructose; metal chloride 10 mol% to fructose; DMSO 1 mL; 80 °C; 30 min.b 4 μL of H2O (67 mol% to fructose) was added.c 8 μL of H2O was added.d Catalyst 20 mol% to fructose (equivalent to the mole of phosphorus in P2O5). | |||
| 1 | P2O5 | 37 | 21 |
| 2 | P2O5 + NiCl2·6H2O | 88 | 75 |
| 3b | P2O5 | 33 | 18 |
| 4c | P2O5 | 29 | 16 |
| 5d | H3PO4 | 6 | 2 |
| 6d | H3PO4 + NiCl2·6H2O | 9 | 3 |
| 7 | P2O5 + NaCl | 66 | 52 |
| 8d | NaH2PO4 | 4 | 0 |
| 9d | Na2HPO4 | 5 | 0 |
Conclusions
In conclusion, this work demonstrated that P2O5/metal chloride mixtures could significantly catalyze the efficient and facile conversion of fructose into 5-HMF under mild conditions. About 75% of 5-HMF yield and 85% of 5-HMF selectivity could be achieved within only 30 min at 80 °C. Inulin was also able to be efficiently converted into 5-HMF under such conditions with a yield of 45%. The studied system was pervasive to many metal salts, especially chlorides, leaving a broad room for catalyst design in the future.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank for the support from the Natural Science Foundation of Liaoning Province (China) (No. 2015020633), Department of Education of Liaoning Province (China) (No. L2015421), the National Natural Science Foundation of China (No. 21673229, 21721004, 21706255) and the CAS/SAFEA International Partnership Program for Creative Research Teams.References
- R. A. Sheldon, ACS Sustainable Chem. Eng., 2018, 6, 4464–4480 CrossRef.
- M. Besson, P. Gallezot and C. Pinel, Chem. Rev., 2014, 114, 1827–1870 CrossRef PubMed.
- P. Y. Dapsens, C. Mondelli and J. Pérez-Ramírez, ACS Catal., 2012, 2, 1487–1499 CrossRef.
- Z. Zhang and G. W. Huber, Chem. Soc. Rev., 2018, 47, 1351–1390 RSC.
- P. Zhou and Z. Zhang, Catal. Sci. Technol., 2016, 6, 3694–3712 RSC.
- B. R. Caes, R. E. Teixeira, K. G. Knapp and R. T. Raines, ACS Sustainable Chem. Eng., 2015, 3, 2591–2605 CrossRef.
- A. Chinnappan, C. Baskar and H. Kim, RSC Adv., 2016, 6, 63991–64002 RSC.
- L. Hu, L. Lin, Z. Wu, S. Zhou and S. Liu, Renewable Sustainable Energy Rev., 2017, 74, 230–257 CrossRef.
- T. Wang, M. W. Nolte and B. H. Shanks, Green Chem., 2014, 16, 548–572 RSC.
- S. Xu, P. Zhou, Z. Zhang, C. Yang, B. Zhang, K. Deng, S. Bottle and H. Zhu, J. Am. Chem. Soc., 2017, 139, 14775–14782 CrossRef PubMed.
- L. Gao, K. Deng, J. Zheng, B. Liu and Z. Zhang, Chem. Eng. J., 2015, 270, 444–449 CrossRef.
- H. Zhao, J. E. Holladay, H. Brown and Z. C. Zhang, Science, 2007, 316, 1597–1600 CrossRef PubMed.
- Y. Lu, H. Li, J. He, Y. Liu, Z. Wu, D. Hu and S. Yang, RSC Adv., 2016, 6, 12782–12787 RSC.
- H. Xin, T. Zhang, W. Li, M. Su, S. Li, Q. Shao and L. Ma, RSC Adv., 2017, 7, 41546–41551 RSC.
- P. Körner, D. Jung and A. Kruse, Green Chem., 2018, 20, 2231–2241 RSC.
- F. N. D. C. Gomes, F. M. T. Mendes and M. M. V. M. Souza, Catal. Today, 2017, 279, 296–304 CrossRef.
