Open Access Article
Open Access ArticleSilica modification of titania nanoparticles enhances photocatalytic production of reactive oxygen species without increasing toxicity potential in vitro
Simona Ortelli a,
Anna L. Costa
a,
Anna L. Costa *a,
Pietro Matteuccib,
Mark R. Millerc,
Magda Blosia,
Davide Gardinia,
Syed A. M. Tofaild,
Lang Trane,
Domenica Tonellib and
Craig A. Polandc
*a,
Pietro Matteuccib,
Mark R. Millerc,
Magda Blosia,
Davide Gardinia,
Syed A. M. Tofaild,
Lang Trane,
Domenica Tonellib and
Craig A. Polandc
aCNR-ISTEC, Institute of Science and Technology for Ceramics – National Research Council of Italy, Via Granarolo 64, I-48018 Faenza, RA, Italy. E-mail: anna.costa@istec.cnr.it
bDepartment of Industrial Chemistry, University of Bologna, Viale del Risorgimento 4, 40136 Bologna, Italy
cQueen's Medical Research Institute, University of Edinburgh, Edinburgh EH16 4TJ, UK
dDepartment of Physics, Bernal Institute, University of Limerick, V94 T9PX, Ireland
eInstitute of Occupational Medicine, Research Avenue North, Riccarton, Edinburgh EH14 4AP, UK
First published on 4th December 2018
Abstract
Titania (TiO2) nanoparticles were surface modified using silica and citrate to implement a ‘safe-by-design’ approach for managing potential toxicity of titania nanoparticles by controlling surface redox reactivity. DLS and zeta-potential analyses confirmed the surface modification, and electron microscopy and surface area measurements demonstrated nanoscale dimensions of the particles. Electron paramagnetic resonance (EPR) was used to determine the exogenous generation of reactive oxygen species (ROS). All the produced spray dried nanotitania lowered levels of ROS when compared to the corresponding dispersed nanotitania, suggesting that the spray drying process is an appropriate design strategy for the control of nano TiO2 ROS reactivity. The modification of nanotitania with silica and with citrate resulted in increased levels of ROS generation in exogenous measurements, including photoexcitation for 60 minutes. The dichlorodihydrofluorescein (DCFH) assay of dose-dependent production of oxidative stress, generated by pristine and modified nanotitania in macrophages and alveolar epithelial cells, found no significant change in toxicity originating from the generation of reactive oxygen species. Our findings show that there is no direct correlation between the photocatalytic activity of nanotitania and its oxidative stress-mediated potential toxicity, and it is possible to improve the former, for example adding silica as a modifying agent, without altering the cell redox equilibrium.
Introduction
In recent years, the presence of nanomaterials (NMs) in biological systems (bio-accessibility or bioavailability) has risen due to the increasing use of these NMs in a large number of advanced applications as a key to industrial innovation. There is also the potential for many of these NMs to produce detrimental effects on human health and the environment. This necessitates paying close attention to safety issues pertaining to NMs and their further development. Nanoparticles (NPs) can exert toxicity through oxidative stress, inflammation, genetic damage, inhibition of cell division and cell death.1–4 For example, photocatalytic NPs of semiconducting oxides, such as titanium dioxide (TiO2) and zinc oxide (ZnO), have found extensive use in applications prohibiting cellular growth and enabling self-sterilization.5 The challenge in the field of using photocatalytic particles remains in finding appropriate conditions, e.g., the amount of local surface charge that will initiate a desired, or inhibit an undesired biological action.6 The above holds true for metal oxide NPs which have widespread use in industry for numerous applications ranging from sunscreens, pigments and construction materials for solar cells.7–11Evidence is abundant within the literature that changes in structural and physicochemical properties of NPs can modify their biological activities, especially in relation to exogenous and endogenous generation of reactive oxygen species (ROS), one of the most frequently reported NP-associated toxicity mechanism.2,12 Relatively rare is the endeavour in using these changes as a means to design NMs that would manage the safety concern of these NPs.13 Reactive oxygen species are known to be able to cause oxidative damages to key structures and components of cells including DNA, proteins and lipids thus leading to significant functional changes of the cell as well as a disruption of cell signalling, induction of inflammatory pathways, apoptosis and cell death.14 Many studies have found that such oxidative stress is a prominent feature of the cellular response to TiO2 NPs,15 particularly when illuminated with ultraviolet (UV) light.16,17 A direct, quantitative, correlation between extracellular and cellular pro-oxidant responses is highly desired, but currently lacking,18 due to the sheer extent of the physicochemical properties of NM (e.g. size, shape, structure, and chemistry of the core and the coating/shell) that can impact biological effects such as toxicity within a complex biological matrix.
Despite the limited knowledge of such a correlation, surface modification of NPs has emerged as a potential method to implement a “Safe-by-Design” (SbD) approach to minimise unwanted biological activity such as NP toxicity. This approach can be a vital tool in the governance of nano-safety. Examples of modification strategies include silica coating of potentially toxic NPs due to the chemical inertia,19 biocompatibility20 and low toxicity21 of silica as well as its ability to create mesoporous structure22 and control ROS production.23 In addition, citrate coating has been used as an organic coating strategy, due to the hydrophilicity and negative surface charge of citrates, that leads to an increased ability of dispersing NPs in an aqueous medium, a strong specific interaction with the hydroxyl groups in NPs, and an electrostatic hindrance to the contact of NPs with negatively charged cellular membrane.24–26
The quantitative determination of the impact of such modifications of physicochemical properties by SbD approach on functional properties is important in forecasting potential biological responses using established in vitro toxicity models.27 The generation of ROS by engineered NPs can be determined either by exogenous, acellular factors or endogenous factors. Exogenous factors include intrinsic reactivity of NPs depending on structural characteristics as well as composition, activation by UV light, and/or a presence of metal impurities on the surface. On the other hand, endogenous factors include the ability to interfere with cellular redox machinery so inducing an excess of ROS production by target and inflammatory cells, processes in which mitochondrial respiration and activation of NAD(P)H-like enzyme systems are thought to be involved.28,29
In the present work, we use the exogenous approach to obtain a quantitative insight into the implementation of SbD approach in managing ROS production by titania NPs to induce cellular oxidative stress. The propensity of different modified TiO2 to produce ROS in abiotic condition is evaluated through electron paramagnetic resonance (EPR) analysis using Tempone-H as the spin trap molecule. EPR provides a sensitive and relatively high-throughput means to test a panel of modified TiO2 for photocatalytic ROS production. Findings from EPR were compared with that of the cellular dichlorodihydrofluorescein diacetate (DCFH-DA) assay for oxidative stress without any photoexcitation as it would be normal in an in vivo condition without any internal photoexcitation source. Both macrophages and epithelial cells were used for in vitro identification of the main predictors (colloidal/structural properties, spin-trapped ROS) which best describe the cellular response.
