Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: A benzylic linker promotes methyltransferase catalyzed norbornene transfer for rapid bioorthogonal tetrazine ligation
F.
Muttach
a,
N.
Muthmann
a,
D.
Reichert
ab,
L.
Anhäuser
a and
A.
Rentmeister
*ab
aUniversity of Münster, Department of Chemistry, Institute of Biochemistry, Wilhelm-Klemm-Str. 2, 48149 Münster, Germany
bCells-in-Motion Cluster of Excellence (EXC1003-CiM), University of Münster, Germany. E-mail: a.rentmeister@uni-muenster.de
First published on 16th November 2017
Abstract
Correction for ‘A benzylic linker promotes methyltransferase catalyzed norbornene transfer for rapid bioorthogonal tetrazine ligation’ by F. Muttach et al., Chem. Sci., 2017, DOI: 10.1039/c7sc03631k.
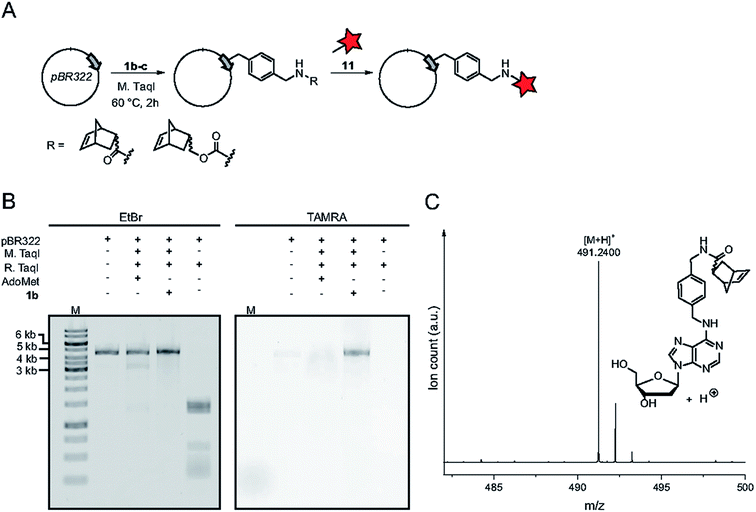
The authors regret that Fig. 4 is incorrect in the original manuscript. In Fig. 4c the chemical structure and mass spectrum of the norbornene-modified adenosine was shown instead of the 2′-deoxyadenosine. The correct figure and caption are displayed below.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2018 |