- X. Zhou, Z. Zhang, B. Liu, Q. Zhou, S. Wang and K. Deng, J. Ind. Eng. Chem., 2014, 20, 644–649 CrossRef.
- Z. Ma, H. Hu, Z. Sun, W. Fang, J. Zhang, L. Yang, Y. Zhang and L. Wang, ChemSusChem, 2017, 10, 1669–1674 CrossRef PubMed.
- X. Guo, J. Tang, B. Xiang, L. Zhu, H. Yang and C. Hu, ChemCatChem, 2017, 9, 3218–3225 CrossRef.
- G. Raveendra, M. Surendar and P. S. S. Prasad, New J. Chem., 2017, 41, 8520–8529 RSC.
- Y. Xiao and X. Huang, RSC Adv., 2018, 8, 18784–18791 RSC.
- A. Pande, P. Niphadkar, K. Pandare and V. Bokade, Energy Fuels, 2018, 32, 3783–3791 CrossRef.
- Z. Zhang, B. Liu and Z. K. Zhao, Carbohydr. Polym., 2012, 88, 891–895 CrossRef.
- J. Y. G. Chan and Y. Zhang, ChemSusChem, 2009, 2, 731–734 CrossRef PubMed.
- X. Qi, M. Watanabe, T. M. Aida and R. L. Smith, Jr., ChemSusChem, 2009, 2, 944–946 CrossRef PubMed.
- L. Lai and Y. Zhang, ChemSusChem, 2010, 3, 1257–1259 CrossRef PubMed.
- Y. Román-Leshkov, J. N. Chheda and J. A. Dumesic, Science, 2006, 312, 1933–1937 CrossRef PubMed.
- W. Li, T. Zhang, H. Xin, M. Su, L. Ma, H. Jameel, H. Chang and G. Pei, RSC Adv., 2017, 7, 27682–27688 RSC.
- C. Bispo, K. D. O. Vigier, M. Sardo, N. Bion, L. Mafra, P. Ferreira and F. Jérôme, Catal. Sci. Technol., 2014, 4, 2235–2240 RSC.
- M. J. Antal, Jr. and W. S. L. Mok, Carbohydr. Res., 1990, 199, 91–109 CrossRef.
- L. Peng, L. Lin, J. Zhang, J. Zhuang, B. Zhang and Y. Gong, Molecules, 2010, 15, 5258–5272 CrossRef PubMed.
- D. Ray, N. Mittal and W. Chung, Carbohydr. Res., 2011, 346, 2145–2148 CrossRef PubMed.
- Y. Yuan, S. Yao, S. Nie and S. Wang, BioResources, 2016, 11, 2381–2392 Search PubMed.
- J. B. Binder and R. T. Raines, J. Am. Chem. Soc., 2009, 131, 1979–1985 CrossRef PubMed.
- G. Tsilomelekis, T. R. Josephson, V. Nikolakis and S. Caratzoulas, ChemSusChem, 2014, 7, 117–126 CrossRef PubMed.
- Z. Cheng, J. L. Everhart, G. Tsilomelekis, V. Nikolakis, B. Saha and D. G. Vlachos, Green Chem., 2018, 20, 997–1006 RSC.
- G. Tsilomelekis, M. J. Orella, Z. Lin, Z. Cheng, W. Zheng, V. Nikolakis and D. G. Vlachos, Green Chem., 2016, 18, 1983–1993 RSC.
- C. Antonetti, D. Licursi, S. Fulignati, G. Valentini and A. M. R Galletti, Catalysts, 2016, 6, 196 CrossRef.
- R. S. Assary, P. C. Redfern, J. R. Hammond, J. Greeley and L. A. Curtiss, J. Phys. Chem. B, 2010, 114, 9002–9009 CrossRef PubMed.
- X. Tong and Y. Li, ChemSusChem, 2010, 3, 350–355 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra07027j |
| This journal is © The Royal Society of Chemistry 2018 |