Results and discussion
Characterisation of colloidal nanosols
The optimal conditions for the self-assembled heterocoagulation process between different colloidal phases occur when such species exhibit, at the working pH, ζ potentials opposite in sign and high enough to preserve colloidal stabilization and avoid homocoagulation. These conditions are fulfilled at pHs lower than the isoelectric point of TiO2 (pH 7.1), where TiO2 and SiO2 present positive and negative ζ potentials, respectively (Table 1, Fig. 1). SiO2 colloidal stabilization was decreased at pH 4.0, promoting the silica coagulation over nanotitania surface. The increase of the hydrodynamic diameter with the progressive addition of SiO2 (Table 1) is caused both by the steric hindrance due to SiO2 particles heterocoagulated on the TiO2 surface40,41 and by the electrostatic destabilization induced by the neutralization of TiO2 positive-charged surface with the SiO2 negative charged particles. Since the ζ potential of the nanocolloidal systems is strictly linked to their agglomeration grade30 the relationship between particle size and the predicted surface charge is complex. However, a decreasing ζ potential was observed for higher amounts of SiO2, and the failure to achieve negative ζ potential for TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2_1
SiO2_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3_COL and TiO2
3_COL and TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2_1
SiO2_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5_COL samples suggested the formation of TiO2/SiO2 matrix encapsulation structures,42 despite a typical core–shell structure, which were confirmed by STEM (Fig. 2c). In fact, TiO2
5_COL samples suggested the formation of TiO2/SiO2 matrix encapsulation structures,42 despite a typical core–shell structure, which were confirmed by STEM (Fig. 2c). In fact, TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2_1
SiO2_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5_COL exhibited a random distribution of small TiO2 nanoparticles (∼5 nm, in the specific 4.82 ± 0.74 nm) within a silica matrix composed by particles of about 20 nm (18.98 ± 1.47 nm) of overall diameter (Fig. 2b). The pristine sample, TiO2_COL, (Fig. 2a) was confirmed to have a diameter of ∼5 nm for the primary particles, which appear clearly agglomerated, as a consequence of the sample preparation process for the microscope analysis.
5_COL exhibited a random distribution of small TiO2 nanoparticles (∼5 nm, in the specific 4.82 ± 0.74 nm) within a silica matrix composed by particles of about 20 nm (18.98 ± 1.47 nm) of overall diameter (Fig. 2b). The pristine sample, TiO2_COL, (Fig. 2a) was confirmed to have a diameter of ∼5 nm for the primary particles, which appear clearly agglomerated, as a consequence of the sample preparation process for the microscope analysis.
| Sample | pH | dDLS (nm) | ζ potential (mV) | pHi.e.p. |
|---|---|---|---|---|
| a nd: not determined. | ||||
| TiO2_COL | 2.8 | 53 ± 0.9 | +38 ± 1.8 | 7.1 |
TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2_1 SiO2_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3_COL 3_COL |
3.1 | 110 ± 0.9 | +31 ± 1.0 | 6.0 |
TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2_1 SiO2_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5_COL 5_COL |
3.4 | 577 ± 39 | +25 ± 0.1 | 5.4 |
TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CIT_1 CIT_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8_COL 0.8_COL |
6.0 | 64 ± 0.9 | −38 ± 1.9 | nd |
| SiO2_COL | 9.6 | 20 ± 0.3 | −42 ± 2.2 | <3 |
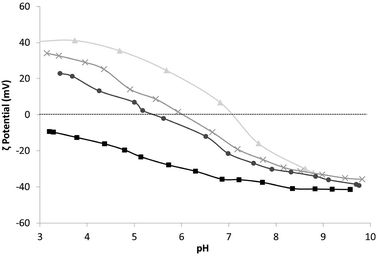 | ||
Fig. 1 ζ potential vs. pH of samples: ▲ TiO2_COL, × TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2_1 SiO2_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3_COL, ● TiO2 3_COL, ● TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2_1 SiO2_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5_COL and ■ SiO2_COL. 5_COL and ■ SiO2_COL. | ||
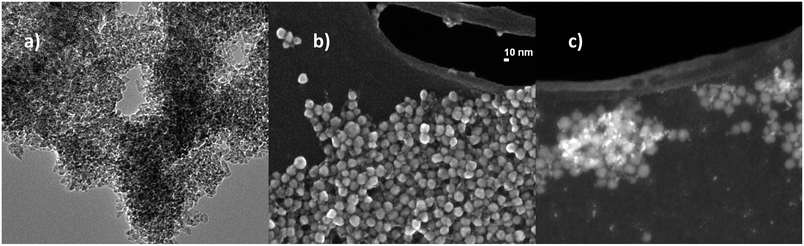 | ||
Fig. 2 Representative electron microscopy images of nanosol samples. (a) TEM image TiO2_COL particles, (b) STEM and (c) STEM-HAADF image of TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2_1 SiO2_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5_COL particles. 5_COL particles. | ||
The citrate added to the sample TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CIT_1
CIT_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8_COL acted as dispersant/capping agent, without any change in hydrodynamic diameter or colloidal stability over time, compared to the pristine titania. The negative ζ potential assessed for this sample (−38 mV) is consistent with the formation of a uniform negatively-charged citrate coating around TiO2 nanoparticles.
0.8_COL acted as dispersant/capping agent, without any change in hydrodynamic diameter or colloidal stability over time, compared to the pristine titania. The negative ζ potential assessed for this sample (−38 mV) is consistent with the formation of a uniform negatively-charged citrate coating around TiO2 nanoparticles.
Characterisation of spray-dried powders
SEM images of the particles prepared from the commercial titania nanosol (TiO2_COL) pointed out dimensions in the range of 1–15 micrometres, with irregular surface hollows, as typical of spray dried particles derived from suspensions containing salts43 (Fig. 5a). Despite the micrometric size, the assessed surface area is of 154 m2 g−1 (Table 2), suggesting that the surface of the micrometric particles is actually nanostructured due to the collapsed nanoparticles contained in the starting nanosol.| Sample | SABET (m2 g−1) | ζ potential (mV) |
|---|---|---|
| TiO2_SD | 154 | +43 ± 0.8 |
TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2_1 SiO2_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3_SD 3_SD |
156 | +22 ± 0.3 |
TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2_1 SiO2_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5_SD 5_SD |
175 | −15 ± 1.2 |
| SiO2_SD | 174 | −34 ± 0.9 |
The nanostructured surface, as demonstrated by a high BET value and SEM analysis (Fig. 3e), was clearly verified for all the spray dried samples. Some differences in the particle shapes were observed for increasing SiO2 contents. For example, the spray-dried sample SiO2_SD, obtained from the sol containing SiO2 only, was micrometric in size with high specific surface area (174 m2 g−1) (Table 2), but with a highly regular and spherical or ‘donut’ shape (Fig. 3d). The spray dried powders TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2_1
SiO2_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3_SD and TiO2
3_SD and TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2_1
SiO2_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5_SD displaying different SiO2
5_SD displaying different SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2 ratios (3 and 5
TiO2 ratios (3 and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively) exhibited a progressive regular morphology (Fig. 3b and c) with SiO2 increasing, with a size from 2–20 micrometres and high BET specific surface areas of 156 and 175 m2 g (Table 2).
1, respectively) exhibited a progressive regular morphology (Fig. 3b and c) with SiO2 increasing, with a size from 2–20 micrometres and high BET specific surface areas of 156 and 175 m2 g (Table 2).
 | ||
Fig. 3 SEM images of spray-dried powders from (a) TiO2_SD, (b) TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2_1 SiO2_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3_SD, (c) TiO2 3_SD, (c) TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2_1 SiO2_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5_SD, (d) SiO2_SD and (e) high magnification of the surface of TiO2 5_SD, (d) SiO2_SD and (e) high magnification of the surface of TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2_1 SiO2_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5_SD. 5_SD. | ||
ζ potential measurements performed after spray drying confirmed both for TiO2 SD and for TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2_1
SiO2_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3_SD positive values, as already assessed on the heterocoagulated suspension form (Table 2). However, the spray dried sample with a large excess of SiO2 (TiO2
3_SD positive values, as already assessed on the heterocoagulated suspension form (Table 2). However, the spray dried sample with a large excess of SiO2 (TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2_1
SiO2_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5_SD) had a negative ζ potential (Table 2), i.e., a charge inversion with respect to the corresponding sample before spray drying, TiO2
5_SD) had a negative ζ potential (Table 2), i.e., a charge inversion with respect to the corresponding sample before spray drying, TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2_1
SiO2_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5_COL. This behaviour is consistent with the presence of a more uniform and shielding SiO2 coating formed on the TiO2 surface during heat drying step.
5_COL. This behaviour is consistent with the presence of a more uniform and shielding SiO2 coating formed on the TiO2 surface during heat drying step.
ROS detection from acellular system by EPR measurements
EPR experiments with different concentrations and UV irradiation times showed a linear correlation between EPR intensity and UV irradiation time. Due to the linearity, using an irradiation time of 60 minutes the optimal EPR signal, was provided and used to compare the different NPs. The SiO2 samples (SiO2_COL and SiO2_SD) did not generate any ROS species under UV irradiation.In colloidal samples, after normalization in TiO2 content, the silica doped surfaces led to greater values of ROS even though SiO2 alone is not photoactive during UV irradiation (Fig. 4). Similarly, a slight ROS production increase if compared to pristine TiO2 (TiO2_COL), was observed in citrate-modified sample (TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CIT_1
CIT_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8_COL). Moreover, among all the samples, the colloidal sols had a greater reactivity and were more photoactive than the spray-dried powders (Fig. 4). The last observation is not surprising, if considering the decrease of free, available, surface reactive sites when passing from the dispersed nano particles to the agglomerated structures of granulated spray-dried micro particles.
0.8_COL). Moreover, among all the samples, the colloidal sols had a greater reactivity and were more photoactive than the spray-dried powders (Fig. 4). The last observation is not surprising, if considering the decrease of free, available, surface reactive sites when passing from the dispersed nano particles to the agglomerated structures of granulated spray-dried micro particles.
