Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
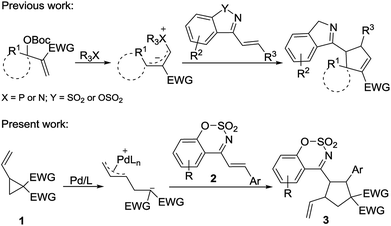
Pd-catalyzed [3 + 2] cycloaddition of vinylcyclopropanes with 1-azadienes: synthesis of 4-cyclopentylbenzo[e][1,2,3]oxathiazine 2,2-dioxides†
Yan Lin,
Qijun Wang,
Yang Wu,
Chang Wang,
Hao Jia,
Cheng Zhang ,
Jiaxing Huang* and
Hongchao Guo
,
Jiaxing Huang* and
Hongchao Guo *
*
Department of Applied Chemistry, China Agricultural University, Beijing 100193, China. E-mail: hchguo@cau.edu.cn; 05084@cau.edu.cn
First published on 5th December 2018
Abstract
The palladium-catalyzed [3 + 2] cycloaddition of vinylcyclopropanes and 1-azadienes has been developed under mild reaction conditions, giving the multisubstituted cyclopentane derivatives in good to excellent yields with moderate to good diastereoselectivities. The relative configuration of both diastereomers of the products have been determined through X-ray crystallographic diffraction.
The cyclopentane framework is ubiquitous in nature and it is also an important structural moiety in many pharmaceuticals, agrochemicals, and materials.1 The development of simple, fast and efficient synthetic methods for highly substituted cyclopentanes has attracted much attention. Among various methods for synthesis of cyclopentane structure, the cycloaddition reaction is a very attractive one.2
Under palladium catalysis conditions, vinylcyclopropane derivatives underwent a ring-opening reaction to generate the zwitterionic allylpalladium intermediate, which reacted with carbon–carbon, carbon–oxygen, carbon–nitrogen double bonds and diazo compounds to provide a variety of five-membered cyclic compounds.3 In the past decades, this type of annulation reactions has emerged as a powerful tool for the synthesis of carbocyclic and heterocyclic compounds.2 Diverse substrates including isocyanates,4 aldehydes,5 isatins,6 3-diazooxindoles,7 electron-deficient alkenes such as para-quinone methides,8 α,β-unsaturated aldehydes,9 β,γ-unsaturated α-keto esters,10 nitroolefins,11 azlactone- and Meldrum's acid alkylidenes,12 and α-nucleobase substituted acrylates,13 have been exploited in palladium-catalyzed [3 + 2] cycloadditions of vinyl cyclopropane, delivering biologically interesting functionalized heterocyclic compounds and cyclopentane derivatives. In 2015, Liu and He reported a palladium-catalyzed [3 + 2] cycloaddition of vinyl cyclopropane and α,β-unsaturated imines generated in situ from aryl sulfonyl indoles, providing the optically enriched spirocyclopentane-1,3′-indolenines with high diastereoselectivity.14 This is the only example where α,β-unsaturated imines were employed in Pd-catalyzed [3 + 2] cycloaddition reaction of vinyl cyclopropane.
As a type of α,β-unsaturated imines, cyclic 1-azadienes such as (E)-4-styrylbenzo[e][1,2,3]oxathiazine 2,2-dioxides 2 are easily accessible and stable (Scheme 1). In particular, they contain the sulfonate-moiety, which is an interesting biologically important motif, and has a great potential in the synthesis of bioactive molecules.15 The cyclic 1-azadienes have been used in a series of annulation reactions such as [2 + n],16–18 [3 + n],19 and [4 + n]20 annulation reactions. Based on the electron-deficient nature of the carbon–carbon double bond in these cyclic 1-azadienes, in 2016, Chen and Ouyang developed cinchona-derived tertiary amine-catalyzed asymmetric [3 + 2] annulation of isatin-derived Morita–Baylis–Hillman (MBH) carbonates with cyclic 1-azadienes to form spirooxindole.17 In the same year, our group demonstrated a phosphine-catalyzed [3 + 2] annulation of MBH carbonates with cyclic 1-azadienes.18 Considering that the 1-azadiene 2 is an electron-deficient alkene with good reactivity, its reaction with a zwitterionic π-allyl Pd complex formed via ring-opening of vinylcyclopropane may be feasible. However, this type of cyclic 1-azadienes have never been used in Pd-catalyzed annulation reactions involving vinylcyclopropanes. As our continuing interest on cycloaddition reactions,21 herein we disclose a [3 + 2] cycloaddition of palladium-catalyzed vinyl cyclopropane with cyclic 1-azadienes to afford the multisubstituted cyclopentane derivatives (Scheme 1).
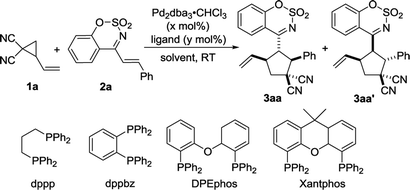
We carried out an initial screening with 2-vinylcyclopropane-1,1-dicarbonitrile 1a and (E)-4-styrylbenzo[e][1,2,3]oxathiazine 2,2-dioxide 2a in CH2Cl2 (DCM) at room temperature in the presence of Pd2(dba)3·CHCl3 (2.5 mol%) (Table 1, entry 1). The reaction worked but required 24 hours to make full conversion, furnishing the desired [3 + 2] cycloadduct 3 in 96% yield with 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (entry 1). Several phosphines including PPh3 and bidentate phosphines were next screened as ligands. It was found that the reaction efficiency was remarkably increased in the presence of phosphines (entries 2–6). With the use of the diphoshhine Xantphos as ligand, the reaction time was shortened to 0.5 h and the yield remained at 96%. Decreasing the catalyst loading to 1%, the product could still be obtained in 86%yield with 6
1 dr (entry 1). Several phosphines including PPh3 and bidentate phosphines were next screened as ligands. It was found that the reaction efficiency was remarkably increased in the presence of phosphines (entries 2–6). With the use of the diphoshhine Xantphos as ligand, the reaction time was shortened to 0.5 h and the yield remained at 96%. Decreasing the catalyst loading to 1%, the product could still be obtained in 86%yield with 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (entry 7). A quick screening of solvents such as toluene, THF, 1,2-dichloroethane (DCE) and MeCN was performed. When toluene and THF were employed as the solvent, the nearly same results as that in CH2Cl2 were observed (entries 8–9). However, using DCE or MeCN as solvent led to a slightly lower diastereoselectivity (entries 10–11). On the basis of the above investigation, the optimal reaction conditions was determined as follow: using Pd2(dba)3·CHCl3 (2.5 mol%) and Xantphos (5.0 mol%) as catalyst in CH2Cl2 at room temperature. The relative configurations of two diastereomers were determined by single crystal X-ray analysis of the product 3aa and 3aa′.22 Under the optimized reaction conditions, we attempted to develop asymmetric variant of this reaction. Unfortunately, the two enantiomers of the product could not be resolved at present stage.23
1 dr (entry 7). A quick screening of solvents such as toluene, THF, 1,2-dichloroethane (DCE) and MeCN was performed. When toluene and THF were employed as the solvent, the nearly same results as that in CH2Cl2 were observed (entries 8–9). However, using DCE or MeCN as solvent led to a slightly lower diastereoselectivity (entries 10–11). On the basis of the above investigation, the optimal reaction conditions was determined as follow: using Pd2(dba)3·CHCl3 (2.5 mol%) and Xantphos (5.0 mol%) as catalyst in CH2Cl2 at room temperature. The relative configurations of two diastereomers were determined by single crystal X-ray analysis of the product 3aa and 3aa′.22 Under the optimized reaction conditions, we attempted to develop asymmetric variant of this reaction. Unfortunately, the two enantiomers of the product could not be resolved at present stage.23
| Entry | x | L (y mol%) | Solvent | t/h | Yield (%) | 3aa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3aa′b 3aa′b |
|---|---|---|---|---|---|---|
| a Unless otherwise stated, all reactions were carried out with 1a (0.12 mmol), 2a (0.10 mmol) and catalyst in solvent (2 mL) at room temperature.b Determined by isolated yield. | ||||||
| 1 | 2.5 | — | DCM | 24 | 96 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | 2.5 | PPh3 (10) | DCM | 5 | 81 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | 2.5 | dppp (5.0) | DCM | 5 | 97 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | 2.5 | DPEphos (5) | DCM | 5 | 89 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | 2.5 | dppbz (5) | DCM | 15 | 95 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | 2.5 | Xantphos (5) | DCM | 0.5 | 96 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | 1.0 | Xantphos (2) | DCM | 0.5 | 86 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | 2.5 | Xantphos (5) | Toluene | 0.5 | 97 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | 2.5 | Xantphos (5) | THF | 0.5 | 92 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | 2.5 | Xantphos (5) | DCE | 1.5 | 99 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11 | 2.5 | Xantphos (5) | MeCN | 5 | 68 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
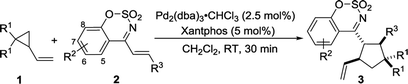
Having the optimized reaction condition in hand, the generality of Pd-catalyzed [3 + 2] cycloaddition of 2-vinylcyclopropane-1,1-dicarbonitrile 1a was scrutinized by using a series of cyclic 1-azadienes 2b–2n (Table 2, entries 2–14). A wide range of substituents on the 1-azadienes 2 were well tolerated in the reaction with the 2-vinylcyclopropane-1,1-dicarbonitrile 1a, giving desired cycloadducts 3aa–3an in high to excellent yields (78–99%) with moderate diastereoselectivities (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–6
1–6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). Regardless of electron-donating groups such as Me and MeO, electron-withdrawing groups such as F, Cl and Br, and their positions on benzene ring, the yields of the corresponding products were satisfactory. Interestingly, 4-Cl, 4-Br or 3,4-dimethoxy substituted 1-azadiene delivered a relative lower yield of product, compared with other substrates (entries 5, 6, 13 vs. 1–4, 7–12). The yield significantly decreased to 78% when a electron-donating methyl group was introducted onto benzo[e][1,2,3] oxathiazine 2,2-dioxide moiety (entry 14). When employing dimethyl 2-vinylcyclopropane-1,1-dicarboxylate 1b instead of 2-vinylcyclopropane-1,1-dicarbonitrile 1a to react with 2a, the corresponding product 3ba was obtained in 86% yield albeit with a 2
1 dr). Regardless of electron-donating groups such as Me and MeO, electron-withdrawing groups such as F, Cl and Br, and their positions on benzene ring, the yields of the corresponding products were satisfactory. Interestingly, 4-Cl, 4-Br or 3,4-dimethoxy substituted 1-azadiene delivered a relative lower yield of product, compared with other substrates (entries 5, 6, 13 vs. 1–4, 7–12). The yield significantly decreased to 78% when a electron-donating methyl group was introducted onto benzo[e][1,2,3] oxathiazine 2,2-dioxide moiety (entry 14). When employing dimethyl 2-vinylcyclopropane-1,1-dicarboxylate 1b instead of 2-vinylcyclopropane-1,1-dicarbonitrile 1a to react with 2a, the corresponding product 3ba was obtained in 86% yield albeit with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (entry 15).
1 dr (entry 15).
| Entry | R1 in 1 | R2, R3 in 2 | 3 | Yield (%) | drb |
|---|---|---|---|---|---|
| a Unless otherwise stated, all reactions were carried out with 1 (0.18 mmol), 2 (0.15 mmol) and catalyst in CH2Cl2 (3 mL) at room temperature for 30 minutes.b Determined by 1H NMR analysis.c After 24 h, the starting material was completely consumed (monitored by TLC). | |||||
| 1 | CN (1a) | H, Ph (2a) | 3aa | 96 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | CN (1a) | H, 2-FC6H4 (2b) | 3ab | 98 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | CN (1a) | H, 3-FC6H4 (2c) | 3ac | 95 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | CN (1a) | H, 4-FC6H4 (2d) | 3ad | 98 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | CN (1a) | H, 4-ClC6H4 (2e) | 3ae | 81 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | CN (1a) | H, 4-BrC6H4 (2f) | 3af | 80 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | CN (1a) | H, 2-MeC6H4 (2g) | 3ag | 99 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | CN (1a) | H, 3-MeC6H4 (2h) | 3ah | 95 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | CN (1a) | H, 4-MeC6H4 (2i) | 3ai | 95 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | CN (1a) | H, 2-OMeC6H4 (2j) | 3aj | 99 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11 | CN (1a) | H, 3-OMeC6H4 (2k) | 3ak | 94 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12 | CN (1a) | H, 4-OMeC6H4 (2l) | 3al | 99 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 13 | CN (1a) | H, 3,4-OMe2C6H3 (2m) | 3am | 80 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 14 | CN (1a) | 6-Me, Ph (2n) | 3an | 78 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 15c | CO2Me (1b) | H, Ph (2a) | 3ba | 86 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
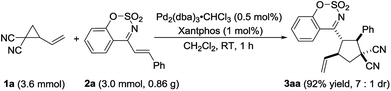
To further demonstrate the reaction to be a practical tool for the synthesis of polysubstituted cyclopentane derivatives, the reaction was carried out on the gram scale. We were satisfied to found that when decreasing the loading of palladium/ligand to 0.5%/1.0%, the reaction still worked very efficiently and completed in one hour to provide the product 3aa in 92% yield with 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (Scheme 2).
1 dr (Scheme 2).
Conclusions
In conclusion, we have developed a new method to access the functionalized polysubstituted cyclopentane derivatives in good to excellent yields, employing palladium-catalyzed [3 + 2] cycloaddition between vinylcyclopropanes and 1-azadienes under mild reaction conditions. The reaction tolerated a wide range of substrates and could be performed on the gram scale, showing that it is a practical tool for synthesis of biologically interesting cyclopentane derivatives.Experimental
General methods
All reactions were performed under argon atmosphere. Infrared spectra were recorded using an FT-IR spectrophotometer. 1H and 13C NMR spectra were recorded using a 300 MHz NMR instrument. Accurate mass measurements were performed on an electrospray ionization (ESI) apparatus using time-of-flight (TOF) mass spectrometry. Melting points were determined on a melting apparatus. 1,1-Disubstituted-2-vinylcyclopropanes 1 were prepared according to the literature procedure.24 (E)-4-Styrylbenzo[e][1,2,3]oxathiazine 2,2-dioxides 2 were synthesized according to the literature procedure.25General procedure for [3 + 2] annulation reaction
An oven-dried 10 mL of Schlenk tube was charged with 1-azadiene 2 (0.15 mmol), vinylcyclopropane 1 (1.2 equiv., 0.18 mmol), Pd2(dba)3·CHCl3 (0.025 equiv., 3.9 mg), Xantphos (0.05 equiv., 4.3 mg) in 3 mL of CH2Cl2 for corresponding time under argon atmosphere at room temperature. Once the starting material was completely consumed (monitored by TLC), the mixture was concentrated to dryness. The residue was purified by flash column chromatography (ethyl acetate/petroleum ether = 1/5) to afford the corresponding cycloaddition product 3.General procedure for preparation of 3aa on the gram scale
Under argon atmosphere, to a mixture of 1-azadiene 2a (3 mmol, 0.86 g), Pd2(dba)3·CHCl3 (0.005 equiv., 15.5 mg), Xantphos (0.01 equiv., 17.4 mg) in 45 mL of acetonitrile, 2-vinylcyclopropane-1,1-dicarbonitrile 1a (1.2 equiv., 3.6 mmol, 0.42 g) were added at room temperature. The resulting mixture was stirred until the starting material was completely consumed (monitored by TLC) and then was concentrated to dryness. The residue was purified through flash column chromatography (EtOAc/PE 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to afford the corresponding annulation product 3aa.
5) to afford the corresponding annulation product 3aa.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) −
−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H]− 416.1074, found 416.1075.
H]− 416.1074, found 416.1075.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the Natural Science Foundation of China (No. 21172253, 21372256, 21572264, 21871293) and Chinese Universities Scientific Fund (No. 2018TC052).Notes and references
- (a) F. C. Biaggio, A. R. Rufino, M. H. Zaim, C. Y. H. Zaim, M. A. Bueno and A. Rodrigues, Curr. Org. Chem., 2005, 9, 419–457 CrossRef CAS; (b) J. González-Sabín, F. Rebolledo and V. Gotor, Chem. Soc. Rev., 2009, 38, 1916–1925 RSC; (c) A. F. Barrero, J. F. Q. Moral, M. M. Herrador, H. Rodriguez and M. C. P. Morales, Curr. Org. Chem., 2009, 13, 1164–1181 CrossRef CAS; (d) B. Heasley, Curr. Org. Chem., 2014, 18, 641–686 CrossRef CAS; (e) Å. Rosenquist, B. Samuelsson, P.-O. Johansson, M. D. Cummings, O. Lenz, P. Raboisson, K. Simmen, S. Vendeville, H. Kock, M. Nilsson, A. Horvath, R. Kalmeijer, G. L. Rosa and M. Beumont-Mauviel, J. Med. Chem., 2014, 57, 1673–1693 CrossRef PubMed.
- (a) P. Tang and Y. Qin, Synthesis, 2012, 44, 2969–2984 CrossRef CAS; (b) L. Jiao and Z.-X. Yu, J. Org. Chem., 2013, 78, 6842–6848 CrossRef CAS PubMed; (c) V. Ganesh and S. Chandrasekaran, Synthesis, 2016, 48, 4347–4380 CrossRef CAS; (d) M. Meazza, H. Guo and R. Rios, Org. Biomol. Chem., 2017, 15, 2479–2490 RSC; (e) B. D. W. Allen, C. P. Lakeland and J. P. A. Harrity, Chem.–Eur. J., 2017, 23, 13830–13857 CrossRef CAS PubMed.
- I. Shimizu, Y. Ohashi and J. Tsuji, Tetrahedron Lett., 1985, 26, 3825–3828 CrossRef CAS.
- K. Yamamoto, T. Ishida and J. Tsuji, Chem. Lett., 1987, 16, 1157–1158 CrossRef.
- A. T. Parsons, M. J. Campbell and J. S. Johnson, Org. Lett., 2008, 10, 2541–2544 CrossRef CAS PubMed.
- L. Mei, Y. Wei, Q. Xu and M. Shi, Organometallics, 2013, 32, 3544–3556 CrossRef CAS.
- (a) L.-Y. Mei, X.-Y. Tang and M. Shi, Chem.–Eur. J., 2014, 20, 13136–13142 CrossRef CAS PubMed; (b) B. Cao, L.-Y. Mei, X.-G. Li and M. Shi, RSC Adv., 2015, 5, 92545–92548 RSC.
- (a) Z. Yuan, W. Wei, A. Lin and H. Yao, Org. Lett., 2016, 18, 3370–3373 CrossRef CAS PubMed; (b) C. Ma, Y. Huang and Y. Zhao, ACS Catal., 2016, 6, 6408–6412 CrossRef CAS.
- (a) H. Zhu, P. Du, J. Li, Z. Liao, G. Liu, H. Li and W. Wang, Beilstein J. Org. Chem., 2016, 12, 1340–1347 CrossRef CAS PubMed; (b) M. Laugeois, S. Ponra, V. Ratovelomanana-Vidal, V. Michelet and M. R. Vitale, Chem. Commun., 2016, 52, 5332–5335 RSC; (c) M. Meazza and R. Rios, Chem.–Eur. J., 2016, 22, 9923–9928 CrossRef CAS PubMed; (d) K. S. Halskov, L. Næsborg, F. Tur and K. A. Jørgensen, Org. Lett., 2016, 18, 2220–2223 CrossRef CAS PubMed.
- L. Mei, Y. Wei, Q. Xu and M. Shi, Organometallics, 2012, 31, 7591–7599 CrossRef CAS.
- (a) A. F. G. Goldberg and B. M. Stoltz, Org. Lett., 2011, 13, 4474–4476 CrossRef CAS PubMed; (b) W.-K. Li, Z.-S. Liu, L. He, T.-R. Kang and Q.-Z. Liu, Asian J. Org. Chem., 2015, 4, 28–32 CrossRef CAS; (c) F. Wei, C.-L. Ren, D. Wang and L. Liu, Chem.–Eur. J., 2015, 21, 2335–2338 CrossRef CAS PubMed; (d) Y. S. Gee, D. J. Rivinoja, S. M. Wales, M. G. Gardiner, J. H. Ryan and C. J. T. Hyland, J. Org. Chem., 2017, 82, 13517–13529 CrossRef CAS PubMed; (e) M. Laugeois, J. Ling, C. Férard, V. Michelet, V. Ratovelomanana-Vidal and M. R. Vitale, Org. Lett., 2017, 19, 2266–2269 CrossRef CAS PubMed; (f) M. Sun, Z.-Q. Zhu, L. Gu, X. Wan, G.-J. Mei and F. Shi, J. Org. Chem., 2018, 83, 2341–2348 CrossRef CAS PubMed; (g) J.-Q. Zhang, F. Tong, B.-B. Sun, W.-T. Fan, J.-B. Chen, D. Hu and X.-W. Wang, J. Org. Chem., 2018, 83, 2882–2891 CrossRef CAS PubMed.
- (a) B. M. Trost and P. J. Morris, Angew. Chem., Int. Ed., 2011, 50, 6167–6170 CrossRef CAS PubMed; (b) B. M. Trost, P. J. Morris and S. J. Sprague, J. Am. Chem. Soc., 2012, 134, 17823–17831 CrossRef CAS PubMed.
- M.-S. Xie, Y. Wang, J.-P. Li, C. Du, Y.-Y. Zhang, E.-J. Hao, Y.-M. Zhang, G.-R. Qu and H.-M. Guo, Chem. Commun., 2015, 51, 12451–12454 RSC.
- Z.-S. Liu, W.-K. Li, T.-R. Kang, L. He and Q.-Z. Liu, Org. Lett., 2015, 17, 150–153 CrossRef CAS PubMed.
- (a) J.-Y. Winum, A. Scozzafava, J.-L. Montero and C. T. Supuran, Med. Res. Rev., 2005, 25, 186–228 CrossRef CAS PubMed; (b) J.-Y. Winum, A. Scozzafava, J.-L. Montero and C. T. Supuran, Expert Opin. Ther. Pat., 2006, 16, 27–47 CrossRef CAS PubMed; (c) L. W. L. Woo, A. Purohit and B. V. L. Potter, Mol. Cell. Endocrinol., 2011, 340, 175–185 CrossRef CAS PubMed; (d) S. J. Williams, Expert Opin. Ther. Pat., 2013, 23, 79–98 CrossRef CAS PubMed.
- (a) C. Ma, J. Gu, B. Teng, Q.-Q. Zhou, R. Li and Y.-C. Chen, Org. Lett., 2013, 10, 2541–2544 Search PubMed; (b) L. Yu, Y. Cheng, F. Qi, R. Li and P. Li, Org. Chem. Front., 2017, 4, 1336–1340 RSC.
- K.-K. Wang, T. Jin, X. Huang, Q. Ouyang, W. Du and Y.-C. Chen, Org. Lett., 2016, 18, 872–875 CrossRef CAS PubMed.
- Y. Wu, Y. Liu, W. Yang, H. Liu, L. Zhou, Z. Sun and H. Guo, Adv. Synth. Catal., 2016, 358, 3517–3521 CrossRef CAS.
- (a) X. Yin, Y. Zheng, X. Feng, K. Jiang, X.-Z. Wei, N. Gao and Y.-C. Chen, Angew. Chem., Int. Ed., 2014, 53, 6245–6248 CrossRef CAS PubMed; (b) X.-L. He, Y.-C. Xiao, W. Du and Y.-C. Chen, Chem.–Eur. J., 2015, 21, 3443–3448 CrossRef CAS PubMed; (c) X. Chen, J.-Q. Zhang, S.-J. Yin, H.-Y. Li, W.-Q. Zhou and X.-W. Wang, Org. Lett., 2015, 17, 4188–4191 CrossRef CAS PubMed.
- (a) X. Feng, Z. Zhou, C. Ma, X. Yin, R. Li, L. Dong and Y.-C. Chen, Angew. Chem., Int. Ed., 2013, 52, 14173–14176 CrossRef CAS PubMed; (b) J. Gu, C. Ma, Q.-Z. Li, W. Du and Y.-C. Chen, Org. Lett., 2014, 16, 3986–3989 CrossRef CAS PubMed; (c) Q. An, J. Shen, N. Butt, D. Liu, Y. Liu and W. Zhang, Adv. Synth. Catal., 2015, 357, 3627–3638 CrossRef CAS; (d) Z. -Q. Liang, D. -L. Wang, C. -L. Zhang and S. Ye, Org. Biomol. Chem., 2016, 14, 6422–6425 RSC; (e) J. Izquierdo and M. A. Pericàs, ACS Catal., 2016, 6, 348–356 CrossRef CAS; (f) Z. Zhou, Z.-X. Wang, Q. Ouyang, W. Xiao, W. Du and Y.-C. Chen, Chem.–Eur. J., 2017, 23, 2945–2949 CrossRef CAS PubMed; (g) Z. Wang, H. Xu, Q. Su, P. Hu, P.-L. Shao, Y. He and Y. Lu, Org. Lett., 2017, 19, 3111–3114 CrossRef CAS PubMed.
- (a) R. Na, C. Jing, Q. Xu, H. Jiang, X. Wu, J. Shi, J. Zhong, M. Wang, D. Benitez, E. Tkatchouk, W. A. Goddard, H. Guo and O. Kwon, J. Am. Chem. Soc., 2011, 133, 13337–13348 CrossRef CAS PubMed; (b) C. Jing, R. Na, B. Wang, H. Liu, L. Zhang, J. Liu, M. Wang, J. Zhong, O. Kwon and H. Guo, Adv. Synth. Catal., 2012, 354, 1023 CrossRef CAS PubMed; (c) H. Guo, H. Liu, F.-L. Zhu, R. Na, H. Jiang, Y. Wu, L. Zhang, Z. Li, H. Yu, B. Wang, Y. Xiao, X.-P. Hu and M. Wang, Angew. Chem., Int. Ed., 2013, 52, 12641–12645 CrossRef CAS PubMed; (d) H. Liu, Y. Wu, Y. Zhao, Z. Li, L. Zhang, W. Yang, H. Jiang, C. Jing, H. Yu, B. Wang, Y. Xiao and H. Guo, J. Am. Chem. Soc., 2014, 136, 2625–2629 CrossRef CAS PubMed; (e) L. Zhang, H. Liu, G. Qiao, Z. Hou, Y. Liu, Y. Xiao and H. Guo, J. Am. Chem. Soc., 2015, 137, 4316–4319 CrossRef CAS PubMed; (f) C. Yuan, L. Zhou, M. Xia, Z. Sun, D. Wang and H. Guo, Org. Lett., 2016, 18, 5644–5647 CrossRef CAS PubMed; (g) Y. Wu, C. Yuan, C. Wang, B. Mao, H. Jia, X. Gao, J. Liao, F. Jiang, L. Zhou, Q. Wang and H. Guo, Org. Lett., 2017, 19, 6268–6271 CrossRef CAS PubMed; (h) W. Yang, W. Sun, C. Zhang, Q. Wang, Z. Guo, B. Mao, J. Liao and H. Guo, ACS Catal., 2017, 7, 3142–3146 CrossRef CAS; (i) C. Yuan, Y. Wu, D. Wang, Z. Zhang, C. Wang, L. Zhou, C. Zhang, B. Song and H. Guo, Adv. Synth. Catal., 2018, 360, 652–658 CrossRef CAS; (j) L. Zhou, C. Yuan, Y. Zeng, H. Liu, C. Wang, X. Gao, Q. Wang, C. Zhang and H. Guo, Chem. Sci., 2018, 9, 1831–1835 RSC.
- The crystallographic data for 3aa and 3aa′ have been deposited with the Cambridge Crystallographic Data Centre as number CCDC 1849601 and 1849602†.
- Chiral HPLC columns such as IA, ID, IE, OD-H, OX-H, AD-H, R&C OD, Lux series, and INC had been used with various proportions of hexane and isopropanol (or ethanol) as eluent to resovle chiral products.
- A. P. Dieskau, M. S. Holzwarth and B. Plietker, J. Am. Chem. Soc., 2012, 134, 5048–5051 CrossRef CAS PubMed.
- M. Tripathi and D. N. Dhar, J. Heterocycl. Chem., 1988, 25, 1191–1192 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectral data and crystallographic data. CCDC 1849601 and 1849602. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8ra08881k |
| This journal is © The Royal Society of Chemistry 2018 |