Open Access Article
Open Access ArticleWater recycling efficacies of extremely hygroscopic, antifouling hydrogels†
Anayet Kabira,
Matthew J. Dunlopab,
Bishnu Acharyab,
Rabin Bissessura and
Marya Ahmed *ab
*ab
aDepartment of Chemistry, University of Prince Edward Island, Charlottetown, PEI C1A 4P3, Canada. E-mail: marahmed@upei.ca
bFaculty of Sustainable Design & Engineering, University of Prince Edward Island, Charlottetown, PEI C1A 4P3, Canada
First published on 13th November 2018
Abstract
Water harvesting, reusable, and antifouling hydrogels have found various applications in the fields of nanotechnology, biomedicine, food production and agriculture. These water-releasing materials are generally comprised of hygroscopic natural polymers, such as alginate blended with ionic salts or thermo-responsive moieties, to aid the release of water from a network of hydrogels. In this report, we propose a simple strategy to develop novel, synthetic, hygroscopic hydrogels (in the absence of ionic salts or thermo-responsive moieties), capable of absorbing copious amount of water and allow the facile release of water at ambient temperatures, as a function of crosslinking density of the polymer chains. The first step in the development of hygroscopic hydrogels is the development of hygroscopic vitamin B5 analogous or pantothenic acid analogous monomer (B5AMA), by ring opening chemistry. The hygroscopic hydrogels are then prepared from B5AMA monomer at different cross-linker densities by free radical polymerization approach and are evaluated for their antifouling properties and for their water absorbing and release efficacies, as a function of temperature. The release of significant amount of water by B5AMA hydrogels at physiological temperature (37 °C), their repeated water absorption and desorption behavior and excellent antifouling properties, suggest their potential usage as water harvesting materials in arid regions.
Introduction
The development of novel materials with superior water absorption and storage capabilities, in well-defined three-dimensional architectures, is a topic of great interest in science, nanotechnology, biomedicine, agriculture, and the food industry.1–9 Superabsorbent hydrogels are an example of highly permeable, and biocompatible, three-dimensional network of polymeric materials, capable of holding large amount of water in comparison to their own molecular weight.2–7 The water releasing hydrogels are a specialized class of superabsorbent hydrogels, which exhibit superior water absorbing and release efficacies, in the presence of external stimulus, such as the temperature.4–7 These water releasing hydrogels, capable of harvesting clean water from rivers, mist, dew, fog and dry air, are generally comprised of hygroscopic natural polysaccharides in the presence of organic salts or are cross-linked in the presence of stimuli responsive polymer chains to aid the release of water at high temperatures.1–8 For example, Miyata and coworkers have recently reported that interpenetrating network of hydrogels of moisture absorbing alginate and thermoresponsive poly N-isopropylacrylamide (PNIPAM) exhibit excellent moisture capturing efficacies and water release due to the collapse of polymeric chains above the lower critical solution temperature (LCST) of PNIPAM.7 Kallenberger et al. also utilized hygroscopic salts in alginate matrix to harvest moisture from air and release the water upon drying.6 Ohno and coworkers, reported that reasonable hydrophobicity of organic salts can be optimized to develop synthetic hydrogels with reversible water absorption/desorption efficacies as a function of temperature.5 The development of a simple and facile strategy to produce novel, cost efficient and simple materials; capable of holding copious amount of water and the release of water upon demand at ambient temperatures, as a function of the crosslinking density of polymer chains, may provide an alternative source to ensure the supply of portable clean water in arid regions. The incorporation of antifouling properties in water harvesting materials further ensures the availability of clean supply of water, upon harvesting the water from natural resources, such as from sea, or rainwater. In this report we intend to develop, reusable, economical, antifouling and water-releasing hydrogels, for the release of significant amount of water by slight changes in the temperature, using highly hygroscopic and biocompatible analogue of pantothenic acid. In comparison to the handful of existing hygroscopic materials, which require higher temperatures for the optimum water release (≥50 °C) and complex synthesis in the presence of stimuli responsive moieties or the presence of ionic molecules (salts) in their polymeric architecture,5–7 the highly hydrophilic and polar nature of pantothenic acid analogous hydrogels may exhibit excellent water absorbing and release efficacies at ambient temperatures (∼37 °C).Pantothenic acid (vitamin B5), a water-soluble vitamin, from vitamin B complex is well known for its hygroscopic, antibacterial, biocompatible and moisturizing activities.9–11 Pantothenic acid, a pantoic acid linked with β-alanine via amide bond, is of biological importance due to its incorporation in acetyl-CoA and is an essential cofactor for cell growth, fatty acid synthesis, carbohydrate metabolism, amino acid catabolism, and heme synthesis.10 Pantothenic acid commercially exists as calcium salt and has seen limited applications in science, due to its instability in salt free conditions and tedious synthetic reaction conditions.11–13 We argue that hygroscopic nature of salt free pantothenic acid analogous hydrogels may represent competitive materials with superior water holding and water release capacities, at ambient temperature, as both of these properties are critically important for the hydrogels to qualify as economical water harvesting materials.7 However, an important step to achieve novel and functional pantothenic acid based materials and to study their role in water recycling efficacies is, the synthesis of salt free pantothenic acid analogous monomer, capable of polymerization under facile reaction conditions.
As discussed above, the structure of pantothenic acid reveals a simple amide bond between β-alanine and of pantolactone: the replacement of β-alanine in pantothenic acid structure with a similar polymerizable methacrylamide moiety might yield interesting properties to pantothenic acid analogous macromolecules. Others have suggested that modification at carboxyl end of β-alanine in pantothenic acid structure yields antibacterial and antifouling efficacies to the resultant materials.14,15 However, due to the difficulties associated with functionalization of ionic salts of pantothenic acid on activated surfaces, such materials have seen limited applications in materials science and biomedicine11,12 and to the best of our knowledge, pantothenic acid analogous monomers, and their corresponding polymerized macromolecules are not yet reported. Herein, a facile method to develop pantothenic acid analogous monomer (indicated as vitamin B5 based monomer or B5AMA) by simple ring opening chemistry is reported. The successful synthesis of B5AMA in reasonable yield encouraged the subsequent development of B5AMA analogous hydrogels of various crosslinking densities, by free radical polymerization method. The hydrogels of B5AMA of varying crosslinking densities produced were analyzed by X-ray diffraction analysis (XRD), thermogravimetric analysis (TGA) and were evaluated for their water absorption and release efficacies as a function of hydrogel cross-linking density and temperature. To the best of our knowledge, this is the first study, where pantothenic acid analogous monomer, and the corresponding hydrogels are prepared and their physiochemical properties (cross-linking density of hydrogels) are investigated for the water release efficacies.
Experimental procedure
Materials
Triethylamine (TEA), anhydrous methanol, potassium persulfate (KPS), N,N,N′,N′-tetramethylethylenediamine (TEMEDA), and N,N′-methylenebisacrylamide were purchased from Sigma-Aldrich. Pantolactone was purchased from Oakwood Chemicals.2-Aminoethyl methacrylamide (AEMA) was synthesized according to previously established procedure.16 Acetone, and ethyl ether were purchased from Fisher Scientific.
Synthesis of B5AMA
23 mmoles of AEMA were dissolved in anhydrous methanol (4 mL) in the presence of triethylamine (20 mL) under inert atmosphere and the mixture was stirred for 3 hours at room temperature to obtain a homogenous solution. 20 mmoles of pantolactone were then added and the reaction was allowed to proceed overnight under inert conditions at room temperature. The solution was precipitated in acetone and the white precipitates of triethylamine hydrochloride were filtered. The B5AMA monomer was purified from the filtrate by silica column chromatography, using acetone as an eluent. The eluent was concentrated and was precipitated in ethyl ether to obtain the final product in the form of pure yellowish oil with 65% yield. The purified B5AMA was analyzed by 1H-NMR and 13C-NMR, using Bruker 300 MHz NMR.1H-NMR (D2O, ppm): δ 0.79 (s, 3H, CH3), 0.83 (s, 3H, CH3), 1.8 (s, 3H, CH3), 3.35 (m, 6H, CH2), 3.9 (s, 1H, CH),5.4 (s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.6 (s, 1H, C
CH2), 5.6 (s, 1H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2) DEPT-45 spectrum of 13C-NMR (D2O, ppm): δ 17.9, 19.4, 20.8, 38.4, 39.3, 68.7, 78.2, 121.6.
CH2) DEPT-45 spectrum of 13C-NMR (D2O, ppm): δ 17.9, 19.4, 20.8, 38.4, 39.3, 68.7, 78.2, 121.6.
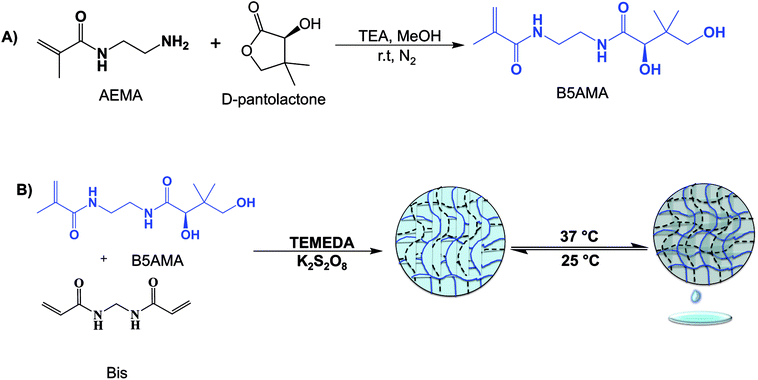
Synthesis of B5AMA hydrogels
B5AMA based hydrogels were prepared by free radical polymerization method. Briefly, the monomer, B5AMA (0.78 M), initiator KPS (10 μM) and the cross-linker N,N′-methylenebisacrylamide (Bis) (5–20 mol%) were dissolved in distilled deionized water and the solution was degassed for 5–10 minutes, followed by the addition of catalyst, TEMEDA (20 μL). B5AMA hydrogels were prepared at various molar ratios of cross-linker, as shown in Table 2. The formation of hydrogels was confirmed by vial inversion test and the gelation time was recorded. The hydrogels obtained were washed three times with deionized water and were dried to obtain pure hydrogel samples. The gravimetric weight of dried hydrogels (10 mol% cross-linked) was compared with the weight of monomer used for hydrogel synthesis and percent monomer conversion was calculated to be 59%.Water release measurements
The hydrogels of various cross-linking densities and of spherical shape (area 1.33 cm2, dimension; diameter 1.3 ± 1 cm and thickness 0.35 ± 0.5 cm) were prepared and water release from hydrogels was measured as a function of time and temperature. Briefly, water-laden hydrogels of varying crosslinking densities (5, 10, and 20 mol%, termed as B5AMA-5, B5AMA-10 and B5AMA-20, respectively) and of initial gravimetric weights 0.342 ± 0.012 g, 0.575 ± 0.07 g, and 0.541 ± 0.032 g (for B5AMA-5, B5AMA-10 and B5AMA-20, respectively) were incubated at 25, 37 and 60 °C in triplicates and water released was collected for the period of 24 hours.Amount of water released was measured as follows:
| amount of water released in gram per gram of hydrogel = WR/WG | (1) |
The percent release of water from the hydrogels was measured as follows:
| % water release from hydrogels = (WR/WL) × 100 | (2) |
Characterization techniques
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.524 Å) was used and the measurements were performed in air at room temperature from 2–60° (2Θ). Lyophilized samples were milled to powder and deposited on double sided scotch tape, which was mounted to glass slides.
1.524 Å) was used and the measurements were performed in air at room temperature from 2–60° (2Θ). Lyophilized samples were milled to powder and deposited on double sided scotch tape, which was mounted to glass slides.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C min−1. Samples were lyophilized, milled to powder and immediately analyzed.
°C min−1. Samples were lyophilized, milled to powder and immediately analyzed.Results and discussion
The water harvesting materials, capable of carrying copious amount of water, its release in the presence of external stimulus and the ability to undergo repeated cycles of absorption and desorption possess multiple applications in agriculture, water harvesting and in household appliances, such as in dehumidifiers.4,6,7 The handful of hydrogels studied for water absorption and release efficacies are summarized in Table 1.| Hydrogel material | Phase of water absorbed | Temperature required for water release (°C) | % water released | Reference |
|---|---|---|---|---|
| B5AMA | Liquid | 37 | 15 | This work |
| CaCl2 and alginate | Moisture | 100 | 90 | 6 |
| Poly(N-isopropyl methacrylamide) and alginate | Moisture | 50 | 15 | 7 |
| Poly-ionic liquids and poly (N-isopropyl methacrylamide) | Liquid | 50 | Not indicated | 5 |
We present here a unique set of hydrogels synthesized from vitamin B5 analogous methacrylamide monomer, termed as B5AMA, which are capable of carrying large amount of water (more than 90% of their own weight) and can repeatedly absorb and desorb significant amount of water at ambient (37 °C) temperature. In comparison to the existing water harvesting materials, which require high temperatures (50 °C and above) for the optimized water release from hydrogels,5–7 the harvesting of water at low temperature (37 °C) provides an ideal and economical method, and can reduce the energy consumption of the system for the supply clean water on demand.
The first step in the synthesis of hydrogels is the synthesis of the monomer B5AMA. To achieve our goal that is to develop pantothenic acid analogous monomer for its subsequent polymerization in the form of hydrogels, 2-aminomethacrylamide, (AEMA) a cationic monomer, a methacrylamide analogue of β-alanine was selected (ESI, Fig. S1†). The replacement of carboxyl group of β-alanine in the form of methacrylamide moiety of AEMA is expected to yield polymerization capability along with other interesting potential applications (such as antibacterial and antifouling properties) to the resultant biomaterials.11,12 AEMA was synthesized according to the previously established protocol16 and was used to prepare B5AMA monomer by facile ring opening chemistry, under inert atmosphere (Scheme 1). The synthesis of B5AMA monomer was confirmed by 1H-NMR, and 13C-NMR spectroscopy (ESI Fig. S2 and S3†).
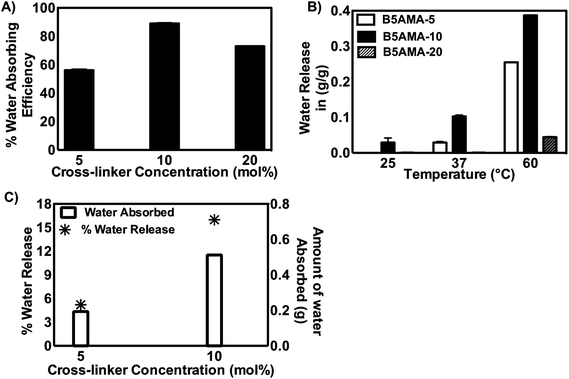
To accomplish the synthesis of highly hygroscopic hydrogels, purified B5AMA monomer was subsequently chemically cross-linked in the presence of cross-linker, N′,N′-methylene bisacrylamide, to yield pantothenic acid analogous hydrogels of varying cross linking densities (Table 2, Scheme 1). The chemically cross-linked hydrogels prepared were then tested for their water retention capacity, as a function of hydrogel crosslinking density (Table 2, Fig. 1a).
| Sample | Monomer (M) | Cross-linker (mol%) | Gelation time (minutes) | Water absorbed (g) | Water released (%) @ 37 °C |
|---|---|---|---|---|---|
| B5AMA-5 | 0.78 | 5 | 15 | 0.1935 ± 0.013 | 5.2 ± 1.71 |
| B5AMA-10 | 0.78 | 10 | 15 | 0.512 ± 0.067 | 15 ± 3.27 |
| B5AMA-20 | 0.78 | 20 | 15 | 0.468 ± 0.049 | 0 |
The water retention capability of hydrogels is generally a function of monomer type and the increase in cross-linking density of hydrogels is reported to reduce their swelling/water absorbing capacity due to the increase in mechanical strength (stiffness) of hydrogels.17 Others have suggested that presence of optimum amount of cross linker in polymeric architecture is required to prevent the dissolution of polymer chains and hence increases the swelling capacity of the hydrogels up to a critical cross linker concentration, however further increase in cross linker concentration is found to have negative effects on water retention efficacy of hydrogels.18 We also report that an optimum concentration of cross-linker is required to achieve maximum water retention capacity of B5AMA hydrogels. The increase in crosslinking density from 5 to 10 mol% in B5AMA hydrogels, increased the water absorption capacity of hydrogels from 59 to 90%, respectively. However, any further increase in cross linker concentration led to the reduction in water holding capacity and water retention efficacy of B5AMA-20 was reduced to 72% (Fig. 1a). Passauer et al. attributed the reduced swelling capability of lignin based xerogels to the small pore volume, and to the stronger polymer–polymer interactions of highly cross-linked hydrogel architecture, which subsequently resulted in lower polymer–solvent interactions.19
The bound water in hydrogels can exist either in the form of polarized molecules around charged groups or is oriented around the polar groups via hydrogen bonding. B5AMA hydrogels contain negligible if any charged groups (sulfate groups on the initiating chains), hence we expect that most of the absorbed water is held by hydrogen bonding around polar groups, such as by the amide and alcohol functional groups. In a recent report, polyionic gels of excellent water absorption efficacies (>90%) have shown the optimum release of water, upon heating at 50 °C.5 The superior water absorption capacity of synthesized B5AMA hydrogels via hydrogen bonding encouraged us to study their water release profiles, as a function of temperature and cross linker density. The B5AMA hydrogels of various cross-linking densities were incubated at different temperatures and the amount of water released as a function of temperature was studied (Table 2, Fig. 1b).
The data reveals that the increase in temperature and crosslinking density (from 5–10 mol% of cross linker), increases the amount of water released from the hydrogels, however further increase in crosslinking density of hydrogels exhibited negative effect on the water release behavior of hydrogels. Jacobson et al. have reported the thermal release of water from poly(methyl vinyledenecyanide) by UV laser assisted thermal desorption and showed that mild changes in solution temperature due to UV radiation, resulted in the diffusion of weakly bound water from poly(methyl vinyledenecyanide). The increase in water release efficacies of poly(methyl vinyledenecyanide) at higher temperatures were associated with the loss of strongly bound water from the polymeric architecture.2 The strikingly similar water release behavior was observed when B5AMA-10 was heated from room temperature to 37 °C, leading to the release of weakly bound water (0.1 g g−1 of hydrogels) from three-dimensional nano-pockets of hydrogels. The further increase in temperature to 60 °C increased the water content (to 0.4 g g−1), possibly due to the release of strongly bound water from the hydrogel sample. The densely cross-linked hydrogels (B5AMA-20), however exhibited poor water release efficacies, despite of high swelling capacities (72%) in deionized water (Fig. 1a and b). The water release of B5AMA-20 was negligible at 37 °C and less than 0.01 g g−1 water (∼9% of total absorbed water) was collected at 60 °C.
We hypothesize that the slightly unrestricted movement of polymer chains in cross-linked hydrogel samples of B5AMA-5 and B5AMA-10, permit the inter and intramolecular hydrogen bonding between polymer chains and water molecules, hence allowing the facile and reversible uptake and release of water under physiological condition. Although, the lower cross-linking density of B5AMA-5 yielded lower water absorption and release efficacies (5% of the total absorbed water), the incubation of B5AMA-10 at 37 °C released 15–18% of total water content absorbed by the hydrogel at room temperature (Fig. 1c). The rearrangement of B5AMA-10 polymeric chains as a function of temperature was clearly visualized by the slight reduction in the diameter (1.3 cm to 1.1 cm) and by the change in opacity of hydrogels (ESI Fig. S4†). The reduced water release behavior of B5AMA-20 may stem from smaller pore sizes, and the restricted flexibility of polymer chain in hydrogel architecture, which in turn results in poor water release efficacies of B5AMA-20.
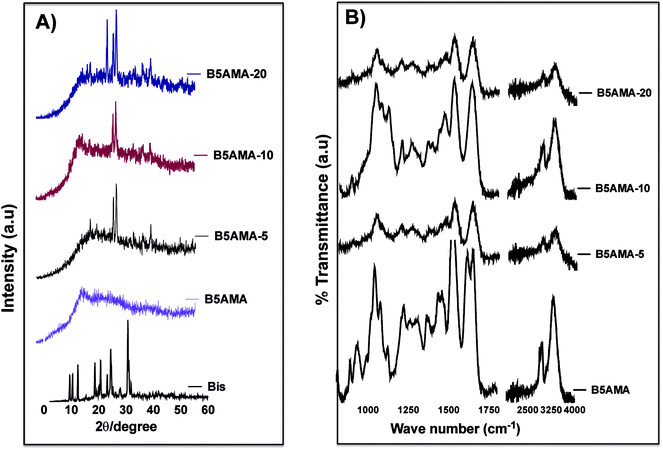
The physical characterization of hydrogels was performed to elucidate the details of crosslinking density of hydrogels and their impact in water release behavior. The changes in physical structure of monomer upon crosslinking with various concentrations of N′,N′-methylene bisacrylamide, were studied by XRD patterns. As expected, the presence of well-defined peaks at 2Θ = 9.25°, 12.3°, 18.7°, 20.6°, 23.1°, 24.3°, and 30.6°, in XRD revealed strongly crystalline behavior of N′,N′-methylene bisacrylamide, while pantothenic acid analogous monomer B5AMA, showed completely amorphous structure, which is indicated by a broad featureless peak at 2Θ = 18–40° (Fig. 2a). The freeze-dried gels prepared at various cross-linked densities maintained the amorphous behavior of B5AMA monomer and a few crystalline peaks corresponding to the structure of the cross-linker appeared around 2Θ = 30° (Fig. 2a). This data is in agreement with other reports, where blended materials prepared with high concentrations of amorphous substances in the presence of crystalline structures, resulted in highly amorphous materials, with only few peaks corresponding to crystalline structures of the starting material.20,21
The presence and availability of polar functional groups in B5AMA monomer and in the corresponding hydrogels was then evaluated by ATR-FTIR spectroscopy. FTIR analysis confirms the presence of hydroxyl and amide bonds in the monomer and in the hydrogel structures. The data reveals the presence of an intense and broad band at 3282 cm−1 originating from stretching of OH groups of B5AMA. The vibrational stretching at 1647 cm−1 is attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O signal of amide bond. The strong stretching at 1050 cm−1 represents C–O of alcohols and C–N bonds exhibit medium stretch at 1127 cm−1. The band at 2923 cm−1 is assigned to asymmetric C–H stretches (Fig. 2b). The presence of hydroxyl stretch at 3282 cm−1, the C–O stretch at 1050 cm−1, and carbonyl signal from amide bonds at 1647 cm−1, suggests the availability of polar groups for water absorption. The strength of % transmittance signal arising from hydroxyl stretch and from other functional groups of the un-crosslinked monomer and of B5AMA-10 is noteworthy and may indicate either the greater concentration of polar groups or their higher polarity/availability in B5AMA-10, as compared to the other hydrogels.22 (Fig. 2b) ATR-FTIR further suggests that greater availability of polar groups in 10% cross-linked hydrogels may have resulted in superior water molecule binding and release efficacies at optimized temperature.
O signal of amide bond. The strong stretching at 1050 cm−1 represents C–O of alcohols and C–N bonds exhibit medium stretch at 1127 cm−1. The band at 2923 cm−1 is assigned to asymmetric C–H stretches (Fig. 2b). The presence of hydroxyl stretch at 3282 cm−1, the C–O stretch at 1050 cm−1, and carbonyl signal from amide bonds at 1647 cm−1, suggests the availability of polar groups for water absorption. The strength of % transmittance signal arising from hydroxyl stretch and from other functional groups of the un-crosslinked monomer and of B5AMA-10 is noteworthy and may indicate either the greater concentration of polar groups or their higher polarity/availability in B5AMA-10, as compared to the other hydrogels.22 (Fig. 2b) ATR-FTIR further suggests that greater availability of polar groups in 10% cross-linked hydrogels may have resulted in superior water molecule binding and release efficacies at optimized temperature.
The thermal stability of hydrogels is an important parameter and may offer an insight on the water retention and release efficacies of hydrogels. The thermal stability of hydrogels was investigated by TGA. The strikingly different thermal decomposition behavior of B5AMA-10, in comparison to B5AMA-5 and B5AMA-20, suggest higher thermal stability of B5AMA-10 at lower temperatures, possibly due to the presence of significant amount of entrapped moisture in B5AMA-10 sample. B5AMA-10 showed a three step degradation profile. The 10% weight loss during the first step occurs upon heating from 50–200 °C and indicates moisture loss from the polymer chains. The second step contributes to the 50% weight loss of B5AMA-10 upon heating from 210–400 °C and is contributed by the thermal decomposition of bulk hydrogel architecture, due to the disruption of both covalent bonds and hydrogen bonds between polymer chains of the hydrogel. The third step is indicated by a sharp weight loss at 400 °C indicating the degradation of polymeric chains. In contrast, the TGA curves of B5AMA-5 and B5AMA-20 showed step-wise sequential weight loss and lower stability, than B5AMA-10 especially at lower temperatures (50–250 °C), indicating the role of moisture and hydrogen bonding in the hydrogel of B5AMA-10. The slower degradation rate and higher stability of B5AMA-5 and B5AMA-20 reflect their densely cross-linked structures, in comparison to B5AMA-10 hydrogel.23,24
To further explore the water release efficacies of B5AMA-10, TGA analysis was performed before and after the release of water. The water laden B5AMA-10 hydrogels, when heated to 53 °C, exhibit rapid weight loss (>90%), indicating that more than 90% of the weight of hydrogel was in the form of water and the cross-linked polymeric chains of pantothenic acid comprised less than 10% of weight of the sample, which were degraded at higher temperatures (between 400–600 °C) (Fig. 3b). In contrast, TGA curve of B5AMA-10 obtained after the in vitro release of water at 37 °C exhibited up to 73% of weight loss at 53 °C, which shows that B5AMA-10 can carry ∼18% water in recyclable form at 37 °C and this water can be retrieved by slight heating of gel from room temperature (22 °C) to 37 °C. The TGA analysis strongly complemented our in vitro water release data obtained and discussed above in Fig. 1, and reinforced that 15–18% of water absorbed by B5AMA-10 is in recyclable form and is accessible upon constant heating the hydrogel at 37 °C. The heating of B5AMA-10 at higher temperature showed two distinct curves between 200-300 °C due to the loss of remaining moisture, disruption of hydrogen bonding and 300–400 °C for the degradation of polymeric chains.
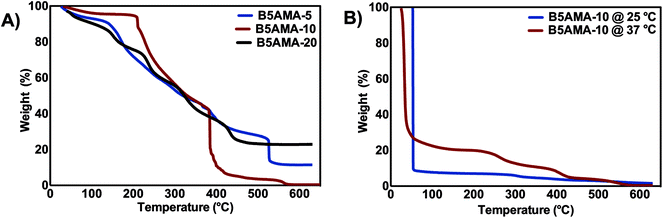 | ||
| Fig. 3 (A) TGA analysis of freeze-dried B5AMA hydrogels, prepared at various cross-linker concentrations. (B) TGA analysis of B5AMA-10 before (25 °C), and after the release of water at 37 °C. | ||
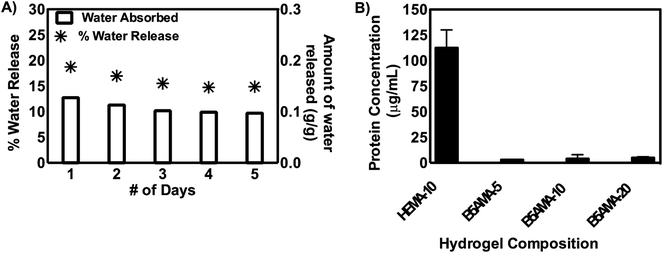
The repeated absorption and desorption of known amount of water in hydrogels in multiple cycles, ensure the reproducibility of the data, recyclability of the materials and their potential applications in various fields of nanotechnology and bioscience.6,7 We performed repeated cycles of water absorption and desorption, by hydrating B5AMA-10 hydrogel samples at room temperature, followed by the release of water at 37 °C. The hydration/dehydration cycle consists of 3 phases: (1) absorption of water at room temperature for 1 hour (2) desorption of water while heating at 37 °C for 24 hours followed by (3) absorption of water at room temperature for 1 hour. The cycles were repeated for 5 days, and 15–18% of the absorbed water was collected after each cycle, as shown in Fig. 4. It should be noted that any change in the weight of gel or physical deformities of hydrogel were not observed for the period of time studied, thus further ensuring the recyclability of our materials (ESI Fig. S5†). The amount of water released from B5AMA-10 at 37 °C (15–18%) is comparable to the reported release of water from alginate based hydrogels (∼20% at 50 °C) and hygroscopic nature of polymeric chains is suggested to prevent the further release of remaining water content from the hydrogels architecture.7 The water release behaviour of B5AMA-10 hydrogels at ambient temperature (37 °C) provides highly efficient energy exchange system with applications in water harvesting and in household appliances such as dehumidifier and sensor.4,7,8
The antifouling properties of pantothenic acid modified materials are well documented in the literature.11,12 The pantothenic acid analogous hydrogels synthesized required their further evaluation of their antifouling properties.11,12 The antifouling properties of B5AMA hydrogels were evaluated by measuring the absorption of bovine serum albumin (BSA) in the three dimensional architecture hydrogels, followed by the release of BSA and its detection by BCA assay. The superior capability of hydrogels to absorb bioactive molecules such as peptides and proteins and their controlled release in the presence of external stimulus are well documented in the literature.25,26 2-Hydroxyethyl methacrylate (HEMA) based non-ionic hydrogels are well-explored for the delivery of small peptides and proteins for ocular therapies, and were used as a positive control for this study.23 The data reveals that B5AMA based hydrogels showed negligible protein absorption, after the 24 hour incubation with bovine serum albumin (BSA) solution. This antifouling behavior of B5AMA hydrogels was not related to their cross-linking density and was attributed to the properties of the material itself. In contrast, as expected, HEMA based hydrogels, prepared under similar conditions, showed significant absorption and release of BSA protein. This result suggests that novel pantothenic acid analogous synthetic materials maintain the inherent antifouling properties of pantothenic acid. Further studies are in progress to assess the applications of these antifouling hydrogels for biomedical applications.
Conclusion
In summary, pantothenic acid analogous monomer, B5AMA and its corresponding hydrogels were prepared at 5, 10 and 20 mol% crosslinking density. The B5AMA based hydrogels prepared by free radical polymerization method showed excellent water retention and release efficacies, as a function of cross-linking density and temperature. The superior water release behavior (15–18% of the total absorbed water) of hydrogels prepared at 10% crosslinking density is associated with optimized three dimensional network of hydrogels, which provides greater availability of polar functional groups and allows flexible polymer chain movement in the hydrogel architecture, hence ensuring superior water absorbance and release behavior. The inter and intra-molecular interactions of polymer chains in B5AMA-10 hydrogel networks is visible by the slight changes in hydrodynamic diameter and opacity of the hydrogels at different temperatures. Moreover, the antifouling properties and the repeated cycles of water absorption and desorption in hydrogels suggest their applications and reusability in the nanosensors, biotechnology, agriculture, and in food industry.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank NSERC and internal research grant of UPEI for the completion of this project.References
- H. Kim, J. Kim and S. J. Lee, ACS Macro Lett., 2018, 7, 387 CrossRef CAS.
- P. A. Jacobson, C. C. Ilie, I. N. Yakovkin, M. Poulsen, D. S. Reddy, J. M. Takacs, P. Greis and A. Dowben, J. Phys. Chem. B, 2006, 110, 15389 CrossRef CAS PubMed.
- K. Bethke, S. Palantöken, V. Andrei, M. Roß, V. S. Raghuwanshi, F. Kettemann, K. Greis, T. T. K. Ingber, J. B. Stückrath, S. Valiyaveettil and K. Rademann, Adv. Funct. Mater., 2018, 28 DOI:10.1002/adfm.201800409.
- D. K. Nandakumar, S. K. Ravi, Y. Zhang, N. Guo, C. Zhang and S. C. Tan, Energy Environ. Sci., 2018, 11, 2179 RSC.
- Y. Deguchi, Y. Kohno and H. Ohno, Chem. Commun., 2015, 51, 9287 RSC.
- P. A. Kallenberger and M. Fröba, Chem. Commun., 2018, 1 DOI:10.1038/s42004-018-0028-9.
- K. Matsumoto, N. Sakikawa and T. Miyata, Nat. Commun., 2018, 9, 2315, DOI:10.1038/s41467-018-04810-8.
- A. Teston, M. Geraldi, B. Colasio and E. Gishi, Water, 2018, 10 DOI:10.3390/w10040471.
- V. Gun'ko, I. Savina and S. Mikhalovsky, Gels, 2017, 3 DOI:10.3390/gels3040037.
- G. S. Kelly, Altern. Med. Rev., 2011, 16, 263 Search PubMed.
- M. N. Zholobak, A. B. Shcherbakov, A. S. Bogorad-Kobelska, A. S. Ivanova, N. Y. Baranchikov and V. K. Ivanov, J. Photochem. Photobiol., B, 2014, 130, 102 CrossRef PubMed.
- L. Fan, Z. Cai, K. Zhang, F. Han, J. Li, C. He, X. Mo, X. Wang and H. Wang, Colloids Surf., B, 2014, 117, 14 CrossRef CAS PubMed.
- M. de Villiers, C. Macuamule, C. Spry, Y. Hyun, E. Strauss and K. J. Saliba, ACS Med. Chem. Lett., 2013, 4, 784 CrossRef CAS PubMed.
- T. O. Akinnusi, K. Vong and K. Auclair, Bioorg. Med. Chem., 2011, 19, 2696 CrossRef CAS PubMed.
- G. Clifton, S. R. Bryant and C. G. Skinner, Arch. Biochem. Biophys., 1970, 137, 523 CrossRef CAS PubMed.
- Z. Deng, H. Bouchékif, K. Babooram, A. Housni, N. Choytun and R. Narain, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 4984 CrossRef CAS.
- N.-T. Nguyen and J.-H. Liu, Eur. Polym. J., 2013, 49, 4201 CrossRef CAS.
- J. R. Witono, I. W. Noordergraaf, H. J. Heeres and L. P. B. M. Janssen, Carbohydr. Polym., 2014, 103, 325 CrossRef CAS PubMed.
- L. Passauer, M. Struch, S. Schuldt, J. Appelt, Y. Schneider, D. Jaros and H. Rohm, ACS Appl. Mater. Interfaces, 2012, 4, 5852 CrossRef CAS PubMed.
- R. Mishra, M. Datt and A. Banthia, AAPS PharmSciTech, 2008, 9, 395 CrossRef CAS PubMed.
- N. Sanabria-DeLong, S. K. Agrawal, S. R. Bhatia and G. N. Tew, Macromolecules, 2006, 39, 1308 CrossRef CAS.
- J. Coates, Encyclopaedia of Analytical Chemistry, R. A. Meyers, John Wiley & Sons, Ltd., 2000 Search PubMed.
- C. Gao, J. Ren, W. Kong, R. Sun and Q. Chen, RSC Adv., 2015, 5, 9671 Search PubMed.
- X. Huang, R. Wang, T. Lu, D. Zhou, W. Zhao, S. Sun and C. Zhao, Biomacromolecules, 2016, 17, 4011 CrossRef CAS PubMed.
- T. Vermonden, R. Censi and W. E. Hennink, Chem. Rev., 2012, 112, 2853 CrossRef CAS PubMed.
- E. V. Hackl, V. V. Khutoryanskiy and I. Ermolina, J. Appl. Polym. Sci., 2017, 134 DOI:10.1002/app.4476.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra07915c |
| This journal is © The Royal Society of Chemistry 2018 |