Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Dye-sensitized electron transfer from TiO2 to oxidized triphenylamines that follows first-order kinetics
Brian N.
DiMarco
,
Ludovic
Troian-Gautier
,
Renato N.
Sampaio
and
Gerald J.
Meyer
*
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599-3290, USA. E-mail: gjmeyer@email.unc.edu
First published on 26th January 2018
Abstract
Correction for ‘Dye-sensitized electron transfer from TiO2 to oxidized triphenylamines that follows first-order kinetics’ by Brian N. DiMarco et al., Chem. Sci., 2018, DOI: 10.1039/c7sc03839a.
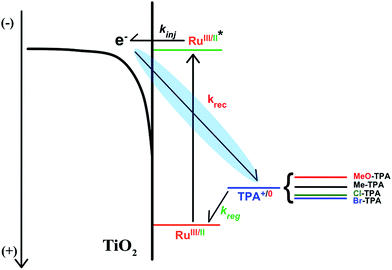
The authors regret that Scheme 1 is incorrect in the original manuscript as an arrow was not properly displayed. The correct scheme is shown below.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2018 |