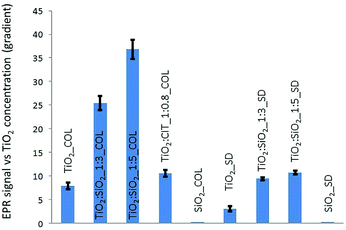 | ||
| Fig. 4 ROS production estimated by the line slope of EPR signal intensity as a function of TiO2 concentration. | ||
In assessing the impact of UV irradiation of coated and uncoated TiO2, analysing the data in Fig. 4, the silica coating appears to cause an enhancement of ROS production, despite silica is not able to produce oxygen radicals on its own. This behaviour could be ascribed to the potential ability of silica to prevent the radical recombination through the formation of hydrogen peroxide molecules, as elsewhere reported.44 Photo-absorption in TiO2 produces, in fact, conduction band electrons (e−) and valence band holes (h+) (eqn (1)). The quantity of H2O2 produced by the chain reactions comes from both the reduction of the oxygen molecules by the conduction band electrons (eqn (2) and (3)) and by water oxidation by the valence band holes (eqn (4) and (5)).
| TiO2 + h+ → TiO2 (e− + h+) | (1) |
| e− + O2 → ·O2− | (2) |
| ·O2− + e− + 2H+ → H2O2 | (3) |
| H2O2 + e− → ·OH + OH− | (3′) |
| h+ + H2O → ·OH + H+ | (4) |
| ·OH + h+ + H2O → H2O2 + H+ | (5) |
The authors hypothesized that silica coating decreased the H2O2 formation, possibly through inhibition of the reactions (3) and (5), thus increasing the availability of intermediate radicals, O2˙− and ·OH, as detected by EPR in the present study.
Cellular oxidative stress
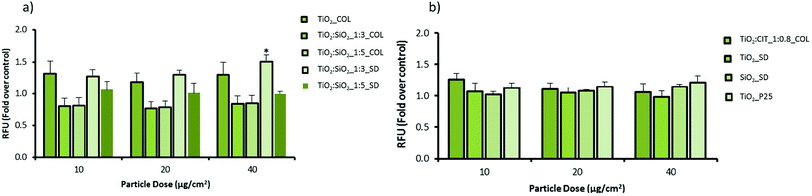
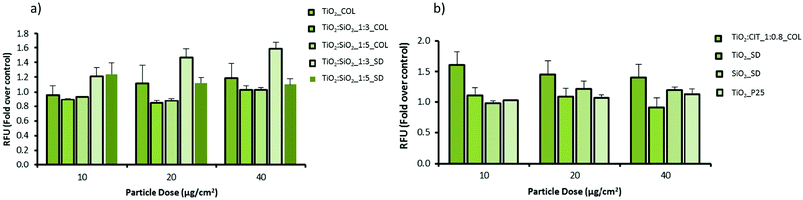
The potential of pristine and modified NPs to induce oxidative stress in cells was assessed using the DCFH-DA assay in both macrophage (RAW 264.7) and epithelial cell (A549) lines (Fig. 5 and 6). In macrophages the pristine material (TiO2_COL) dose dependently increased DCFH oxidation. However, due to the variations in the amplitude of the response the results were not statistically significant from control. In contrast, TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2_1
SiO2_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3_SD dose dependently and significantly induced oxidative stress in macrophages, and the effect was statistically significant at the highest dose tested (40 μg cm−2). None of the other tested NPs induced significant levels of DCFH oxidation in macrophages. The benchmark material P25 TiO2 slightly increased the level of oxidative stress in macrophages (up to 1.2-fold), however, the results were not statistically significant compared to the control (Fig. 5).
3_SD dose dependently and significantly induced oxidative stress in macrophages, and the effect was statistically significant at the highest dose tested (40 μg cm−2). None of the other tested NPs induced significant levels of DCFH oxidation in macrophages. The benchmark material P25 TiO2 slightly increased the level of oxidative stress in macrophages (up to 1.2-fold), however, the results were not statistically significant compared to the control (Fig. 5).
In alveolar epithelial cells (Fig. 6), none of the tested compounds dose-dependently changed the degree of DCFH oxidation in a significant way. Importantly, TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2_1
SiO2_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3_SD increased the levels of ROS in alveolar epithelial cells, as seen in macrophages, but the results did not reach statistical significance (at 40 μg cm−2).
3_SD increased the levels of ROS in alveolar epithelial cells, as seen in macrophages, but the results did not reach statistical significance (at 40 μg cm−2).
In colloidal nanosols, the presence of silica gave indications of oxidative stress, albeit modestly. In contrast, for spray-dried samples, the presence of silica slightly increased the oxidative stress, with the highest activity again shown by TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2_1
SiO2_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3_SD sample. However, a dose dependent trend in oxidative stress in response to silica content or in the presence of citrate as surface modifier was not observed (Fig. 5 and 6).
3_SD sample. However, a dose dependent trend in oxidative stress in response to silica content or in the presence of citrate as surface modifier was not observed (Fig. 5 and 6).
The effects of P25 TiO2 on oxidative stress, as assessed by the DCFH assay, have been reported in the literature. However, contradictory findings are frequent, demonstrating the difficulty to determine specific reactivity inherent to ROS and oxidative stress in nano-sized samples, especially due to the lack of consistency between experimental conditions and time points. For example, Kroll et al. showed that P25 TiO2 induced oxidative stress to some extent in four different cell lines after 1 h incubation, but they did not find evidence of any effect either in macrophages or in alveolar epithelial cells.45
In macrophages, Kang et al. showed that a 4 hour incubation of 0.5 to 100 μg mL−1 P25 TiO2 induced oxidative stress, yet by 24 hours, oxidative stress was evident only for 5 and 25 μg mL−1 P25 TiO2. The authors also used fluorescent microscopy with another probe, dihydroethidium, demonstrating oxidative stress in macrophages treated with P25 TiO2 for 30 minutes.46 Contrary to the findings of Kroll et al.45 using alveolar epithelial cells, signs of oxidative stress have been reported47 following incubation for 1 h with P25 TiO2 1.5 μg cm−2. Other studies using alveolar epithelial cells have shown induction of oxidative stress following incubation with P25 TiO2 at 2 h and 24 h, however the doses used were not directly comparable.48 Here, amorphous silica on its own did not induce any form of oxidative stress in alveolar epithelial cells and macrophages. In macrophages, Yang et al. showed that 20 nm silica nanoparticles induced oxidative stress at concentrations higher than those tested in the present study.49 Sohaebuddin et al.50 showed that 30 nm SiO2 nanoparticles induced oxidative stress in macrophages after 2 h incubation at 100 μg mL−1. Others found out that SiO2 nanoparticles dose dependently induced oxidative stress after longer periods of incubation (48–72 h) in alveolar epithelial cells.51,52 In some cases, these results were gathered from longer incubation time using particles concentrations higher than 40 μg cm−2, the highest dose of the present study.
Experimental
Materials
The following commercial products were used for the preparation of TiO2/SiO2 samples: TiO2 colloidal nano-suspension (“TiO2_COL”) containing 6 wt% TiO2, (anatase 84%, brookite 16%)30 was provided by Colorobbia, Italy and SiO2 colloidal nano-suspension (“SiO2_COL”) Ludox HS-40® containing 40 wt% SiO2 by Grace Davison, USA. The nanopowder AEROXIDE® TiO2 P25 (anatase 83% rutile 17%)31 was used as reference material. Tempone-H hydrochloride (1-hydroxyl-2,2,6,6-tetramethyl-4-oxo-piperidine·HCl) was purchased from Enzo Life Sciences (Exeter, UK).Preparation of modified TiO2 samples
The commercial colloidal nano-suspensions (nanosols) were diluted with deionised water (TiO2 and SiO2 nanosols to 3 wt%) and, in the case of SiO2 nanosol, acidified by cationic exchange on resin at pH 4. Titania and silica nanosols were then mixed in defined ratios (TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2 weight ratios 1
SiO2 weight ratios 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) and ball-milled for 24 hours with 5 mm diameter zirconia spheres as milling media in order to form silica modified “TiO2
5) and ball-milled for 24 hours with 5 mm diameter zirconia spheres as milling media in order to form silica modified “TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2_1
SiO2_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3_COL” and “TiO2
3_COL” and “TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2_1
SiO2_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
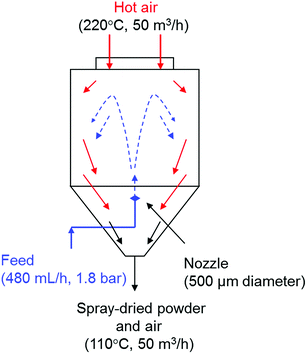
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5_COL” samples through a self-assembled heterocoagulation process. In order to increase the adhesion of silica NPs to the surfaces of TiO2 NPs, the heterocoagulated nanosols were spray-dried in counterflow with a stream of hot air (220 °C) through a nozzle of 500 μm of diameter (Fig. 7) to obtain spray-dried powder with TiO2
5_COL” samples through a self-assembled heterocoagulation process. In order to increase the adhesion of silica NPs to the surfaces of TiO2 NPs, the heterocoagulated nanosols were spray-dried in counterflow with a stream of hot air (220 °C) through a nozzle of 500 μm of diameter (Fig. 7) to obtain spray-dried powder with TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2 weight ratios 1
SiO2 weight ratios 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (“TiO2
5 (“TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2_1
SiO2_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3_SD” and “TiO2
3_SD” and “TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2_1
SiO2_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5_SD”, respectively). Pristine nanosols: TiO2 and SiO2 were also spray-dried, (“TiO2_SD” and “SiO2_SD”).
5_SD”, respectively). Pristine nanosols: TiO2 and SiO2 were also spray-dried, (“TiO2_SD” and “SiO2_SD”).
Organic modification was achieved through citrate coating. The sample was prepared adding trisodium citrate dihydrate (Cit) to TiO2_COL with TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cit weight ratio 1
Cit weight ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8, with a TiO2 concentration of 3 wt% via self-assembled monolayer formation32 obtaining “TiO2
0.8, with a TiO2 concentration of 3 wt% via self-assembled monolayer formation32 obtaining “TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CIT_1
CIT_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8_COL” sample. The mechanical stirring process was continued for 15 h to promote the re-dispersion of TiO2 NPs.
0.8_COL” sample. The mechanical stirring process was continued for 15 h to promote the re-dispersion of TiO2 NPs.
Characterisation of colloidal nanosols
Hydrodynamic diameter and ζ potential of tested NPs (1 g L−1 in deionized water) were evaluated with a Zetasizer Nano ZSP (model ZEN5600, Malvern Instruments, UK). Hydrodynamic diameters obtained from dynamic light scattering (DLS) data (dDLS) were derived from a measurement angle of 173° and automatic measurement duration. After 2 min temperature equilibration step (25 °C), 1 mL of sample volume was subjected to three consecutive measurements and particle size distribution by intensity was obtained by averaging these measurements. ζ potential measurements were performed on 700 μL sample at 25 °C by Electrophoretic Light Scattering (ELS) technique. Smoluchowski equation was applied to convert the electrophoretic mobility to ζ potential. After a 2 min temperature equilibration step, the samples underwent three measurements and ζ potential value was obtained by averaging these measurements. ζ potential of nanosols as a function of pH, was derived from Zetasizer Nano ZSP equipped with an automatic titrating system, by addition of 0.1 M KOH solution to TiO2 based nanosols and 0.1 M HCl to SiO2 nanosol (experimental uncertainty: 1 mV for ζ potential and 0.2 for pH). The ζ potential vs. pH titrations allowed identification of the isoelectric point (i.e.p.); the pH at which ζ potential sets to zero (pH i.e.p.). Morphological analysis was carried out by transmission electron microscopy (TEM) using a JEOL JEM-2100F multipurpose, high resolution, electron microscope with a field emission source operating between 80–200 kV. The nanoparticles were taken directly from the sols, placed on holey carbon grids for TEM analysis and air-dried at room temperature in a sealed environment for 2 hours.Characterisation of spray-dried powders
The powder morphology was analysed using a scanning electron microscope (Leica, Cambridge Stereoscan 360, UK). Samples were prepared by placing powders on graphite double-sided adhesive into the aluminium stub. Surface area of spray-dried powders was measured by nitrogen adsorption (BET) (Sorpty 1750, Carlo Erba).EPR analysis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, in order to avoid a destabilization of NP dispersion due to the electrostatic double layer compression. All the suspensions were dispersed by sonication bath (US 70; Philip Harris Scientific, Lichfield, UK) for 15 min, at room temperature. The following suspensions at concentrations of: 200, 300, 500, 1000, 2000, 3000 and 5000 μg mL−1, starting from a stock suspension of 10 mg L−1, were tested in order to obtain an analytical signal significantly different from the blank one. The analysed samples are reported in Table 3.
100, in order to avoid a destabilization of NP dispersion due to the electrostatic double layer compression. All the suspensions were dispersed by sonication bath (US 70; Philip Harris Scientific, Lichfield, UK) for 15 min, at room temperature. The following suspensions at concentrations of: 200, 300, 500, 1000, 2000, 3000 and 5000 μg mL−1, starting from a stock suspension of 10 mg L−1, were tested in order to obtain an analytical signal significantly different from the blank one. The analysed samples are reported in Table 3.
| Sample name | Physical form | Chemical composition | pH |
|---|---|---|---|
| a nd: not determined. | |||
| TiO2_SD | Spray-dried | TiO2 | nd |
| SiO2_SD | Spray-dried | SiO2 | nd |
TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2_1 SiO2_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5_SD 5_SD |
Spray-dried | TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2 = 1 SiO2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
nd |
TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2_1 SiO2_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3_SD 3_SD |
Spray-dried | TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2 = 1 SiO2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
nd |
| TiO2_COL | Colloidal | TiO2 | 1.6 |
| SiO2_COL | Colloidal | SiO2 | 4.1 |
TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2_1 SiO2_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5_COL 5_COL |
Colloidal | TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2 = 1 SiO2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
2.0 |
TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2_1 SiO2_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3_COL 3_COL |
Colloidal | TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2 = 1 SiO2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
1.9 |
TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CIT_1 CIT_1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8_COL 0.8_COL |
Colloidal | TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) citrate = 1 citrate = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 0.8 |
4.9 |
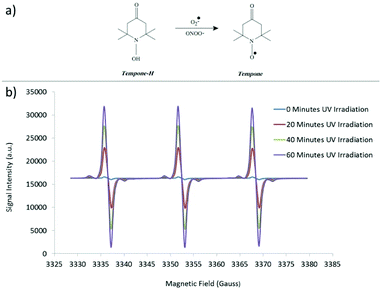 | ||
| Fig. 8 (a) Use of Tempone-H as a spin trap to detect superoxide free radicals and (b) representative EPR spectrum. | ||
Pyrogallol (320 μM in physiological saline solution) was used as positive control to assess the generation of superoxide radicals during the time analysis.37 Samples were kept at 37 °C (in order to simulate body temperature) and the measurements taken by withdrawing a 50 μL sample into a capillary tube (Scientific Laboratory Ltd., Coatbridge, UK) plugged with Cristaseal (VWR International, Lutterwoth, UK). The intensity of peaks of the characteristic 3 peaked EPR spectra (Fig. 8b) was taken at 0, 20, 40 and 60 minutes of irradiation through a mercury UV lamp (λmax = 254 nm). The blank value (PSS and spin-trap alone) was subtracted. For the colloidal nanosol samples, the value of blank in presence of HCl 0.01 M was subtracted in order to simulate the pH of TiO2 suspensions. All the samples were analysed three times and the values were obtained by averaging these measurements. In order to make a comparison of the reactivity of the different samples, the EPR signal intensity was plotted as a function of nanoparticles concentration at 60 minutes UV irradiation time, normalizing all the data to the TiO2 content, and reporting the slope of the relative curves.
The typical EPR parameters used were as follows: microwave frequency 9.3–9.55 Hz, microwave power 20 MW, modulation frequency 100 kHz, modulation amplitude 1500 Mg, center field 3365 G, sweep width 50 G, sweep time 30 s and number of passes 133.
Cellular oxidative stress
The ability of the samples to induce ROS production in an acellular system was tested using the DCFH assay in both murine RAW 264.7 macrophages and human A549 epithelial cells (ATCC, LGC Standards, UK). The cells were sub-cultured in RPMI 1640 Medium (Lonza, UK), supplemented with 10% heat inactivated foetal bovine serum (Gibco, UK), penicillin/streptomycin (100 U mL−1; 100 μg mL−1) and 2 mM L-glutamine (Sigma, Poole, UK).Contrary to the gathered EPR data, the DCFH assay is performed in cell culture medium which contains some anti-oxidants. The membrane permeable dichlorodihydrofluorescein diacetate (DCFH-DA) probe was used to measure the ability of materials to induce oxidative stress in cells. After internalization, intracellular esterases cleave the diacetate moiety, thus causing probe retention and making it sensitive to ROS. DCFH was determined fluorometrically in cell lysates according to a previously described procedure,38,39 with minor modifications. Cells were seeded in 96-well plates and treated for 24 h with RPMI cell culture medium with or without the tested materials at concentrations of 10, 20 and 40 μg cm−2. These sub-lethal concentrations were selected based on dose–response (2.5–80 μg cm−2) cytotoxicity analysis using the AlamarBlue® (Invitrogen, UK) and lactate dehydrogenase (Roche Diagnostics Ltd., Burgess Hill, UK) assays (data not shown). After being washed twice in sodium chloride (0.9%), cells were incubated for 1 h at room temperature in a solution of DCFH-DA (10 μM in sodium chloride) to allow internalization of the probe into the cell cytoplasm. Cells were then washed with sodium chloride and lysed in 90% DMSO in PBS. Plates were centrifuged at 300g for 15 min to remove cellular debris and particulates. The fluorescence was measured in the supernatants (λex 485 nm; λem 530 nm) using a plate reader (Fluostar Optima, BMG Labtech, Aylesbury, UK). Results were expressed as change in relative fluorescence units (RFU) compared to untreated control. Using the same procedure, cells were prepared without the probe to check the material interference.
Conclusions
The influence of different TiO2 surface coatings and agglomeration state on ROS production was investigated using an acellular (EPR) and cellular assay (DCFH assay). The data of EPR analysis showed that silica coating increased the amount of ROS generated, proportionally to the amount of silica added. This trend was verified both for liquid samples in form of colloidal suspensions and for powdered samples achieved after spray drying processing. These findings likely derive from the ability of the silica layer to hinder radicals' recombination with the development of H2O2, so causing the accumulation of by-products O2˙− and ·OH. The spray drying process provided an important action in terms of ROS mitigation, in fact the spray dried samples showed lower levels of ROS production as compared to the colloidal suspensions which was observed both for TiO2 and for TiO2–SiO2 samples. Despite the antioxidant property of citrate, the citrate coating only caused a slight improvement in ROS production compared to uncoated TiO2, although the increase was minor compared to the silica effect.In vitro assessment of oxidative stress in alveolar epithelial cells and macrophages showed a decrease of ROS in the silica-added samples, but in contrast a ROS increasing detected on all the spray dried samples. The trends found in acellular tests are, however, different from those in DCFH-DA cellular assay, due to the absence of any photo-excitation in the cellular assay. Otherwise in cells exposed to silica modified NPs, it was detected a slight decrease of oxidative stress even if no significant relationships between surface modifiers and the DCFH response could be detected. More generally, the modified forms did not attenuate the modest oxidative stress elicited by the pristine material in vitro cellular assay. This is significant from a SbD approach that shows that modifying pristine titania by silica can actually enhance photocatalytic activity without any significant deterioration of its biological impact.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research received support from the FP7 EU SANOWORK (Safe Nano Worker Exposure Scenarios) Collaborative Project (NMP4-SL-2012-280716).Notes and references
- Y. Ju-Nam and J. R. Lead, Sci. Total Environ., 2008, 400, 396–414 CrossRef CAS PubMed.
- N. Li, T. Xia and A. E. Nel, Free Radical Biol. Med., 2008, 44, 1689–1699 CrossRef CAS PubMed.
- V. Stone, H. Johnston and M. J. D. Clift, IEEE Trans. Nanobioscience, 2007, 6, 331–340 Search PubMed.
- H. J. Johnston, G. Hutchison, F. M. Christensen, S. Peters, S. Hankin and V. Stone, Crit. Rev. Toxicol., 2010, 40, 328–346 CrossRef CAS PubMed.
- T. Syed, Electrically Active Materials for Medical Devices, Imperial College Press, New Jersey, 1 edn, 2016 Search PubMed.
- S. A. M. Tofail and J. Bauer, Adv. Mater., 2016, 28, 5470–5484 CrossRef CAS PubMed.
- R. Kaegi, A. Ulrich, B. Sinnet, R. Vonbank, A. Wichser, S. Zuleeg, H. Simmler, S. Brunner, H. Vonmont, M. Burkhardt and M. Boller, Environ. Pollut., 2008, 156, 233–239 CrossRef CAS PubMed.
- J. Lee, S. Mahendra and P. J. J. Alvarez, ACS Nano, 2010, 4, 3580–3590 CrossRef CAS PubMed.
- X. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891–2959 CrossRef CAS PubMed.
- G. Li, S. Ciston, Z. Saponjic, L. Chen, N. Dimitrijevic, T. Rajh and K. Gray, J. Catal., 2008, 253, 105–110 CrossRef CAS.
- Z. Barbieriková, M. Mihalíková and V. Brezová, Photochem. Photobiol., 2012, 88, 1442–1454 CrossRef PubMed.
- A. Nel, Science, 2006, 311, 622–627 CrossRef CAS PubMed.
- R. Hjorth, L. van Hove and F. Wickson, Nanotoxicology, 2017, 11, 305–312 CrossRef.
- M. Valko, D. Leibfritz, J. Moncol, M. T. D. Cronin, M. Mazur and J. Telser, Int. J. Biochem. Cell Biol., 2007, 39, 44–84 CrossRef CAS PubMed.
- L. Reijnders, Polym. Degrad. Stab., 2009, 94, 873–876 CrossRef CAS.
- S. George, H. Gardner, E. K. Seng, H. Chang, C. Wang, C. H. Yu Fang, M. Richards, S. Valiyaveettil and W. K. Chan, Environ. Sci. Technol., 2014, 48, 6374–6382 CrossRef CAS PubMed.
- G. Janer, E. Mas del Molino, E. Fernández-Rosas, A. Fernández and S. Vázquez-Campos, Toxicol. Lett., 2014, 228, 103–110 CrossRef CAS.
- N. von Moos, V. B. Koman, C. Santschi, O. J. F. Martin, L. Maurizi, A. Jayaprakash, P. Bowen and V. I. Slaveykova, RSC Adv., 2016, 6, 115271–115283 RSC.
- P. Verlooy, A. Aerts, O. I. Lebedev, G. Van Tendeloo, C. Kirschhock and J. A. Martens, Chem. Commun., 2009, 4287 RSC.
- S. P. Hudson, R. F. Padera, R. Langer and D. S. Kohane, Biomaterials, 2008, 29, 4045–4055 CrossRef CAS PubMed.
- J.-H. Park, L. Gu, G. von Maltzahn, E. Ruoslahti, S. N. Bhatia and M. J. Sailor, Nat. Mater., 2009, 8, 331–336 CrossRef CAS.
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710–712 CrossRef CAS.
- S. Ortelli, C. A. Poland, G. Baldi and A. L. Costa, Environ. Sci.: Nano, 2016, 3, 602–610 RSC.
- A. A. Torrano, Â. S. Pereira, O. N. Oliveira and A. Barros-Timmons, Colloids Surf., B, 2013, 108, 120–126 CrossRef CAS PubMed.
- J. I. Kwak, W.-M. Lee, S. W. Kim and Y.-J. An, J. Appl. Toxicol., 2014, 34, 1145–1154 CrossRef CAS.
- V. Mangini, M. Dell'Aglio, A. D. Stradis, A. D. Giacomo, O. D. Pascale, G. Natile and F. Arnesano, Chem.–Eur. J., 2014, 20, 10745–10751 CrossRef CAS PubMed.
- Environmental Health Perspectives – Efficacy of Simple Short-Term In Vitro Assays for Predicting the Potential of Metal Oxide Nanoparticles to Cause Pulmonary Inflammation, https://ehp.niehs.nih.gov/11811/, accessed October 3, 2017.
- A. M. Knaapen, P. J. A. Borm, C. Albrecht and R. P. F. Schins, Int. J. Cancer, 2004, 109, 799–809 CrossRef CAS PubMed.
- A. A. Shvedova, A. Pietroiusti, B. Fadeel and V. E. Kagan, Toxicol. Appl. Pharmacol., 2012, 261, 121–133 CrossRef CAS PubMed.
- A. L. Costa, S. Ortelli, M. Blosi, S. Albonetti, A. Vaccari and M. Dondi, Chem. Eng. J., 2013, 225, 880–886 CrossRef CAS.
- B. M. Rotoli, O. Bussolati, A. L. Costa, M. Blosi, L. Di Cristo, P. P. Zanello, M. G. Bianchi, R. Visigalli and E. Bergamaschi, J. Nanopart. Res., 2012, 14, 1069 CrossRef.
- D. K. Schwartz, Annu. Rev. Phys. Chem., 2001, 52, 107–137 CrossRef CAS PubMed.
- M. R. Miller, S. J. Borthwick, C. A. Shaw, S. G. McLean, D. McClure, N. L. Mills, R. Duffin, K. Donaldson, I. L. Megson, P. W. F. Hadoke and D. E. Newby, Environ. Health Perspect., 2009, 117, 611–616 CrossRef CAS PubMed.
- G. Bartosz, Clin. Chim. Acta, 2006, 368, 53–76 CrossRef CAS PubMed.
- V. Brezová, S. Gabčová, D. Dvoranová and A. Staško, J. Photochem. Photobiol., B, 2005, 79, 121–134 CrossRef PubMed.
- S. Dikalov, M. Skatchkov and E. Bassenge, Biochem. Biophys. Res. Commun., 1997, 230, 54–57 CrossRef CAS PubMed.
- S. Marklund and G. Marklund, Eur. J. Biochem., 1974, 47, 469–474 CrossRef CAS PubMed.
- M. T. Siu, A. M. Shapiro, M. J. Wiley and P. G. Wells, Toxicol. Appl. Pharmacol., 2013, 273, 508–515 CrossRef CAS PubMed.
- Y. Li, X. Liu, T. Zhou, M. R. Kelley, P. Edwards, H. Gao and X. Qiao, Redox Biol., 2014, 2, 485–494 CrossRef CAS PubMed.
- Q. Wu, Z. Wang, X. Kong, X. Gu and G. Xue, Langmuir, 2008, 24, 7778–7784 CrossRef CAS PubMed.
- J. Sun and L. Gao, Carbon, 2003, 41, 1063–1068 CrossRef CAS.
- S. Ortelli and A. L. Costa, Nano-Struct. Nano-Objects, 2018, 13, 155–162 CrossRef CAS.
- G. L. Messing, S.-C. Zhang and G. V. Jayanthi, J. Am. Ceram. Soc., 1993, 76, 2707–2726 CrossRef CAS.
- J. Oguma, Y. Kakuma, S. Murayama and Y. Nosaka, Appl. Catal., B, 2013, 129, 282–286 CrossRef CAS.
- A. Kroll, C. Dierker, C. Rommel, D. Hahn, W. Wohlleben, C. Schulze-Isfort, C. Göbbert, M. Voetz, F. Hardinghaus and J. Schnekenburger, Part. Fibre Toxicol., 2011, 8, 9 CrossRef CAS PubMed.
- J. L. Kang, C. Moon, H. S. Lee, H. W. Lee, E.-M. Park, H. S. Kim and V. Castranova, J. Toxicol. Environ. Health, Part A, 2008, 71, 478–485 CrossRef CAS PubMed.
- T. A. J. Kuhlbusch, A. C. John and U. Quass, Biomarkers, 2009, 14, 23–28 Search PubMed.
- B. Ekstrand-Hammarström, C. M. Akfur, P. O. Andersson, C. Lejon, L. Österlund and A. Bucht, Nanotoxicology, 2012, 6, 623–634 CrossRef PubMed.
- H. Yang, Q. Wu, M. Tang, L. Kong and Z. Lu, J. Biomed. Nanotechnol., 2009, 5, 528–535 CrossRef CAS PubMed.
- S. K. Sohaebuddin, P. T. Thevenot, D. Baker, J. W. Eaton and L. Tang, Part. Fibre Toxicol., 2010, 7, 22 CrossRef PubMed.
- W. Lin, Y. Huang, X.-D. Zhou and Y. Ma, Toxicol. Appl. Pharmacol., 2006, 217, 252–259 CrossRef CAS PubMed.
- M. J. Akhtar, M. Ahamed, S. Kumar, H. Siddiqui, G. Patil, M. Ashquin and I. Ahmad, Toxicology, 2010, 276, 95–102 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |