Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
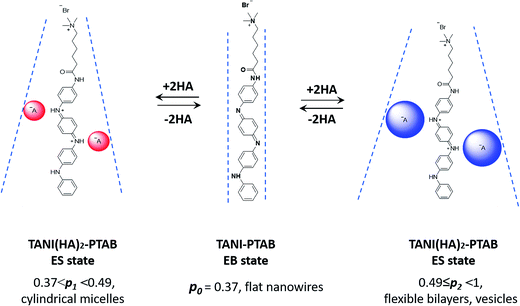
An addressable packing parameter approach for reversibly tuning the assembly of oligo(aniline)-based supra-amphiphiles†
Wei
Lyu
 ab,
Maha
Alotaibi
ac,
O. Alexander
Bell
a,
Kazuyoshi
Watanabe
a,
Robert
Harniman
a,
Benjamin M.
Mills
ab,
Maha
Alotaibi
ac,
O. Alexander
Bell
a,
Kazuyoshi
Watanabe
a,
Robert
Harniman
a,
Benjamin M.
Mills
 a,
Annela M.
Seddon
df,
Sarah E.
Rogers
a,
Annela M.
Seddon
df,
Sarah E.
Rogers
 e,
Stephen M.
King
e,
Stephen M.
King
 e,
Wei
Yan
b and
Charl F. J.
Faul
e,
Wei
Yan
b and
Charl F. J.
Faul
 *a
*a
aSchool of Chemistry, University of Bristol, Bristol, BS8 1TS, UK. E-mail: charl.faul@bristol.ac.uk
bDepartment of Environmental Science and Engineering, Xi'an Jiaotong University, 710049, Xi'an, P. R. China
cChemistry Department, Faculty of Science, King Abdul Aziz University, Jeddah, Kingdom of Saudi Arabia
dSchool of Physics, H. H. Wills Physics Laboratory, University of Bristol, Tyndall Avenue, Bristol, BS8 1FD, UK
eISIS Pulsed Neutron & Muon Source, STFC Rutherford Appleton Laboratory, Harwell Campus, Didcot, OX11 0QX, UK
fBristol Centre for Functional Nanomaterials, H. H. Wills Physics Laboratory, University of Bristol, Tyndall Avenue, Bristol, BS8 1FD, UK
First published on 2nd April 2018
Abstract
We present a newly developed approach to non-covalently address the packing parameter of an electroactive amphiphile. The pH-responsive reversible switching of a tetra(aniline)-based cationic amphiphile, TANI-pentyl trimethylammonium bromide (TANI-PTAB), between self-assembled vesicles and nanowires by acid/base chemistry in aqueous solution is used to exemplify this approach. Trifluoroacetic acid (TFA) was selected as a prototypical acid to form emeraldine salt (ES) state (TANI(TFA)2-PTAB) vesicles for this new class of small-molecule supramolecular amphiphiles. UV-vis-NIR spectroscopy, transmission electron microscopy (TEM), tapping-mode atomic force microscopy (AFM), and fluorescence spectroscopy were used to investigate the reversible structural transformation from vesicles to nanowires. We show that utilising different protonic acid-dopants for TANI-PTAB can regulate the packing parameter, and thus the final self-assembled structures, in a predictable fashion. We envisage potential application of this concept as smart and switchable delivery systems.
Introduction
The formation of well-defined self-assembled supramolecular nanostructures continues to attract considerable attention as a strategy to prepare active and addressable materials for a wide range of applications and devices.1,2 Amphiphiles, a well-known class of materials whose self-assembly behaviour has been thoroughly studied in aqueous environments, are of interest because of their applications in the consumer products, personal care, and pharmaceutical industries.3 Their development has included the study and application of polymeric surfactants,4 and novel sugar-based and CO2-philic amphiphiles5 for sustainable and green chemistry applications.A recent development in the area of novel amphiphiles has been the advent of the so-called supra-amphiphiles, a new class of supramolecular amphiphilic constructs. To produce such constructs, carefully designed sub-units produced using covalent chemistry are allowed to interact via various non-covalent interactions to form new amphiphiles. Such non-covalent interactions include hydrogen-bonding, host-guest, π-stacking, charge-transfer and electrostatic interactions.6–8 Once formed, these supra-amphiphiles can assemble, just like classical amphiphiles, into micelles, vesicles, or nanofibers through the careful choice and tailoring of further non-covalent interactions, or even dynamic covalent bonds.9 Such control enables the design of dynamic and smart materials with the capability to respond to external stimuli, such as photo-irradiation,10 changes in temperature,11 pH,12 redox potential,13 solvent type,14 and concentration,15 and form the basis for investigations into the production of novel tuneable materials.
Oligo(aniline)s are a class of conjugated oligomeric materials that act as model compounds of the well-known conducting polymer poly(aniline) (PANI). These conjugated materials have attracted increasing academic interest as conjugated cores for the construction of new functional materials, amphiphilic copolymers, and surfactants.16–21 Compared with PANI, oligo(aniline)s possess well-defined and monodisperse molecular structures, and exhibit excellent solubility and processability. These features have enabled the design of oligo(aniline)-based materials with well-defined functionalities, as well as new properties and applications that are unavailable to PANI.22–26 Among oligo(aniline)s, tetra(aniline) (TANI) is the shortest oligomer that can exhibit the full range of oxidation states,22 including the emeraldine base (EB) state, which is required for pH-responsive switching. The unique pH- and redox-switchable properties of oligo(aniline)s endow amphiphiles based on this motif with stimuli-responsive self-assembly characteristics.16,17 To date, several reports exist of oligo(aniline)-containing amphiphiles that show redox-induced structural changes. Park and co-workers reported TANI-b-poly(ethylene glycol), which showed reversible electrochemical switching between self-assembled vesicles and micelles.16 They recently reported redox-responsive aggregation–disaggregation behavior between the EB and the leucoemeraldine base state (LEB) of a diaminohepta(aniline)-based multi-block rod-coil structure.27 Wu et al. obtained electrochemically switchable vesicles from a TANI-b-poly(N-isopropyl acrylamide) polymer. Application of an oxidizing voltage at 0.6 V led to the destruction of these vesicles, reportedly by the removal of the structure-directing amine–imine hydrogen bonding.17 These reports are, to the best of our knowledge, the only previous studies of redox-responsive self-assembly of oligo(aniline) amphiphiles.
Compared with traditional pH-responsive systems, the unique pH-switchable properties of oligo(aniline)s endow an additional means of control in the form of TANI's well-known switchable conductivity,22 as well as the more recently explored switchable optical properties (specifically refractive indices).28 It was therefore surprising to find that despite the unique doping properties of aniline-based materials, reports of pH-induced structural changes (i.e., acid doping and base de-doping) directly affecting the self-assembly of oligo(aniline)s (both amphiphilic and non-amphiphilic) are rare. Previous work has relied solely on the structural differences produced by switching between the fully reduced LEB and the half-oxidised EB states of TANI, while the doped and conducting emeraldine salt (ES) state has been largely overlooked. Consequently, we sought to further develop a general strategy to design oligo(aniline)-based pH-responsive smart systems.
One aspect of the unique ability of oligo(aniline)-based materials to be doped that has not been exploited is the use of dopants to address not only the redox state, but also the volume of the electroactive component (induced volume changes in PANI have been exploited widely for the production of soft actuators29). In the case of classical amphiphiles, such changes in molecular volume (v) will lead to changes of the well-known surfactant packing parameter p. The dimensionless packing parameter, p, is widely used to link the structure of an amphiphile to predicted micelle morphology in aqueous solutions.
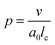 | (1) |
As defined in eqn (1), v is the volume of the hydrophobic section, a0 is the optimal headgroup area per amphiphile and lc is the (critical) hydrophobic tail length.30 The packing parameter, p, predicts that spherical micelles are favoured when 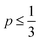 , cylindrical micelles are formed at
, cylindrical micelles are formed at 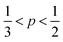 , flexible bilayers and vesicles arise when
, flexible bilayers and vesicles arise when 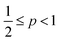 , planar bilayers are favoured at p = 1 and inverted micelles appear when p > 1.31
, planar bilayers are favoured at p = 1 and inverted micelles appear when p > 1.31
Changes in p are generally limited to tuning the headgroup area for classical covalent amphiphiles (through changes in concentration or counterions). For polymeric supra-amphiphiles this approach (i.e., changes in p) has led to reversible switching of the assembled nanostructures.32–34
It is therefore envisaged that this strategy will also be suitable for amphiphiles based on our TANI motif: the electroactive tail of a TANI-based amphiphile will be available for reversible protonic acid doping and de-doping, and thus lead to reversibly tuneable changes in v and p and, consequently, the formed self-assembled structures; i.e., a reversibly addressable low molecular weight electrostatic supra-amphiphile (eSA).35 In addition, changing the type (and thus volume) of the dopant would provide a simple strategy to tune packing parameters and thus formed supramolecular assembled structures in a facile fashion, as shown in Scheme 1. We term this approach the “addressable packing parameter (APP)” approach.
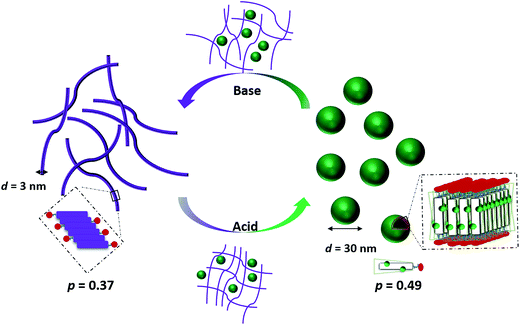
We recently reported the aqueous self-assembly of a TANI-based cationic amphiphile, TANI-PTAB with a packing parameter p = 0.37, see Scheme 1, into 3 nm-wide flat nanowires or nanobelts. This assembly was driven primarily by the hydrophobic effect, with structure-directing hydrogen bonding and π–π stacking interactions occurring between TANI molecules.19
Here, based on our previous work and the expected influence of noncovalent dopants on the volume of the TANI-based surfactant tail and thus the packing parameter, we explore an unprecedented method of tuning the self-assembly of small-molecule amphiphiles. Starting with TANI-PTAB in the EB state, p = 0.37, trifluoroacetic acid (TFA) was selected as an initial model acid to form the doped ES-state amphiphile, TANI(TFA)2-PTAB, with a calculated packing parameter p = 0.49. We demonstrate reversible switching of this system between nanowires (p = 0.37) and vesicles (p = 0.49), in response to changes in pH that control the reversible protonic doping of the TANI-based surfactant tail. Moreover, we show the generality of this approach by using dopants of different sizes (and volumes) to change the packing parameter p, leading to the reversible tuning of the self-assembled amphiphile structures, e.g., from wires to worm-like micelles and bilayer structures and back to wires.
Experimental section
Synthesis
TANI-PTAB was synthesized according to the previously reported route.19 Full synthetic details and characterization are available in the ESI.†Theoretical estimation of packing parameter of EB TANI-PTAB and ES TANI(HX)2-PTAB
The calculated packing parameters of EB TANI-PTAB and ES TANI(HX)2-PTAB (HX = protonic acid dopant) species were estimated according to eqn (1). The parameters v and lc were estimated by QSAR calculations based on DFT models.36 For TANI-based amphiphiles bearing an alkyltrimethylammonium bromide headgroup, the cross-sectional area of the polar group, a0, was estimated to be 0.54 nm2 by considering the bond lengths and bond angles, as in previous reports.37 Full computational details and results are shown in the ESI.†Doping studies
An aqueous solution of TFA (2 eq., 32 mM, 125 μL) was added to EB TANI-PTAB solid (1 eq., 1.2 mg, 2 mmol) to achieve a doping ratio of 2 TFA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 TANI-PTAB, followed by dilution with the required volume of deionised water to achieve the desired final concentration. The solutions were shaken and sonicated for 5 min to ensure homogeneity, and then left undisturbed overnight (RT) before analysis. Doped samples were stored in the fridge between experiments.
1 TANI-PTAB, followed by dilution with the required volume of deionised water to achieve the desired final concentration. The solutions were shaken and sonicated for 5 min to ensure homogeneity, and then left undisturbed overnight (RT) before analysis. Doped samples were stored in the fridge between experiments.
Characterization
UV-vis-NIR spectra were recorded using a Shimadzu UV2600 spectrophotometer. FT-IR spectra were collected using a Perkin Elmer Spectrum 100 FT-IR spectrometer with attenuated total reflectance cell; samples were freeze-dried before measurements. Transmission electron microscopy (TEM) was performed on a JEOL 1400 TEM with a tungsten filament, operated at a frequency of 120 kV and equipped with a 4 Mp Gatan Orius 830 digital camera using the Gatan DigitalMicrograph software. Samples (5 μL) were drop-cast onto carbon-coated copper grids and allowed to rest for 60 s, before the excess solution was wicked away. Samples were stained with a 1% aqueous uranyl acetate solution for 30 s. Dynamic light scattering (DLS) was carried out on a Malvern Zetasize Nano-S apparatus with a 532 nm laser. Atomic force microscopy (AFM) was performed using a Bruker Multimode with a Nanoscope V controller and Picoforce Extender. Samples were drop-cast onto either carbon-coated copper grids or freshly cleaved mica. Fluorescence spectra were obtained using a Varian Cary Eclipse Fluorimeter. Samples for fluorescence analysis were prepared by adding a solution of pyrene in acetone (20 μL, 0.5 mg mL−1) to aqueous ES TANI(TFA)2-PTAB (2 mL) solutions of varying concentrations, followed by purging with argon. A pyrene emission spectrum (between 360 nm and 600 nm, excitation at 337 nm) was recorded for each solution. Please see ESI Page S18† for additional comments on attempts to determine the critical aggregation concentration (CAC).X-ray scattering data were collected on a Ganesha SAXS/WAXS instrument (SAXSLAB) at room temperature over a Q-range of 0.001 < Q < 0.7 Å−1 where
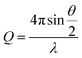 | (2) |
Q is the scattering vector, θ is the scattering angle and λ is the incident wavelength. The source is a GeniX3D source (made by Xenocs) with a wavelength of 1.5406 Å. The detector is a Pilatus 300 K by Dectris. Samples were loaded into borosilicate glass capillaries (Capillary Tube Supplies) and sealed with UV-curable adhesive (Norland Optical Adhesive). Data were collected over an exposure time of 3 hours and background-corrected with TFA, and intensity measurements were corrected for tube thickness. Data analysis was performed using SasView (versions 3.0x).38
Small-angle neutron scattering (SANS) data were obtained on the LOQ small-angle diffractometer at the ISIS Pulsed Neutron Source (STFC Rutherford Appleton Laboratory, Didcot, U.K.).39,40 This is a fixed-geometry “white beam” time-of-flight instrument, which utilizes neutrons with wavelengths between 2.2 and 10 Å. Data are simultaneously recorded on two, two-dimensional, position-sensitive, neutron detectors, to provide a simultaneous Q range of 0.008–1.6 Å−1. Each sample and background was placed in 2 mm path length quartz cuvettes and was measured for a total of 5 hours to gather data of high statistical precision. The beam diameter was 8 mm. Each raw scattering data set was then corrected for the detector efficiencies, sample transmission and background scattering and converted to scattering cross-section data (∂Σ/∂Ω vs. Q) using the instrument-specific software.41 These data were placed on an absolute scale (cm−1) using the scattering from a standard sample (a solid blend of hydrogenous and perdeuterated polystyrene) in accordance with established procedures.42 SANS data were subsequently collected, over the much lower Q-range of 0.0015–0.25 Å−1, on the SANS2D small-angle diffractometer at ISIS, utilizing an incident wavelength range of 1.75–12.5 Å and employing an instrument configuration of L1 = L2 = 12 m (where L1 = source-sample distance, and L2 = sample-detector distance), with the 1 m2 main detector offset vertically by 60 mm and sideways by 100 mm.39,43 The beam diameter was again 8 mm. These data were processed in an analogous manner to that from LOQ. The SANS data were also analysed using SasView (versions 3.0x).38
Results and discussion
Self-assembly of ES TANI(TFA)2-PTAB
In our previously reported studies into the self-assembly of TANI-PTAB,19 we focused mainly on the undoped EB state, forming infinitely long 3 nm wide π-stacked nanowires in solution. Modelling of solution SAXS data indicated that these structures could be described as flat and tape-like, with widths of 3 nm. Initial doping studies with CSA, the prototypical PANI acid dopant,19 showed the formation of thicker (6 nm) less well-defined conductive wire-like constructs. Simple calculations (see Scheme 1 and Table 1) suggested that p changed from 0.37 (for EB TANI-PTAB) to 0.73 for ES TANI(CSA)2-PTAB on the addition of CSA, but these changes and their potential influence on assembly was not further explored in any way.| Molecule | pKa | V(Å3) | l c(Å) | a 0(Å2) | p | Expected self-assembled structure |
|---|---|---|---|---|---|---|
| TANI-PTAB | — | 474.10 | 23.91 | 54.00 | 0.37 | Cylindrical micelles |
| TANI(HCl)2-PTAB | −7.00 | 535.31 | 24.28 | 54.00 | 0.41 | Cylindrical micelles |
| TANI(HNO3)2-PTAB | −1.40 | 569.71 | 24.01 | 54.00 | 0.44 | Cylindrical micelles |
| TANI(AcOH)2-PTAB | 4.76 | 607.62 | 24.04 | 54.00 | 0.47 | Cylindrical micelles |
| TANI(TFA)2-PTAB | 0.23 | 634.87 | 24.03 | 54.00 | 0.49 | Flexible bilayers, vesicles |
| TANI(DCA)2-PTAB | 1.35 | 663.39 | 23.94 | 54.00 | 0.51 | Flexible bilayers, vesicles |
| TANI(CSA)2-PTAB | 1.20 | 933.91 | 23.78 | 54.00 | 0.73 | Flexible bilayers, vesicles |
| TANI(BinPO4H)2-PTAB | 3.37 | 1160.28 | 24.06 | 54.00 | 0.89 | Flexible bilayers, vesicles |
Careful examination and further investigations into a range of acid dopants, to explore this proposed switchability, led us to TFA as the dopant of choice for the following reasons: (a) TFA possessed the capacity to dope TANI-based materials with a pKa = 0.23; (b) the calculated value of p after doping, i.e. for the doped ES TANI(TFA)2-PTAB, is = 0.49; and (c) a series of further related acids with different volumes would be available for additional investigations.
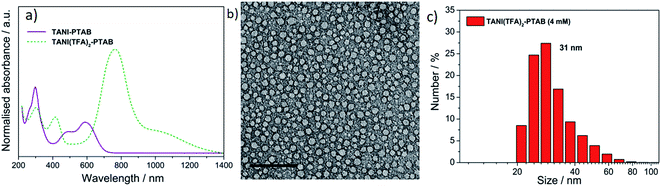
As expected, doping EB TANI-PTAB with TFA has a profound effect on the formed self-assembled structures: the addition of TFA converts EB TANI-PTAB with its well-defined 3 nm-wide nanowire morphology to doped ES TANI(TFA)2-PTAB, now with a very well-defined vesicular structure. The capacity of TFA to dope EB TANI-PTAB to the ES state was confirmed by UV-vis-NIR absorption spectroscopy.44 As shown in Fig. 1a, three maxima were observed in the UV-vis-NIR spectrum of TANI(TFA)2-PTAB at 1 mM in water, located at 302 nm, 420 nm, and 780 nm. These maxima originate from π-electron transitions in the TANI-PTAB molecule that are comparable to the π–π*, polaron-π*, and π-polaron band transitions in PANI-ES, respectively,22,45 and are characteristic of the ES state of doped oligo(aniline) species. Recent46 TD-DFT calculations on the core TANI electroactive unit have shown that absorption maxima in the range 780–800 nm correspond to the presence of the triplet ES state. The presence of these bands, as well as the absence of the absorption features typical for EB-state materials (especially the band at 600 nm) confirm that fully doped ES TANI(TFA)2-PTAB was formed. These conclusions were further confirmed by FT-IR spectra of TANI-PTAB and 2 mM TANI(TFA)2-PTAB, recorded in the solid state (Fig. S7†). Circular objects with average diameters of 27 ± 2 nm were observed by conventional TEM (Fig. 1b, S9a†), and DLS was used to confirm the existence of objects with similar size in solution. The average hydrodynamic diameter was determined to be 31 ± 7 nm (Fig. 1c), which agrees very well with the measurements from TEM, since the hydrodynamic diameter is expected to be larger than that of the dried sample.47
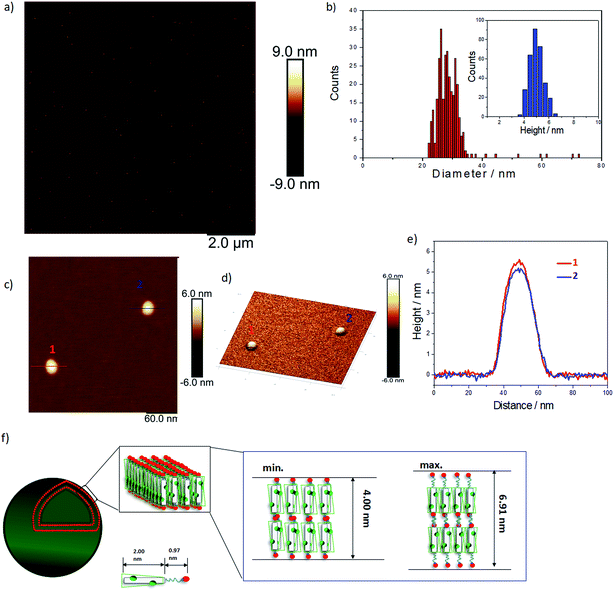
We used AFM to further investigate the topology of these objects. AFM images of a TANI(TFA)2-PTAB solution (2 mM), drop-cast on a mica surface, are shown in Fig. 2. Highly monodisperse circular structures were observed on the surface (Fig. 2a, also embedded in films – see Fig. S8†), indicating that these objects were not droplets formed by drying. The average diameter and height of these objects, calculated from 317 counted objects, were 28.80 ± 3.33 nm and 5.01 ± 0.57 nm, respectively (Fig. 2b). The value of the average diameter was found to be in excellent agreement with those measured by both DLS and TEM. Two representative objects were selected to show their detail in higher resolution and 3D images (Fig. 2c, 2d, respectively). The measured heights of these two objects were 5.5 nm and 5 nm, respectively (Fig. 2e). Given the theoretical stretched length of an upright TANI(TFA)2-PTAB molecule (2.97 nm, of which the rigid TANI block makes up 2 nm with 0.97 nm of flexible alkyl chains), we deduce that the height is determined by the thickness of an interdigitated double layer of TANI(TFA)2-PTAB. This layer is calculated to have a minimum height of ∼4 nm (with fully interdigitated and overlapping π-conjugated surfaces and no tilt with respect to the surface) and maximum height of ∼6.9 nm (an upright, non-interdigitated state), as shown in the proposed molecular packing models in Fig. 2f. Moreover, the absence of the characteristic adsorption of strong asymmetric inter-chain NH+⋯N hydrogen bonds in the FT-IR spectrum of TANI(TFA)2-PTAB, shown in Fig. S7,† support the proposed packing model. Therefore, combining the measured average diameter with the proposed interdigitated double-layer packing model, we deduced that these objects are hollow double-layer vesicles.
SAXS and SANS investigations (in H2O- and D2O-based media, respectively) were also used to probe the shape and size of the objects formed in the 4 mM TANI(TFA)2-PTAB system; see the ESI† for representative data. The SANS data possessed superior scattering contrast, but both sets of measurements showed a pronounced ∼Q−2 dependence. This behaviour is consistent with either the overall scattering from discoidal particles/platelets, or the sheet-like scattering from a unilamellar structure such as might surround a vesicle, but unfortunately there is no way to unambiguously differentiate between the two. However, if the TEM and DLS size data are to be believed, and the objects were discs/platelets, then one would expect to see a levelling off in the low-Q scattering in the measurement window of the SANS instruments; this behaviour was not observed. Instead, the SANS data start to increase more steeply than Q−2 below Q ∼ 0.004 Å−1, equivalent to a length scale of ∼160 nm. The explanation for this observation is unclear. The absence of any oscillations in the SANS data at high-Q is likely due to the significant polydispersity of the system (see Fig. 1c), but the absence of a pronounced diffraction peak at high-Q also rules out any multilamellar structures.
Reversible pH-responsive self-assembly
Confirmation was sought that the observed changes in packing parameter, and thus self-assembly behaviour, are reversible and driven by an acid-dopant-induced packing parameter change of the addressable amphiphile, rather than a simple change in volume or concentration.48 Our starting point was thus to first prepare the ES TANI(TFA)2-PTAB vesicular structures by doping an EB TANI-PTAB fibre-containing solution with TFA, as discussed above (Fig. 1b). A small amount of concentrated NaOH solution (0.1 M, 0.03 mL) was then added to TANI(TFA)2-PTAB (1 mM,49 1.5 mL) to de-dope and alter the pH with minimal change in volume. We observed the full conversion of ES TANI(TFA)2-PTAB to EB TANI-PTAB, as shown in the UV-vis-NIR spectra in Fig. 3a, with the pH changing from 3.0 to 6.2. In a control experiment designed to evaluate the influence of the ionic strength on structure formation, we added identical amounts of NaCl to doped 1 mM TANI(TFA)2-PTAB. Addition of NaCl produced aggregated vesicles with an average diameter of 29 ± 2 nm (Fig. 3b, S9b†), with no perceptible change in morphology.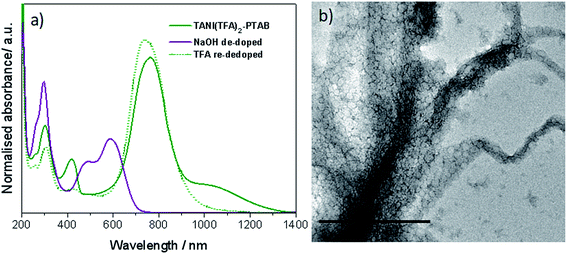 | ||
| Fig. 3 (a) UV-vis-NIR spectra of TANI(TFA)2-PTAB sample, NaOH de-doped sample and TFA re-doped sample. (b) Control with the addition of NaCl to ES TANI(TFA)2-PTAB. Scale bar: 500 nm. | ||
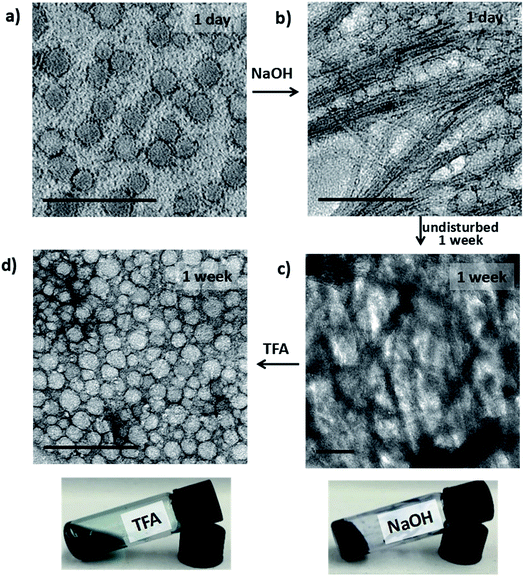
Investigation of the structures formed during the following series of doping/de-doping cycles (see Fig. 4) showed the initial co-existence of vesicles and nanowires after addition of NaOH (Fig. 4b). We propose that these structures are indicative of a kinetically trapped state, rather than a thermodynamic minimum, as the UV-vis-NIR data showed that all TANI moieties were fully de-doped. This suggestion was confirmed by leaving the sample undisturbed for 1 week to equilibrate, after which a weak gel with a high concentration of bundles of nanowires, visible by TEM (Fig. 4c), formed. We added TFA to the fibre-containing gelled samples to further explore the usefulness of the switchable nature of our oligo(aniline) functional unit for structural changes. After leaving the samples to stand undisturbed for a further week, we obtained a low-viscosity solution containing vesicles again, as clearly shown in Fig. 4d (and the inset photos of the gel and solution, respectively). This finding indicates that a fully reversible doping/de-doping-induced morphology switching system was achieved. The average diameter of the obtained vesicular structures was 42 ± 2 nm, although this increase in size (vs. initial vesicles) could be attributed to the change in ionic strength.
As shown in Scheme 2, the removal of the TFA counterions and protons (de-doping) modifies the packing parameter by changing the volume of the TANI-containing amphiphile, causing the vesicles formed by the ES state to revert to nanowires.
 | ||
| Scheme 2 Schematic showing the proposed doping/de-doping responsive self-assembly behaviour of TANI(TFA)2-PTAB in aqueous solution. | ||
Non-covalent packing parameter tuning: generalising the approach
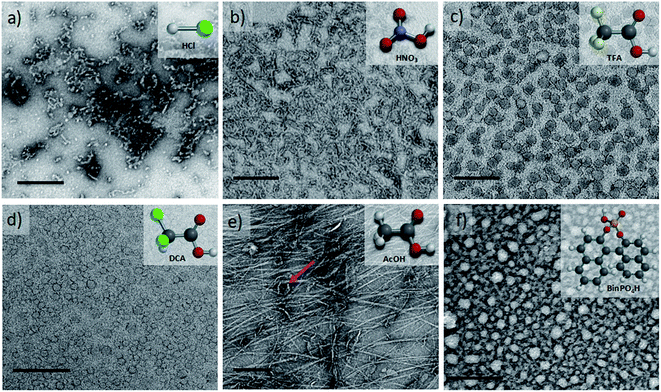
Upon doping with acid, the volume of the hydrophobic section of TANI increased, which was reflected in an increased surfactant packing parameter. However, a number of factors (including the size, polarity and chemical nature of the conjugate base) could affect the range of non-covalent interactions among TANI molecules, making the doping-induced self-assembly of TANI-based eSAs a complex process. In an attempt to gain insight and to start to explore simple variations, dopants of different size and polarity were carefully selected to vary the estimated packing parameter from 0.37 (EB TANI-PTAB) through to 0.89 (ES TANI(HX)2-PTAB), thus providing an exciting opportunity to reversibly tune p for our low molecular weight eSA. Please see Table 1 for an overview of the chosen acids, as well as a summary of the relevant molecular parameters.We started our packing parameter investigations with the small inorganic acid dopants HCl and HNO3, where p was calculated to be 0.41 and 0.44, respectively. Slightly elongated worm-like cylindrical micelles were observed for both TANI(HCl)2-PTAB (Fig. 5a) and TANI(HNO3)2-PTAB (Fig. 5b), which agreed with the morphology suggested by the packing parameters in Table 1.50
Acetic acid (AcOH, p = 0.47) and dichloroacetic acid (DCA, p = 0.51) were used as dopants that are analogous to TFA and whose packing parameters bracket the value calculated for TFA (p = 0.49). We found a significant difference in the formed self-assembled structures: AcOH was unable to fully dope TANI-PTAB (with a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) because of its high pKa value (4.76). Partial doping was shown by the presence of peaks attributed to both the EB and ES-state absorption bands in the UV-vis spectra (Fig. S10†), indicating a mixture of the two species. TEM investigations showed that TANI(AcOH)2-PTAB formed a mixture of nanowires, vesicles and some intermediate states, as shown in Fig. 5e (red arrow). It is clear that the low level of doping (a consequence of the high pKa) led to an undesired mixture of self-assembled structures, and is therefore not a true reflection of the influence of the dopant on the surfactant tail volume. Increasing the amount of AcOH to a mole ratio of 4
1) because of its high pKa value (4.76). Partial doping was shown by the presence of peaks attributed to both the EB and ES-state absorption bands in the UV-vis spectra (Fig. S10†), indicating a mixture of the two species. TEM investigations showed that TANI(AcOH)2-PTAB formed a mixture of nanowires, vesicles and some intermediate states, as shown in Fig. 5e (red arrow). It is clear that the low level of doping (a consequence of the high pKa) led to an undesired mixture of self-assembled structures, and is therefore not a true reflection of the influence of the dopant on the surfactant tail volume. Increasing the amount of AcOH to a mole ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 AcOH
1 AcOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TANI-PTAB yielded doped vesicular TANI(AcOH)2-PTAB. However, the simultaneous presence of a small feature at 580 nm in the UV-vis-NIR spectrum, typical of undoped materials, was congruent with the observation by TEM that nanowires remained in the system (Fig. S11a and b†), indicating incomplete doping within the sample. However, as shown in Fig. 5d, the strong acid DCA (pKa = 1.35) caused the doped TANI(DCA)2-PTAB supra-amphiphile to form well-defined vesicles with an average diameter of 34 nm (Fig. S12†), very similar to those obtained from TANI(TFA)2-PTAB. In addition, the TANI(DCA)2-PTAB system exhibits similar doping/de-doping responsive self-assembly behaviour in aqueous solution as found for TANI(TFA)2-PTAB sample (Fig. S13†).
TANI-PTAB yielded doped vesicular TANI(AcOH)2-PTAB. However, the simultaneous presence of a small feature at 580 nm in the UV-vis-NIR spectrum, typical of undoped materials, was congruent with the observation by TEM that nanowires remained in the system (Fig. S11a and b†), indicating incomplete doping within the sample. However, as shown in Fig. 5d, the strong acid DCA (pKa = 1.35) caused the doped TANI(DCA)2-PTAB supra-amphiphile to form well-defined vesicles with an average diameter of 34 nm (Fig. S12†), very similar to those obtained from TANI(TFA)2-PTAB. In addition, the TANI(DCA)2-PTAB system exhibits similar doping/de-doping responsive self-assembly behaviour in aqueous solution as found for TANI(TFA)2-PTAB sample (Fig. S13†).
To ensure that we explored the boundary conditions and limitations of our proposed APP approach, we also considered the use of the very bulky phosphoric acid (R)-(−)-1,1′-binaphthyl-2,2′-diyl hydrogen phosphate, BinPO4H, (p = 0.89, pKa = 3.37). UV-vis spectra showed that BinPO4H doped TANI-PTAB completely (Fig. S14†). Attempting doping and assembly with this acid produced grain-like structures, which may be considered as aggregated structures of spherical structures (see Fig. 5f and S15†).
Finally, we compared the structures of the oligo(aniline)-based eSAs (HNO3, DCA, and BinPO4H) obtained here with those found for TANI(CSA)2-PTAB (CSA, p = 0.73), for which we expected either lamellar or vesicular structures. However, doping with CSA led to the formation of 6 nm-thick wire-like structures.19 One aspect of the molecular design and non-covalent interactions we have not considered in any of our discussions are secondary additional interactions between the dopant and the oligo(aniline) moiety. Previous molecular modelling investigations of the successful doping of PANI with CSA51 suggested an exceptionally serendipitous fit of CSA into the molecular cavity formed between adjacent phenyl rings (Ph-N-Ph), with additional hydrogen-bonding interactions to the PANI backbone structure. These postulated fit interactions, in combination with additional hydrogen-bonding capability, could play a significant role in the formation of stable wire-like structures (rather than the expected classical lamellar or vesicular structures). We are currently investigating the perceived anomalous behaviour found for the CSA-doped system in more detail.
However, these initial investigations indicate that the strategy employed here, i.e., using a suitable dopant to regulate packing parameters and influence intermolecular assembly forces, is a feasible route produce controllable and tuneable self-assembled structures of oligo(aniline)-based eSAs (Scheme 3).
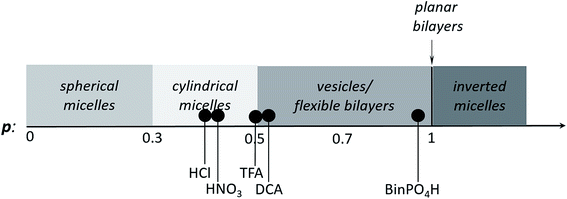 | ||
| Scheme 3 Scheme showing the relationship between packing parameter and the obtained nanostructures using different dopants for our TANI-PTAB system. | ||
Conclusion
We have developed a reversibly switchable self-assembling system using an electroactive oligo(aniline)-based eSA, TANI(TFA)2-PTAB, that exhibits dopant-dependent morphology transitions between vesicles and nanowires. This behavior arises from changes in the packing parameter of the protonated oligo(aniline) vs. the un-protonated EB form, caused by non-covalent association of the conjugate base as counter-ion to the protonated species. Cycling the system between basic and acidic states allowed reversible switching between nanowires and vesicles, respectively. Further exploration of these addressable self-assembly concepts using acids with different volumes provided packing parameters ranging from 0.41 to 0.89. This reversibly switchable self-assembly behaviour in aqueous solution therefore provides initial design rules that will enable the development of novel switchable and addressable systems for use in encapsulation and delivery, as well as novel biomaterial applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the United Kingdom Engineering and Physical Sciences Research Council (EPSRC, grant numbers EP/K502996/1 and EP/J500379/1). Mass spectrometric analysis was performed on instrumentation bought through the Core Capability for Chemistry Research – Strategic Investment in Mass Spectrometry EPSRC grant (EP/K03927X/1). The facilities and staff of the Mass Spectrometry service and the NMR service at the University of Bristol, as well as computing resources from the Centre for Computational Chemistry, are gratefully acknowledged. PeakForce atomic force microscopy was carried out in the Chemical Imaging Facility, University of Bristol with equipment funded by EPSRC under “Atoms to Applications” grant EP/K035746/1, as well as Ganesha. The SANS data were acquired under ISIS Xpress Access proposal XB1690307. The SasView application was originally developed under US NSF award DMR-0520547, but also contains code developed with funding from the EU Horizon 2020 research and innovation programme under the SINE2020 project (grant number 654000). WL acknowledges the support of the Chinese Scholarship Council. MA acknowledges the support of King Abdulaziz University. KZ acknowledges the support of the Kyoto University and Murata Overseas Scholarship Foundation.References
- C. F. J. Faul, Acc. Chem. Res., 2014, 47(12), 3428–3438 CrossRef CAS PubMed.
- X. Li, P. J. Wolanin, L. R. MacFarlane, R. L. Harniman, J. Qian, O. E. C. Gould, T. G. Dane, J. Rudin, M. J. Cryan, T. Schmaltz, H. Frauenrath, M. A. Winnik, C. F. J. Faul and I. Manners, Nat. Commun., 2017, 8, 15909–15917 CrossRef CAS PubMed.
- H. J. Kim, T. Kim and M. Lee, Acc. Chem. Res., 2011, 44(1), 72–82 CrossRef CAS PubMed.
- V. Dichiarante, R. Milani and P. Metrangolo, Green Chem., 2018, 20, 13–27 RSC.
- J. Eastoe, S. Gold, S. Rogers, P. Wyatt, D. C. Steytler, A. Gurgel, R. K. Heenan, X. Fan, E. J. Beckman and R. M. Enick, Angew. Chem., Int. Ed., 2006, 45(22), 3675–3677 CrossRef CAS PubMed.
- X. Zhang and C. Wang, Chem. Soc. Rev., 2011, 40(1), 94–101 RSC.
- C. Wang, Z. Wang and X. Zhang, Acc. Chem. Res., 2012, 45(4), 608–618 CrossRef CAS PubMed.
- G. Yu, K. Jie and F. Huang, Chem. Rev., 2015, 115(15), 7240–7303 CrossRef CAS PubMed.
- T. Fenske, H.-G. Korth, A. Mohr and C. Schmuck, Chem.–Eur. J., 2012, 18(3), 738–755 CrossRef CAS PubMed.
- D. Xia, G. Yu, J. Li and F. Huang, Chem. Commun., 2014, 50(27), 3606–3608 RSC.
- C. Zhou, X. Cheng, Y. Yan, J. Wang and J. Huang, Langmuir, 2014, 30(12), 3381–3386 CrossRef CAS PubMed.
- P. Moitra, K. Kumar, P. Kondaiah and S. Bhattacharya, Angew. Chem., Int. Ed., 2014, 53(4), 1113–1117 CrossRef CAS PubMed.
- X. Chang, R. Dong, B. Ren, Z. Cheng, J. Peng and Z. Tong, Langmuir, 2014, 30(29), 8707–8716 CrossRef CAS PubMed.
- N. Chandrasekhar and R. Chandrasekar, Angew. Chem., Int. Ed., 2012, 51(15), 3556–3561 CrossRef CAS PubMed.
- X. Yan, