Open Access Article
Open Access ArticleNatural phosphate-supported Cu(II), an efficient and recyclable catalyst for the synthesis of xanthene and 1,4-disubstituted-1,2,3-triazole derivatives†
Abbas Amini *ab,
Azadeh Fallah
*ab,
Azadeh Fallah *cd,
Chun Chenge and
Mahmood Tajbakhsh
*cd,
Chun Chenge and
Mahmood Tajbakhsh f
f
aCentre for Infrastructure Engineering, Western Sydney University, Kingswood Campus, Bld Z, Locked Bag 1797, Penrith 2751, NSW, Australia. E-mail: a.amini@westernsydney.edu.au; a.amini@ack.edu.kw; Fax: +61-2-9685-9298; Tel: +61-2-404-060-787
bDepartment of Mechanical Engineering, Australian College of Kuwait, Mishref, Kuwait
cDepartment of Chemistry, Payame Noor University, Tehran, Iran
dPharmaceutical Sciences Research Center, Department of Medicinal Chemistry, Mazandaran University of Medical Sciences, Sari, Iran. E-mail: azadehfallah84@gmail.com
eDepartment of Materials Science and Engineering, South University of Science and Technology, Shenzhen, China
fDepartment of Organic Chemistry, University of Mazandaran, Babolsar, Iran
First published on 12th December 2018
Abstract
Cu(NO3)2 supported on natural phosphate, Cu(II)/NP, was prepared by co-precipitation and applied as a heterogeneous catalyst for synthesizing xanthenes (2–3 h, 85–97%) through Knoevenagel–Michael cascade reaction of aromatic aldehydes with 1,3-cyclic diketones in ethanol under refluxing conditions. It was further used for regioselective synthesis of 1,4-disubstituted-1,2,3-triazoles (1–25 min, 95–99%) via a three-component reaction between organic halides, aromatic alkynes and sodium azide in methanol at room temperature. The proposed catalyst, Cu(II)/NP, was characterized using X-ray fluorescence, X-ray diffraction, Fourier-transform infrared spectroscopy, scanning electron microscopy, Brunauer–Emmett–Teller, Barrett–Joyner–Halenda and inductively coupled plasma analyses. Compared to other reports in literature, the reactions took place through a simple co-precipitation, having short reaction time (<3 hours), high reaction yield (>85%), and high recyclability of catalyst (>5 times) without significant decrease in the inherent property and selectivity of catalyst. The proposed protocols provided significant economic and environmental advantages.
Introduction
In recent years, methodological developments have significantly enhanced syntheses of heterocyclic compounds via separation/purification processes.1,2 Recycling catalysts can minimize the consumption of auxiliary substances, concluding in economic and environmental benefits.3,4To significantly enhance the catalytic activity, a support is added to the reaction system; that is, the intimate contact between the supported ions and the support surface can favorably affect the reaction process.5 Over the past decade, considerable attention has been given to recycling catalysts and heterogeneous metal-based systems,6 including homogenous metal complexes or noble metals (e.g., Pt, Pd, Au, Ag, Ru, Cu) on inorganic substances (e.g., metal oxides, silica, clay, alumina, carbon) catalysts.7–9 These exhibit high catalytic activities in a wide range of chemical reactions, for instance, in synthesizing aromatic nitriles from aromatic aldehydes,10 CO oxidation,11 carbon dioxide reduction,12 asymmetric addition of aryl boronic acids to α,β-unsaturated carbonyl compounds,13 and hydrogenation of CO2.14
Natural phosphate (NP) is a widely available resource in many countries.15 As it is cheap, nontoxic and non-polluting, major efforts have focused on evaluating its applications in the synthesis of essential compounds.16 Ma et al. reported the adsorption capacity of natural phosphate in immobilizing heavy metals from aqueous solutions.17,18 Aklil et al. examined natural phosphate for the removal of heavy metals from aqueous solution.18 Natural phosphate was examined to treat soil, waste and wastewater resources contaminated with heavy metals,19 for the sorption affinity of Pb, Cu and Zn.20 In these applications, natural phosphate was considered as a support for homogeneous catalysts, due to its chemical and thermal stability, high surface area, and potential sorbents for numerous heavy metals.21
Xanthene (1,8-dioxo-octahydroxanthene) is a phenyl with pyran ring fused on either sides along with two other cyclohexanone rings. This compound has biological capability for anti-inflammatory, antibacterial and antiviral activities,22,23 dye applications,24 corrosion inhibitory,25 pH-sensitive fluorescence26 and laser technologies.27 For the synthesis of xanthenes, Knoevenagel condensation has been employed followed by the Michael addition of carbonyl compounds to 1,3-dicarbonyl compounds along with using a wide range of solid catalysts and inorganic salts. Various catalysts have been reported to promote the synthesis of xanthene, including ICl3/SiO2 and In(CF3SO3)3,28 lactic acid,29 2,6-pyridinedicarboxylic acid,30 poly(N,N′-dibromo-N-ethylnaphtyl-2,7-sulfonamide) (PDNES),31 W-doped ZnO,32 silica-supported Preyssler nanoparticles,33 ceric ammonium nitrate-supported HY-zeolite,34 and Fe3O4@SiO2-imid-H3PMo12O40 nanoparticles.35 However, these catalysts possess several disadvantages, such as less availability, difficult preparation process, containing toxic organic solvents and harsh reaction conditions (microwave irradiation and high temperature). New catalysts are required to synthesize xanthenes via simple, efficient and environmentally friendly processes.
1,2,3-Triazoles belong to an important class of nitrogen-containing heterocyclic compounds with enormous applications in biology, pharmaceutics,36,37 and medicinal chemistry.38–41 They are used as corrosion inhibitors,42 agrochemicals,43 optical brighteners and dyes.44 The Huisgen (3 + 2) cycloaddition reaction of azide and alkyne (AAC)45 was facilitated by Cu(I) catalysts (CuAAC) to afford 1,4-disubstituted-1,2,3-triazoles as a regioselective product.46,47 CuAAC-reaction was catalyzed by various copper sources, such as Cu(I) salts,48 in situ reduction of Cu(II),49 oxidation of Cu(0) metal,50 and Cu(II)/Cu(0) comproportionation.51 To establish reusable catalysts, Cu(I) AAC catalysts were immobilized onto polymers,52 zeolite,53 montmorillonite,54 silica-supported N-heterocyclic carbine,55 activated charcoal,56 alumina,57 titanium dioxide58 and CuO nanoparticles.59,60 Nevertheless, heterogeneous catalysts, immobilized with Cu(I) species, suffer from inherent thermodynamic instability of Cu(I) species, which results in an easy oxidation to Cu(II) and/or disproportionation to Cu(0) and Cu(II)61 as well as copper nanoparticles.62 For the AAC reaction, in which Cu(II) species are directly employed, a protocol without deliberate addition of a reducing agent is necessitated.63
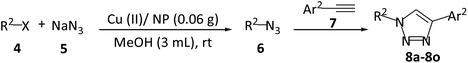
The present study introduces a new surface modified natural phosphate, Cu(II) supported-natural phosphate, for the synthesis of xanthenes via Knoevenagel–Michael cascade reaction of aromatic aldehydes with 1,3-cyclic diketones under refluxing in ethanol (Scheme 1a). This catalyst is further used for the region-selective generation of 1,4-disubstituted-1,2,3-triazoles in a three-component reaction from terminal alkynes, benzyl halides, and NaN3 in methanol at room temperature (Scheme 1b). With this method, no additional base is needed for the preparation of 1,4-disubstituted-1,2,3-triazoles. The proposed protocols for the synthesis of xanthene and 1,4-disubstituted-1,2,3-triazole present an efficient and robust chemical processes with high yield products at considerable short reaction times. The utilized catalyst in this study (Cu(II)/NP) is recyclable by a simple filtration process, readily available, and easy to prepare with high stability.
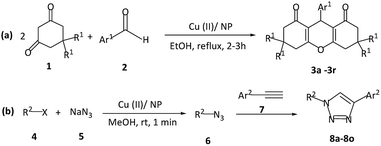 | ||
| Scheme 1 Preparation of (a) xanthene 3a–3r, and (b) 1,4-disubstituted-1,2,3-triazole 8a–8o by Cu(II)/NP, as the heterogeneous catalyst. | ||
Experimental
Reagents and materials
All chemicals were purchased from Merck, Germany, and Sigma Aldrich, USA. The structural data of all synthesized products were compared with the authentic data from literature.Instrumentation
The chemical compositions of NP and Cu(II)/NP were determined using a PANalytical's XRF spectrometer Venus 200. X-ray powder diffraction analyses were performed at room temperature on a vertical Philips PW1050/25 goniometer mounted with Bragg–Brentano configuration (θ, 2θ) using Ni-filtered Cu-Kα radiation (λ = 1.54 Å) and facilitated with the PANalytical X'Pert High Score Plus software. The surface imaging was performed using a scanning electron microscope (SEM) EM-3200. Surface areas were characterized at 77 K using a Coulter SA 31000 instrument through an automated gas volumetric method with nitrogen as the adsorbate. Fourier Transform Infrared (FT-IR) spectra were recorded on a Bruker Tensor 27 spectrometer, using KBr pellets for solids. 1H and 13C Nuclear Magnetic Resonance (NMR) spectra were recorded using a Bruker Advance DRX spectrometer at 400 MHz in DMSO-d6 and CDCl3 with tetra-methyl-silane as the internal reference.Preparation of catalyst
Different metal salts, including NaNO3, NaCl, KF, Cu(NO3)2, Ni(NO3)2, CuCl2, CoCl2, were doped on NP. They were prepared by adding 5 g NP to the aqueous metal salts solution (50 mL, 0.4 M). The mixture stirred at room temperature for 1 hour, then, the water content was evaporated at vacuum pressure. The suspended solid was calcined in air at three different temperatures of 160 °C for NaCl, 150 °C for KF, NaNO3, Cu(NO3)2, Ni(NO3)2, CuCl2, CoCl2 and 105 °C for Cu(NO3)2.64Preparation and characterization of Cu(II)/NP catalyst
NP was collected from ore resources in Yazd (Iran). NP was refluxed in water for 4 hours, then, filtered and washed with water, dried at 80 °C overnight and calcined at 900 °C for another 2.5 hours. The obtained solid was washed again and recalcined at 900 °C for 30 minutes. To obtain Cu(II)/NP, NP (5 g) was added to an aqueous Cu(NO3)2·3H2O solution (50 mL, 0.4 M). The mixture was stirred at room temperature for 30 minutes, then, evaporated in vacuum. The final solid was calcined under air at 105 °C for 2 hours.Synthesis of xanthenes
To a solution of aromatic aldehyde (1 mmol) and diketone (2 mmol) in ethanol (3 mL), Cu(II)/NP catalyst (0.2 g) was added and refluxed 2–3 hours to result xanthene 3, while the reaction progress was characterized by TLC (silica gel, hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 7
EtOAc, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). After the completion of reaction, the mixture was filtered and washed with hot ethanol (boiling). Evaporation of the ethanoic filtrate provided xanthene 3 (Scheme 1(a)), the melting point of the final product was characterized through FT-IR, 1H and 13C NMR spectra. Selected spectral data of some obtained xanthenes are as follow:
3). After the completion of reaction, the mixture was filtered and washed with hot ethanol (boiling). Evaporation of the ethanoic filtrate provided xanthene 3 (Scheme 1(a)), the melting point of the final product was characterized through FT-IR, 1H and 13C NMR spectra. Selected spectral data of some obtained xanthenes are as follow:
Synthesis of 1,4-disubstituted-1,2,3-triazoles
Cu(II)/NP catalyst (0.06 g) was added to a stirred suspension of NaN3 (78 mg, 1.2 mmol), alkyl halide (1.0 mmol) and methanol (2 mL). The procedure was followed by slow drop-wise addition of a solution of terminal alkyne (1.0 mmol) and methanol (2 mL). The mixture was stirred at room temperature, after the completion of reaction, it was characterized by TLC (silica gel, hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 7
EtOAc, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). The mixture was filtered and washed with methanol. All the products were carefully isolated from methanol or a mixture of ethanol/water (1
3). The mixture was filtered and washed with methanol. All the products were carefully isolated from methanol or a mixture of ethanol/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) through recrystallization. The spectral data (1H, 13C NMR) of the obtained products were evaluated with the spectra of authentic samples reported in literature. Selected spectral data of some obtained 1,4-disubstituted-1,2,3-triazoles are presented below:
3, v/v) through recrystallization. The spectral data (1H, 13C NMR) of the obtained products were evaluated with the spectra of authentic samples reported in literature. Selected spectral data of some obtained 1,4-disubstituted-1,2,3-triazoles are presented below:
Results and discussion
Characterization of Cu(II)/NP catalyst
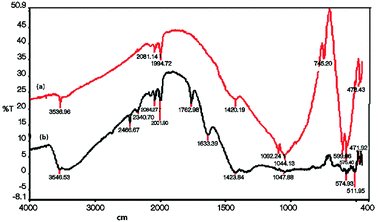
The FT-IR spectrum of NPs shows strong peaks at: 1092, 1044, 600, 576 and 473 cm−1 which can be referred to the vibrational modes of P–O, P–O–P bands in PO43− groups of the apatite structure (Fig. 1a).68 In the spectrum of Cu(II)/NPs, these bands are weakened or disappeared (Fig. 1b). The FT-IR characterization of Cu(II)/NP shows strong bands with the maximums at ∼1633 and 1423 cm−1, which are associated to N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, O–NO–O bands and nitrate groups of Cu(NO3)2·3H2O. The strong absorption peak at 3480 cm−1 attributes to H2O absorbed either in the samples or in the KBr pellet. These spectra show that the decomposition of nitrate does not happen at the temperature of calcination (105 °C).15
O, O–NO–O bands and nitrate groups of Cu(NO3)2·3H2O. The strong absorption peak at 3480 cm−1 attributes to H2O absorbed either in the samples or in the KBr pellet. These spectra show that the decomposition of nitrate does not happen at the temperature of calcination (105 °C).15
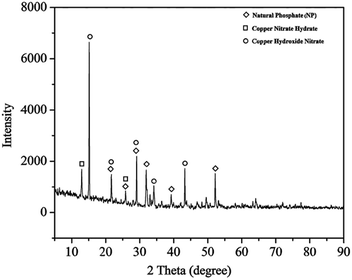
The X-ray diffraction (XRD) patterns of Cu(II)/NP in Fig. 2 show sharp and distinct peaks which confirm a high degree of crystallinity.69 The structure of NP is shown to be modified by the solid phase reaction with copper nitrate (Cu(NO3)2). The XRD peaks fit with standard patterns of fluorapatite JCPDS 15-876 (Fig. 3c), and some other new crystalline phases, including copper nitrate hydrate, Cu(NO3)2(H2O)2.5, JCPDS 75-1493 (Fig. 3d), and copper hydroxide nitrate, Cu2(OH)3(NO3), JCPDS 75-1779 (Fig. 3e). Compared to NPs, the intensity of typical diffraction peaks of Cu(II)/NP significantly differs, which indicates disordering the crystalline structure of Cu(II)/NP. No detection of Cu(NO3)2 phase on the doped materials implies that Cu(NO3)2 is highly dispersed in NP.70 CaO phase (2θ = 32.2, 37.5 and 54.0°) has no peaks, which verifies no solid phase reaction between the supported copper nitrate and the calcium phosphate of NP during the copper nitrate decomposition process.12,70 From the XRD curves of Cu(II)/NP (Fig. 2), it is concluded that copper phosphate or any other types of mixed calcium copper phosphate does not combine with the NP crystalline framework. The remarkable fact is that the main modifications in the pattern are produced in the range of 2θ = 12–15°, with new lines at 12.92° and 15.13° together with an increase in the intensity of other lines (Fig. 2). The intense lines related to NP are weakened against fluorapatite (see Fig. 3a–c); the lack of intense bands may account for the formation of very small crystals, partially amorphous materials or mixed phosphates with different stoichiometry.16
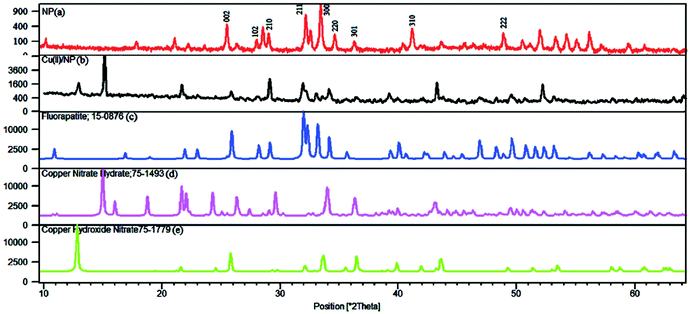 | ||
| Fig. 3 X-ray diffraction of (a) NP, (b) Cu(II)/NP, (c) fluorapatite, (d) copper nitrate hydrate, (e) copper hydroxide nitrate. | ||
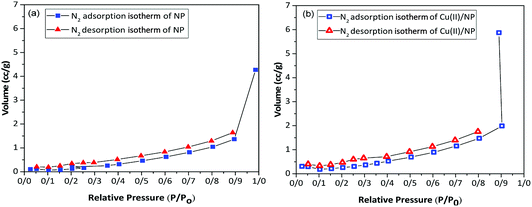
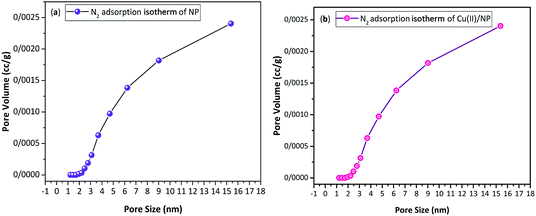
The porosity of Cu(II)/NP is investigated via Brunauer–Emmett–Teller (BET) (Fig. 4) and Barrett–Joyner–Halenda (BJH) (Fig. 5) methods using N2 adsorption–desorption isotherms. The type IV isotherm of Cu(II)/NP possesses a hysteresis loop and typical property of mesoporous materials (2–50 nm), such as high surface areas (>1000 m2 g−1), large internal pore volumes and unique ordered porous structures, similar to NP, but at a higher relative pressure of N2 gas. The surface area of Cu(II)/NP is higher compared to other mesoporous NPs with no Cu particles (Fig. 4). The N2 adsorption–desorption isotherms of NP and Cu(II)/NP are measured by BET and BJH methods, where the special surface area (S), the total pore volume (Vtot BET), micro pore volume (VBJH), and the average pore diameter (Daverage) are calculated using the adsorption data. The special surface area (SBET) of NP and Cu(II)/NP is calculated through BET method and the surface area of meso-pore SBJH, and the meso-pore volume VBJH of NP and Cu(II)/NP are calculated using BJH method (Table 1). According to the classification standard of International Union of Pure and Applied Chemistry (IUPAC), the characteristics of NP and Cu(II)/NP as mesoporous solids display a type IV isotherm with H3 hysteresis loop.71,72 According to BET and BJH results, the shape of Cu(II)/NP was similar to NPs, and the porosity of Cu(II)/NP did not change during the stabilization of Cu(II) on the surface of NP. These results introduced permanent porosity of the Cu(II)/NP surface. From Table 1, according to the BET calculation, the specific surface area decreased from 2.081 m2 g−1 for NP to 1.849 m2 g−1 for Cu(II)/NP. These observations verified no clogging of pores with copper species.73 The specific pore volume increased after supporting NP with copper nitrate. After loading Cu(II), larger pores generated; as a result, the average pore diameter increased from 12.73 to 19.70 nm.74 The BJH curves of nitrogen adsorption displayed a decline in pore diameter from 3.67 to 3.08 nm, which was originated from introducing copper species complex to the interior side of some pores in NP.75 High surface area and large pore size of Cu(II)/NP favoured a high dispersion of active elements, and made the large amounts of the reactive molecules to be easily accessible.76
| Sample | Specific surface area [m2 g−1] | Specific pore volume [cm3 g−1] | Pore diameter [nm] | |||
|---|---|---|---|---|---|---|
| SBET | SBJH | Vtot BET | VBJH | Daverage | DBJH | |
| NP | 2.081 | 1.948 | 0.00662 | 0.00722 | 12.73 | 3.675 |
| Cu(II)/NP | 1.849 | 2.513 | 0.00912 | 0.00972 | 19.70 | 3.089 |
Surface structure of Cu(II)/NP was investigated by SEM method in order to visualise the textural modifications after adding copper nitrate to natural phosphate (Fig. 6). Compared to NP (Fig. 6a), Cu(II)/NP (Fig. 6b) had a rougher surface with more distorted areas, along with uniform spherical particles. In fact, the addition of copper nitrate increased the size of catalyst particles in Cu(II)/NP.15,72,77
Coupling reaction-based synthesis of xanthenes
Authors have introduced NP as a catalyst for synthesizing xanthene derivatives through the reaction of 4-chlorobenzaldehyde (1 mmol) with dimedone (2 mmol) in refluxing ethanol.78 To afford xanthene 3 with a high yield, relatively high weight of NP catalyst (1.5 g) and long reaction time are required (Table 1, no. 1). Here, to increase the NP activity, it is doped with different metal salts (i.e., sodium nitrate, NaNO3; sodium chloride, NaCl; potassium fluoride, KF; nickel nitrate, Ni(NO3)2; copper nitrate, Cu(NO3)2; cobalt chloride, CoCl2; and copper chloride, CuCl2) (Table 2). The results show that copper salts are the best candidates to synthesize xanthene 3a (Table 2, entries 7 and 9). Table 2 (entries 5–7) represents the results of calcination temperatures for preparing Cu(NO3)2/NP. The treatment of Cu(NO3)2/NP at 150 and 500 °C led to catalysts with poor or no catalytic activities (Table 2, entries 5 and 6). This is due to thermal decomposition of nitrate groups of Cu(NO3)2/NP at temperatures over 200 °C as well as the formation of the copper oxide (CuO).77 The decomposition of d-metal nitrates hydrates proceeded with slow pace that rarely led to anhydrous salts. Usually the dehydration process is not complete and the beginning of nitrate (V) groups decomposition occurs simultaneously with the evolution of water hydration.79 The following molecular and fragmentation ions of H2O+, NO+, N2O+, NO2+ can be formed during the decomposition of nitrates. These ionic products from the decomposition of copper nitrate concluded in the formation of copper hydroxide nitrate, Cu2(OH)3(NO3) and copper nitrate hydrate, Cu(NO3)2(H2O)2.5)77 which in our study were confirmed by XRD analysis (Fig. 2). At the lower calcination temperature, e.g., 105 °C, a high yield (98%) of 3a was created in a short reaction time (Table 2, entry 7). In fact, the initial state of Cu on the support was Cu(NO3)2·3H2O and the final state of Cu on the support was copper nitrate hydrate, Cu(NO3)2(H2O)2.5, copper hydroxide nitrate and Cu2(OH)3(NO3), supported on the surface of NP. The decomposition of nitrate groups proceeded at the same temperature range as dehydration did.| Entry | Catalyst | Calcination temperature (oC) | Time (h) | Isolated yield 3a (%) |
|---|---|---|---|---|
| a Reaction conditions: 4-chlorobenzaldehyde (1 mmol), dimedone (2 mmol), catalyst (0.2 g) in refluxing EtOH (3–5 mL). | ||||
| 1 | NP78 | 900 | 6.5 | 90 |
| Present study | ||||
| 2 | NaNO3/NP | 150 | 6.5 | 15 |
| 3 | NaCl/NP | 160 | 5 | 70 |
| 4 | KF/NP | 150 | 5 | 10 |
| 5 | Cu(NO3)2/NP | 500 | 48 | 15 |
| 6 | Cu(NO3)2/NP | 150 | 6 | 50 |
| 7 | Cu(NO3)2/NP | 105 | 2 | 98 |
| 8 | Ni(NO3)2/NP | 150 | 5 | 25 |
| 9 | CuCl2/NP | 150 | 3.5 | 98 |
| 10 | CoCl2/NP | 150 | 6.5 | 20 |
The treatment at 500 °C resulted in a very low yield 15% of 3a after a long period of reaction time 48 hours (Table 2, entry 5). The decomposition of Cu(II) species to copper oxide was confirmed by the decreased weight of catalyst, its black color and reported results from other researchers.77 The non-catalytic activity of Cu(NO3)2/NP [calcinated at 500 °C] might be due to the formation of copper oxide on NP and its non-porous surface morphology of copper oxide.80 As previously reported,78 this reaction under the same condition is carried out by using NP as catalyst, which provides 90% high yield of 3a in a little long reaction time 6.5 hours (Table 2, entry 1). When NP is modified by Cu(NO3)2, the reaction time decreases to 2 hours and the yield of the products increases to 98% (Table 2, entry 5). In short, compared to NP, Cu(NO3)2/NP calcined at 105 °C produces xanthenes with much higher yields at shorter reaction times.
In order to determine the best ratio of Cu(NO3)2/NP, supported copper catalyst was prepared by impregnating NP with different concentrations of Cu(NO3)2·3H2O aqueous solution (0.1, 0.2, 0.3, and 0.4 M). These supported copper catalysts were prepared according to the procedures introduced in Sections 2.3 and 2.4. Their catalytic effect on the yield and time of the reaction of 4-chlorobenzaldehyde with dimedone for the synthesis of xanthene 3a as model reaction were investigated (Table 3). As shown in Table 3, the best result was obtained by Cu(NO3)2/NP, prepared with 0.4 molar concentration of Cu(NO3)2·3H2O aqueous solution; we name it Cu(II)/NP (Table 3, entry 7). To determine the effect of support in the reaction, the catalytic effect of sole Cu(NO3)2 was investigated in the model reaction. The unsupported copper catalyst was prepared via calcination of Cu(NO3)2·3H2O at 105 °C for 2 hours. In order to determine the role of NP in the catalytic activity of Cu(II)/NP, the amount of copper nitrate existed in 0.2 g Cu(II)/NP was calculated as 0.064 g. So, 0.064 g of Cu(NO3)2 was used as catalyst in the model reaction; it afforded 3a only with 50% of yield after 4 hours (Table 3, entry 8). The result show that, in contrast with NP, the sole modified Cu(NO3)2 cannot positively affect the reaction time and yield of 3a.
| Entry | Catalyst | Catalyst loading (g) | Time (h) | Yield 3a (%) |
|---|---|---|---|---|
| a Reaction conditions: dimedone (2 mmol), 4-chlorobenzaldehyde (1 mmol), catalyst in refluxing EtOH (3–5 mL). | ||||
| 1 | Cu(NO3)2(0.2 M)/NP | 0.2 | 7 | 70 |
| 2 | Cu(NO3)2(0.2 M)/NP | 0.5 | 7 | 90 |
| 3 | Cu(NO3)2(0.3 M)/NP | 0.1 | 4 | 90 |
| 4 | Cu(NO3)2(0.3 M)/NP | 0.2 | 3 | 89 |
| 5 | Cu(NO3)2(0.4 M)/NP | 0.1 | 4 | 92 |
| 6 | Cu(NO3)2(0.4 M)/NP | 0.15 | 3 | 95 |
| 7 | Cu(NO3)2(0.4 M)/NP | 0.2 | 2 | 98 |
| 8 | Cu(NO3)2 | 0.064 | 4 | 50 |
Click-reaction-base synthesis of 1,4-disubstituted-1,2,3-triazoles
The catalytic activity of Cu(II)/NP was studied in the 1,3-dipolar cycloaddition reactions using benzyl bromide (4a), sodium azide (5, 1.1 mmol) and phenyl acetylene (7a) as model substrates under various conditions. This three-component reaction proceeded via in situ formation of benzyl azide from benzyl bromide and sodium azide. Benzyl azide underwent the 1,3-dipolar cycloaddition reaction with phenyl acetylene (7a) to afford 1-benzyl-4-phenyl-1H-1,2,3-triazole (8a). This model reaction was carried out in different solvents with Cu(II)/NP (0.05 g) at room temperature (Table 4). Among the used solvents, the best result was earned from using methanol with 95% yield of the desired product 8a after 15 minutes (Table 4, entry 3). The model reaction with EtOH synthesized the product 8a with an excellent yield (90%) after a longer reaction time (60 minutes) (Table 4, entry 2).The catalyst loading of the model reaction was investigated in the synthesis of 1,4-disubstituted-1,2,3-triazole 8a (Table 5). 0.06 g of Cu(II)/NP (with 6.7 mol% Cu content) afforded the best yields with ∼100% isolated yield of 1,4-disubstituted-1,2,3-triazole 8a after 1 minute (Table 5, entry 6), whereas Cu(NO3)2·3H2O (0.026 g, 6.7 mol%) afforded 70% yield after 2 hours.
By implementing the optimized conditions (Cu(II)/NP 0.06 g, methanol (3 mL), room temperature), various aryl halides and sodium azide were reacted with various terminal acetylenes for the synthesis of 1,4-disubstituted-1,2,3-triazole 8 (Table 6). The desired 1,4-disubstituted-1,2,3-triazole derivatives were synthesized and characterized by checking reported melting points of related products in the literatures and spectroscopic methods. As shown in Table 6, corresponding products were obtained with excellent yields in short reaction times at room temperature.
| Entry | X | R2 | Ar2 | Product 8 | Time (min) | Isolated yield 8 (%) |
|---|---|---|---|---|---|---|
| 1 | Br | C6H5CH2 | C6H5 | 8a | 1 | 99 |
| 2 | Br | 4-BrC6H4CH2 | C6H5 | 8b | 5 | 99 |
| 3 | Br | 4-NO2C6H4CH2 | C6H5 | 8c | 10 | 97 |
| 4 | Cl | 3,5-Cl2C6H3CH2 | C6H5 | 8d | 10 | 98 |
| 5 | Cl | C6H5CH2 | C6H5 | 8e | 15 | 98 |
| 6 | Br | 2-ClC6H4CH2 | C6H5O | 8f | 20 | 96 |
| 7 | Cl | C6H5CH2 | C6H5O | 8g | 20 | 96 |
| 8 | Br | 4-BrC6H4CH2 | C6H5O | 8h | 20 | 95 |
| 9 | Br | 4-NO2C6H4CH2 | C6H5O | 8i | 25 | 95 |
| 10 | Cl | 3,5-Cl2C6H3CH2 | C6H5O | 8j | 25 | 96 |
| 11 | Cl | 2-ClC6H4CH2 | C6H5O | 8k | 20 | 96 |
| 12 | Br | C6H5CH2 | 4-Br-C6H4O | 8l | 15 | 97 |
| 13 | Br | C6H5CH2 | 3,5-Cl2-C6H3O | 8m | 20 | 95 |
| 14 | Br | 4-NO2C6H4CH2 | 4-Br-C6H4O | 8n | 10 | 96 |
| 15 | Br | 4-NO2C6H4CH2 | 3,5-Cl2-C6H3O | 8o | 15 | 94 |
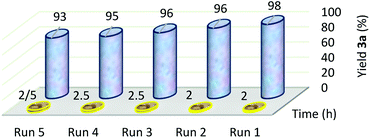
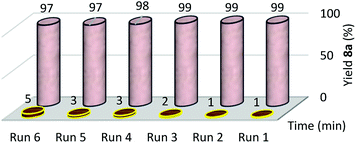
A proper supported catalyst should not leach to the reaction mixture. The recyclability of the supported catalyst is another important characteristic which should not suffer from mechanical degradation.81 As such, Cu(II)/NP did not suffer from extensive mechanical degradation after running the tests. Here, the separated Cu(II)/NP from the reaction was dried at room temperature and calcined at 105 °C for half an hour for its recovery and reuse. The catalyst leaching in the reaction media was evaluated by measuring the copper content in recycled Cu(II)/NP, by means of Inductively Coupled Plasma (ICP) analysis. From the ICP analysis, the copper contents of fresh catalyst and 5th run reused catalyst were 20.3 and 16.4 w/w%, respectively. The difference of copper contents between fresh and reused catalyst (5th run) was only 4 w/w%.20,82 After the calculation, 0.056 g of Cu(II) existed in 5th recycled Cu(II)/NP rather than 0.064 g of Cu(II) in fresh Cu(II)/NP, so 0.008 g of Cu(II) was removed (Table 3, entry 8). This indicated very low leaching amount of copper catalyst into the reaction mixture. So, Cu(II)/NP can be reused up to 5 runs without any significant decline in the yield during the synthesis of xanthene 3a (Fig. 7), and 6 runs during the synthesis of 1,4-disubstituted-1,2,3-triazole 8a (Fig. 8).
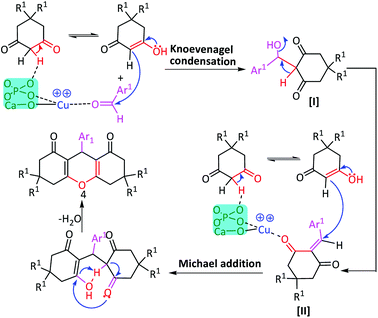
The surface of Cu(II)/NP possesses multiple catalytic active sites.83 The excellent catalytic efficiency was mainly corresponded to the surface cooperation between the Lewis basic sites of NP (oxygen in phosphate PO4 and calcium oxide CaO)84 and the Lewis acidic site of adjacent Cu+/Cu++ species (copper ion Cu2+ coordinated with the basic sites of NP surface).84,85 The basic sites on NP held the key role in the abstraction of protons from the methylene in 1,3-diketones.84 Further, the carbonyl group of aldehyde interacted with the acidic sites (Cu2+) of Cu(II)/NP, thereby, activated the carbonyl carbon for the nucleophilic attack. So, the Knoevenagel–Michael cascade reactions accelerated the Knoevenagel–Michael cascade reactions for the synthesis of xanthenes86 (Scheme 2). According to previous reports,83 a stepwise mechanism can be proposed for synthesizing 1,4-disubstituted-1,2,3-triazoles (Scheme 3). In stage 1, benzyl azide was formed in situ beside the terminal hydrogen of alkyne. It deprotonated in the basic medium of NP, while Cu(I) acetylide formed through the π-alkyne copper complex intermediate I. This π-coordination of alkyne with copper was activated to form σ-acetylide (stage 2). Then, the azide was activated by the coordination with copper to form intermediate II (stage 3). The coordination phenomena were synergistic for both reactive partners in intermediates I and II. Coordination of azide revealed the β-nucleophilic which resulted in vinylidene-like properties of acetylide. So, the azide's terminus became even more electrophilic and a strained copper triazolide B was formed (stage 4). Protonation of B, followed by the dissociation of product 8, completed the reaction, transferred the dehydrogenation of basic sites on NP (stage 5), and regenerated the catalyst Cu(II)/NP.
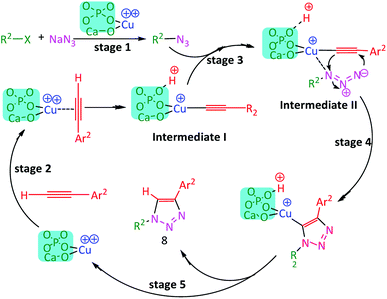 | ||
| Scheme 3 Plausible mechanism for one-pot CuAAC protocol synthesis of 1,4-disubstituted-1,2,3-triazole while using Cu(II)/NP catalyst. | ||
To illustrate the efficiency of present protocols for the synthesis of xanthene, our results are compared with the existing results in literature (Table 7). As shown in Table 7, the synthesis of xanthene with Fe+3-montmorillonite, alumina-sulfuric acid and SBSSA, the preparation of catalyst needs more time (at least 48 hours). It also includes several steps and working with hazardous and expensive materials (Table 7, entries 1, 2 and 5). By using other catalysts, e.g., SBSAN, ICl3/SiO2, In(CF3SO3)3, PANI-PTSA and Nano ZnAl2O4, the reactions are completed in a little shorter period of time than our process, however, they suffer from their difficult, multi-step and costly routs as well as extra material preparation (Table 7, entries 3–8). Using LUS-Pr-SO3H is accomplished under harsh reaction conditions, in a longer period, and multi-step preparation process which requires more devices (Table 7, entry 9). The reaction by [cmmim][BF4] as the catalyst takes place under microwave condition and suffers from the harsh reaction conditions (Table 7, entry 10). The reaction by NP needs a high amount of NP at a long reaction time (Table 7, entry 11).
| Entry | Catalyst | Condition | Time | Yield 3a (%) | Ref. |
|---|---|---|---|---|---|
| a Reaction conditions: dimedone (2 mmol), 4-chlorobenzaldehyde (1 mmol), catalyst.b Silica boron-sulfuric acid nano particles (SBSAN).c Nano ferrite-glutathione-copper (PANI-PTSA).d Silica bonded S-sulfonic acid (SBSSA).e Propyl sulfonic acid functionalized LUS-1 (Laval University silica) (LUS-Pr-SO3H). | |||||
| 1 | Fe+3-montmorillonite (15 wt%) | 100 °C/EtOH | 6 h | 97 | 87 |
| 2 | Alumina-sulfuric acid (200 mg) | Reflux/EtOH | 4 h | 83 | 88 |
| 3 | SBSANb (0.5 g) | 50 °C/solvent-free | 2 h | 95 | 89 |
| 4 | PANI-PTSAc (25 wt%) | Reflux/H2O | 6 h | 75 | 90 |
| 5 | SBSSAd (0.1 g) | Reflux/EtOH | 10 h | 98 | 91 |
| 6 | Nano ZnAl2O4 (90 mg) | Reflux/EtOH | 15 min | 88–98 | 92 |
| 7 | ICl3/SiO2 (5 mol%) | 75 °C/solvent free | 1 h | 90 | 28 |
| 8 | In(CF3SO3)3 (2 mol%) | 100 °C/solvent free | 1 h | 95 | 28 |
| 9 | LUS-Pr-SO3He (0.02 g) | 140 °C/solvent free | 15 min | 90 | 93 |
| 10 | [cmmim][BF4] (0.2 g) | 80 °C/solvent free, MW | 2 h | 94 | 94 |
| 11 | NP (1.5 g) | Reflux/EtOH | 6.5 h | 90 | 78 |
| 12 | Cu(II)/NP (0.2 g) | Reflux/EtOH | 2 h | 98 | This study |
To illustrate the efficiency of present protocols for the synthesis of 1,4-disubstituted-1,2,3-triazole derivatives, the results are compared with the existing results in literature (Table 8). Different catalysts are used for the synthesis of 1,4-disubstituted-1,2,3-triazole derivatives which suffer from several disadvantages. For example, by using Cu/Al2O3 and Cu/SiO2 as catalysts, the reactions are performed under harsh conditions, such as ball-milling and MW irradiation (Table 8, entries 2 and 4). The preparation process of some other catalysts, i.e., Cu(II)-PBS–HPMO and SiO2–NHC–Cu(I), is multistep at a long period of time under 100 °C and 80 °C, respectively (Table 8, entries 5 and 9). CuNP/C as the catalyst can result in a desired product with 99% yield but it needs 70 °C temperature and more time (Table 8, entry 6). Although, the reactions by using Cu(II)-TD@nSiO2 and PS-C22-Cu(I) complexes as catalysts are completed at room temperature but they suffer from hard and lengthy preparation process (Table 8, entries 8 and 10). Although the syntheses in the presence of some catalysts, like 2-pyrrole carbaldiminato-Cu(II) complex, Cu(I)-zeolite, Cu NP and PS-C22-CuI, were carried out at room temperature, they took much longer reaction times (Table 8, entries 1,3,7 and 10). The preparations of CuNPs/Mag Silica, MNP@PILCu, MNP@PDMA-Cu and Cu@βCD-PEG-mesoGO are also difficult or costly (Table 8, entries 11–14). The advantages of Cu(II)/NP as catalyst for the synthesis of 1,4-disubstituted-1,2,3-triazole 8a are listed in Table 8, entry 15.
| Entry | Catalyst | Condition | Time | Yield 8a (%) | Ref. |
|---|---|---|---|---|---|
| a Reaction condition: three-component reaction of the benzyl bromide, phenyl acetylene and NaN3.b Porphyrin-bridged silsesquioxane (PBS).c Copper nanoparticles on activated carbon (CuNP/C).d Nano-copper (CuNP).e Copper Immobilized on Nano silica Triazine Dendrimer.f N-heterocyclic carbene.g Polystyrene resin-supported copper(I) iodide-cryptand-22 complex.h Magnetic nanoparticles into the cross-linked poly(imidazole/imidazolium) immobilized Cu(II).i Copper sulphate onto multi-layered poly (2-dimethylaminoethyl acrylamide)-coated Fe3O4 nanoparticles.j Copper supported β-cyclodextrin functionalized PEGylated mesoporous silica nanoparticle-graphene oxide hybrid. | |||||
| 1 | 2-Pyrrole carbaldiminato–Cu(II) complex (0.005 mmol) | Rt/H2O | 13 h | 97 | 63 |
| 2 | Cu/Al2O3 (10 mol%) | Ball-milling, rt/neat | 1 h | 92 | 95 |
| 3 | Cu(I)-zeolite (10 mol%) | 20 °C/MeOH | 15 h | 80 | 96 |
| 4 | Cu/SiO2 (20 mol%) | MW, 70 °C/H2O | 10 min | 92 | 97 |
| 5 | Cu(II)-PBSb–HPMO (10 mg) | 100 °C/H2O | 3.5 h | 96 | 98 |
| 6 | CuNP/Cc (0.5 mol%) | 70 °C/H2O | 6 h | 99 | 99 |
| 7 | CuNPd (0.025 mmol) | rt/MeOH | 8 h | 93 | 100 |
| 8 | Cu(II)-TD@nSiO2e (0.3 mol%) | rt/sodium ascorbate, H2O/EtOH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
20 min | 99 | 101 |
| 9 | SiO2–NHCh-Cu(I)f (1 mol%) | 80 °C/H2O | 6 h | 98 | 102 |
| 10 | PS-C22-CuIg (0.6 mol%) | Rt/H2O | 15 h | 99 | 61 |
| 11 | CuNPs/Mag silica (4.3 mol%) | 70 °C/H2O | 1 h | 98 | 103 |
| 12 | MNP@PILCuh (4 mg) | 50 °C/H2O | 2.5 h | 95 | 104 |
| 13 | MNP@PDMA-Cui (0.3 mol%) | 50 °C/H2O, sodium ascorbate | 2 h | 96 | 74 |
| 14 | Cu@βCD-PEG-mesoGOj (5 mol%) | 25 °C/H2O | 1 h | 90 | 105 |
| 15 | Cu(II)/NP (0.06 g) | rt/MeOH | 1 min | 99 | This study |
Conclusions
In summary, we established copper nitrate, supported on meso-pores of natural phosphate (Cu(II)/NP), as new, inexpensive, eco-friendly and recyclable heterogeneous catalyst for the synthesis of heteroatom-containing chemicals including xanthene and 1,4-disubstituted-1,2,3-triazole in good to excellent yields. Cu(II)/NP efficiently catalyzed the synthesis of xanthenes by the condensation of aromatic aldehydes with 1,3-cyclic diketones in ethanol under the reflux condition. Cu(II)/NP also showed as an efficient catalyzer in the regioselective synthesis of 1,4-disubstituted-1,2,3-triazoles via azide/alkyne ‘‘click’’-reaction or CuAAC reaction at room temperature. The reaction for the synthesis of 1,4-disubstituted-1,2,3-triazoles proceeded by using stable Cu(II) instead of unstable Cu(I) species in the absence of any ligand or base. By using the heterogeneous catalyst Cu(II)/NP, the processes afforded the products with high yields in short reaction times, while the preparation process of the catalyst was easy, using inexpensive materials, recyclable, environmentally benign, stable, and almost leaching-free. The use of natural phosphate (NP) as a support for Cu(II) provides the advantages due to its excellent stability (both chemical and thermal), high surface area, low cost, and availability.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We are grateful of Payame Noor University (PNU) for their partial support, Mazandaran University of Medical Sciences for providing laboratory facilities to carry out this research and Australian College of Kuwait for the funding of IRC-2017-18-SOE-ME-PR01 and PR02 is also acknowledged.Notes and references
- M. J. Hülsey, H. Yang and N. Yan, ACS Sustainable Chem. Eng., 2018, 6, 5694–5707 CrossRef.
- X. Chen, H. Yang, M. J. Hülsey and N. Yan, ACS Sustainable Chem. Eng., 2017, 5, 11096–11104 CrossRef CAS.
- S. Rostamnia and E. Doustkhah, RSC Adv., 2014, 4, 28238–28248 RSC.
- F. Rajabi, M. Pinilla-de Dios and R. Luque, Catalysts, 2017, 7, 216 CrossRef.
- F. Bazi, H. El Badaoui, S. Tamani, S. Sokori, L. Oubella, M. Hamza, S. Boulaajaj and S. Sebti, J. Mol. Catal. A: Chem., 2006, 256, 43–47 CrossRef CAS.
- M. Zhang, Y. Zhao, Q. Liu, L. Yang, G. Fan and F. Li, Dalton Trans., 2016, 45, 1093–1102 RSC.
- A. Chen and P. Holt-Hindle, Chem. Rev., 2010, 110, 3767–3804 CrossRef CAS PubMed.
- D. Kim, C. S. Kley, Y. Li and P. Yang, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 10560–10565 CrossRef CAS PubMed.
- R. van den Berg, G. Prieto, G. Korpershoek, L. I. van der Wal, A. J. van Bunningen, S. Lægsgaard-Jørgensen, P. E. de Jongh and K. P. de Jong, Nat. Commun., 2016, 7, 13057 CrossRef CAS PubMed.
- B. S. Anandakumar, M. B. M. Reddy, C. N. Tharamani, M. A. Pasha and G. T. Chandrappa, Chin. J. Catal., 2013, 34, 704–710 CrossRef CAS.
- M. Domínguez, F. Romero-Sarria, M. Centeno and J. Odriozola, Appl. Catal., B, 2009, 87, 245–251 CrossRef.
- Z. Weng, Y. Wu, M. Wang, J. Jiang, K. Yang, S. Huo, X.-F. Wang, Q. Ma, G. W. Brudvig, V. S. Batista, Y. Liang, Z. Feng and H. Wang, Nat. Commun., 2018, 9, 415 CrossRef PubMed.
- T. Yasukawa, A. Suzuki, H. Miyamura, K. Nishino and S. Kobayashi, J. Am. Chem. Soc., 2015, 137, 6616–6623 CrossRef CAS PubMed.
- J. Jia, C. Qian, Y. Dong, Y. F. Li, H. Wang, M. Ghoussoub, K. T. Butler, A. Walsh and G. A. Ozin, Chem. Soc. Rev., 2017, 46, 4631–4644 RSC.
- A. Smahi, A. Solhy, R. Tahir, S. Sebti, J. A. Mayoral, J. I. García, J. M. Fraile and M. Zahouily, Catal. Commun., 2008, 9, 2503–2508 CrossRef CAS.
- S. d. Sebti, A. Solhy, R. Tahir, S. Abdelatif, S. d. Boulaajaj, J. A. Mayoral, J. I. García, J. M. Fraile, A. Kossir and H. Oumimoun, J. Catal., 2003, 213, 1–6 CrossRef CAS.
- Q. Y. Ma, T. J. Logan and S. J. Traina, Environ. Sci. Technol., 1995, 29, 1118–1126 CrossRef CAS PubMed.
- A. Aklil, M. Mouflih and S. Sebti, J. Hazard. Mater., 2004, 112, 183–190 CrossRef CAS PubMed.
- M. Vila, S. Sánchez-Salcedo, M. Cicuéndez, I. Izquierdo-Barba and M. Vallet-Regí, J. Hazard. Mater., 2011, 192, 71–77 CAS.
- X. Cao, L. Q. Ma, D. R. Rhue and C. S. Appel, Environ. Pollut., 2004, 131, 435–444 CrossRef CAS PubMed.
- M. Šljivić, I. Smičiklas, I. Plećaš and M. Mitrić, Chem. Eng. J., 2009, 148, 80–88 CrossRef.
- E. Vieira, J. Huwyler, S. Jolidon, F. Knoflach, V. Mutel and J. Wichmann, Bioorg. Med. Chem. Lett., 2005, 15, 4628–4631 CrossRef CAS PubMed.
- H. Hafez, M. Hegab, I. Ahmed-Farag and A. El-Gazzar, Bioorg. Med. Chem. Lett., 2008, 18, 4538–4543 CrossRef CAS PubMed.
- Y. Kitahara and K. Tanaka, Chem. Commun., 2002, 932–933 RSC.
- B. Maleki, A. Davoodi, M. V. Azghandi, M. Baghayeri, E. Akbarzadeh, H. Veisi, S. S. Ashrafi and M. Raei, New J. Chem., 2016, 40, 1278–1286 RSC.
- C. G. Knight and T. Stephens, Biochem. J., 1989, 258, 683–687 CrossRef CAS PubMed.
- M. Ahmad, T. A. King, D.-K. Ko, B. H. Cha and J. Lee, J. Phys. D: Appl. Phys., 2002, 35, 1473 CrossRef CAS.
- B. Karami, K. Esk and G. Ansari, Chem. Sin., 2017, 8, 342–354 CAS.
- F. N. Sadeh, M. Fatahpour, N. Hazeri, M. T. Maghsoodlou and M. Lashkari, Acta Chem. Iasi, 2017, 25, 24–37 Search PubMed.
- S. Rezayati, H. Seifournia and E. Mirzajanzadeh, Asian J. Green Chem., 2017, 1, 24–33 CrossRef.
- A. Khazaei, M. Rezaei, A. R. Moosavi-Zare and S. Saednia, J. Chin. Biochem. Soc., 2017, 64, 1088–1095 CrossRef CAS.
- F. S. Arbosara, F. Shirini, M. Abedini and H. F. Moafi, J. Nanostruct. Chem., 2015, 5, 55–63 CrossRef CAS.
- A. Javid, M. M. Heravi and F. F. Bamoharram, J. Chem., 2011, 8, 910–916 CAS.
- P. Sivaguru and A. Lalitha, Chin. Chem. Lett., 2014, 25, 321–323 CrossRef CAS.
- M. Esmaeilpour, J. Javidi, F. Dehghani and F. Nowroozi Dodeji, New J. Chem., 2014, 38, 5453–5461 RSC.
- R. N. Baig and R. S. Varma, Green Chem., 2012, 14, 625–632 RSC.
- L. Kashmiri and Y. Pinki, Anti-Cancer Agents Med. Chem., 2018, 18, 21–37 CrossRef PubMed.
- B. Wang, B. Zhao, Z.-S. Chen, L.-P. Pang, Y.-D. Zhao, Q. Guo, X.-H. Zhang, Y. Liu, G.-Y. Liu, Z. Hao, X.-Y. Zhang, L.-Y. Ma and H.-M. Liu, Eur. J. Med. Chem., 2018, 143, 1535–1542 CrossRef CAS PubMed.
- I. Mohammed, I. R. Kummetha, G. Singh, N. Sharova, G. Lichinchi, J. Dang, M. Stevenson and T. M. Rana, J. Med. Chem., 2016, 59, 7677–7682 CrossRef CAS PubMed.
- C. Wu, B. Eck, S. Zhang, J. Zhu, A. D. Tiwari, Y. Zhang, Y. Zhu, J. Zhang, B. Wang, X. Wang, X. Wang, J. You, J. Wang, Y. Guan, X. Liu, X. Yu, B. D. Trapp, R. Miller, J. Silver, D. Wilson and Y. Wang, J. Med. Chem., 2017, 60, 987–999 CrossRef CAS PubMed.
- D. Dheer, V. Singh and R. Shankar, Medicinal Attributes of 1,2,3-Triazoles, Current Developments, 2017 Search PubMed.
- A. Espinoza-Vazquez, F. J. Rodriguez-Gomez, B. I. Vergara-Arenas, L. Lomas-Romero, D. Angeles-Beltran, G. E. Negron-Silva and J. A. Morales-Serna, RSC Adv., 2017, 7, 24736–24746 RSC.
- N. P. Debia, M. T. Saraiva, B. S. Martins, R. Beal, P. F. B. Gonçalves, F. S. Rodembusch, D. Alves and D. S. Lüdtke, J. Org. Chem., 2018, 83, 1348–1357 CrossRef CAS PubMed.
- K. D. Gavlik, E. S. Sukhorukova, Y. M. Shafran, P. A. Slepukhin, E. Benassi and N. P. Belskaya, Dyes Pigm., 2017, 136, 229–242 CrossRef CAS.
- F. Alonso, Y. Moglie, G. Radivoy and M. Yus, Adv. Synth. Catal., 2010, 352, 3208–3214 CrossRef CAS.
- C. W. Tornøe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057–3064 CrossRef.
- M. S. Singh, S. Chowdhury and S. Koley, Tetrahedron, 2016, 72, 5257–5283 CrossRef CAS.
- M. Meldal and C. W. Tornøe, Chem. Rev., 2008, 108, 2952–3015 CrossRef CAS PubMed.
- V. O. Rodionov, S. I. Presolski, S. Gardinier, Y.-H. Lim and M. Finn, J. Am. Chem. Soc., 2007, 129, 12696–12704 CrossRef CAS PubMed.
- F. Himo, T. Lovell, R. Hilgraf, V. V. Rostovtsev, L. Noodleman, K. B. Sharpless and V. V. Fokin, J. Am. Chem. Soc., 2005, 127, 210–216 CrossRef CAS PubMed.
- S. Quader, S. E. Boyd, I. D. Jenkins and T. A. Houston, J. Org. Chem., 2007, 72, 1962–1979 CrossRef CAS PubMed.
- A. Sarkar, T. Mukherjee and S. Kapoor, J. Phys. Chem. C, 2008, 112, 3334–3340 CrossRef CAS.
- S. Chassaing, M. Kumarraja, A. Sani Souna Sido, P. Pale and J. Sommer, Org. Lett., 2007, 9, 883–886 CrossRef CAS.
- I. Jlalia, H. Elamari, F. Meganem, J. Herscovici and C. Girard, Tetrahedron Lett., 2008, 49, 6756–6758 CrossRef CAS.
- T. Miao and L. Wang, Synthesis, 2008, 2008, 363–368 CrossRef.
- B. H. Lipshutz and B. R. Taft, Angew. Chem., Int. Ed., 2006, 45, 8235–8238 CrossRef CAS.
- M. L. Kantam, V. S. Jaya, B. Sreedhar, M. M. Rao and B. M. Choudary, J. Mol. Catal. A: Chem., 2006, 256, 273–277 CrossRef CAS.
- K. Yamaguchi, T. Oishi, T. Katayama and N. Mizuno, Chem.–Eur. J., 2009, 15, 10464–10472 CrossRef CAS PubMed.
- G. Molteni, C. L. Bianchi, G. Marinoni, N. Santo and A. Ponti, New J. Chem., 2006, 30, 1137–1139 RSC.
- D. Wang, N. Li, M. Zhao, W. Shi, C. Ma and B. Chen, Green Chem., 2010, 12, 2120–2123 RSC.
- B. Movassagh and N. Rezaei, Tetrahedron, 2014, 70, 8885–8892 CrossRef CAS.
- K. Lal and P. Rani, ARKIVOC, 2016, i, 307–341 Search PubMed.
- C. Zhou, J. Zhang, P. Liu, J. Xie and B. Dai, RSC Adv., 2015, 5, 6661–6665 RSC.
- J. M. Fraile, J. I. García, J. A. Mayoral, S. Sebti and R. Tahir, Green Chem., 2001, 3, 271–274 RSC.
- S. Kantevari, R. Bantu and L. Nagarapu, J. Mol. Catal. A: Chem., 2007, 269, 53–57 CrossRef CAS.
- V. Thanikachalam, A. Arunpandiyan, J. Jayabharathi, C. Karunakaran and P. Ramanathan, RSC Adv., 2014, 4, 62144–62152 RSC.
- C. W. Tornøe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057–3064 CrossRef.
- J. C. Elliott, Structure and chemistry of the apatites and other calcium orthophosphates, Elsevier, 2013 Search PubMed.
- H. R. Ramananarivo, A. Solhy, J. Sebti, A. Smahi, M. Zahouily, J. Clark and S. Sebti, ACS Sustainable Chem. Eng., 2013, 1, 403–409 CrossRef CAS.
- A. Saber, A. Smahi, A. Solhy, R. Nazih, B. Elaabar, M. Maizi and S. d. Sebti, J. Mol. Catal. A: Chem., 2003, 202, 229–237 CrossRef CAS.
- S. Brunauer, L. S. Deming, W. E. Deming and E. Teller, J. Am. Chem. Soc., 1940, 62, 1723–1732 CrossRef CAS.
- A. J. Nathanael, D. Mangalaraj, S. Hong, Y. Masuda, Y. Rhee and H. Kim, Mater. Chem. Phys., 2013, 137, 967–976 CrossRef.
- S. Kim, S. W. Kang, A. Kim, M. Yusuf, J. C. Park and K. H. Park, RSC Adv., 2018, 8, 6200–6205 RSC.
- N. Zohreh, S. H. Hosseini, A. Pourjavadi and C. Bennett, Appl. Organomet. Chem., 2016, 30, 73–80 CrossRef CAS.
- M. Bagherzadeh, H. Mahmoudi, M. Amini, S. Gautam and K. H. Chae, Sci. Iran., 2018, 25, 1335–1343 Search PubMed.
- B. J. Borah, D. Dutta, P. P. Saikia, N. C. Barua and D. K. Dutta, Green Chem., 2011, 13, 3453–3460 RSC.
- B. Małecka, A. Łącz, E. Drożdż and A. Małecki, J. Therm. Anal. Calorim., 2015, 119, 1053–1061 CrossRef.
- A. Fallah, M. Tajbakhsh, H. Vahedi and A. Bekhradnia, Res. Chem. Intermed., 2017, 43, 29–43 CrossRef CAS.
- A. Małecki, R. Gajerski, S. Łabuś, B. Prochowska-Klisch and K. Wojciechowski, J. Therm. Anal. Calorim., 2000, 60, 17–23 CrossRef.
- X. Gao, X. Chen, J. Zhang, W. Guo, F. Jin and N. Yan, ACS Sustainable Chem. Eng., 2016, 4, 3912–3920 CrossRef CAS.
- J. Albadi, M. Keshavarz, M. Abedini and M. Vafaie-Nezhad, Chin. Chem. Lett., 2012, 23, 797–800 CrossRef CAS.
- A. Corami, S. Mignardi and V. Ferrini, Acta Geol. Sin. (Engl. Ed.), 2008, 82, 1223–1228 CrossRef CAS.
- B. A. Dar, A. Bhowmik, A. Sharma, P. R. Sharma, A. Lazar, A. Singh, M. Sharma and B. Singh, Appl. Clay Sci., 2013, 80, 351–357 CrossRef.
- J. Bennazha, M. Zahouily, S. Sebti, A. Boukhari and E. Holt, Catal. Commun., 2001, 2, 101–104 CrossRef CAS.
- J. C. Védrine, Catalysts, 2017, 7, 341 CrossRef.
- T. R. Mandlimath, B. Umamahesh and K. I. Sathiyanarayanan, J. Mol. Catal. A: Chem., 2014, 391, 198–207 CrossRef CAS.
- G. Song, B. Wang, H. Luo and L. Yang, Catal. Commun., 2007, 8, 673–676 CrossRef CAS.
- A. Pramanik and S. Bhar, Catal. Commun., 2012, 20, 17–24 CrossRef CAS.
- A. Khalafi-Nezhad, F. Panahi, S. Mohammadi and H. O. Foroughi, J. Iran. Chem. Soc., 2013, 10, 189–200 CrossRef CAS.
- A. John, P. J. P. Yadav and S. Palaniappan, J. Mol. Catal. A: Chem., 2006, 248, 121–125 CrossRef CAS.
- K. Niknam, F. Panahi, D. Saberi and M. Mohagheghnejad, J. Heterocycl. Chem., 2010, 47, 292 CAS.
- T. R. Mandlimath, B. Umamahesh and K. I. Sathiyanarayanan, J. Mol. Catal. A: Chem., 2014, 391, 198–207 CrossRef CAS.
- M. Rahimifard, G. M. Ziarani, A. Badiei, S. Asadi and A. A. Soorki, Res. Chem. Intermed., 2016, 42, 3847–3861 CrossRef CAS.
- A. N. Dadhania, V. K. Patel and D. K. Raval, J. Saudi Chem. Soc., 2017, 21, S163–S169 CrossRef CAS.
- N. Mukherjee, S. Ahammed, S. Bhadra and B. C. Ranu, Green Chem., 2013, 15, 389–397 RSC.
- V. Bénéteau, A. Olmos, T. Boningari, J. Sommer and P. Pale, Tetrahedron Lett., 2010, 51, 3673–3677 CrossRef.
- C. S. Radatz, L. d. A. Soares, E. R. Vieira, D. Alves, D. Russowsky and P. H. Schneider, New J. Chem., 2014, 38, 1410–1417 RSC.
- A. N. Prasad, B. M. Reddy, E.-Y. Jeong and S.-E. Park, RSC Adv., 2014, 4, 29772–29781 RSC.
- F. Alonso, Y. Moglie, G. Radivoy and M. Yus, Org. Biomol. Chem., 2011, 9, 6385–6395 RSC.
- L. Huang, W. Liu, J. Wu, Y. Fu, K. Wang, C. Huo and Z. Du, Tetrahedron Lett., 2014, 55, 2312–2316 CrossRef CAS.
- M. Nasr-Esfahani, I. Mohammadpoor-Baltork, A. R. Khosropour, M. Moghadam, V. Mirkhani, S. Tangestaninejad and H. Amiri Rudbari, J. Org. Chem., 2014, 79, 1437–1443 CrossRef CAS PubMed.
- P. Li, L. Wang and Y. Zhang, Tetrahedron, 2008, 64, 10825–10830 CrossRef CAS.
- F. Nador, M. A. Volpe, F. Alonso, A. Feldhoff, A. Kirschning and G. Radivoy, Appl. Catal., A, 2013, 455, 39–45 CrossRef CAS.
- A. Pourjavadi, S. H. Hosseini, N. Zohreh and C. Bennett, RSC Adv., 2014, 4, 46418–46426 RSC.
- S. Bahadorikhalili, L. Ma'mani, H. Mahdavi and A. Shafiee, Microporous Mesoporous Mater., 2018, 262, 207–216 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra08260j |
| This journal is © The Royal Society of Chemistry 2018 |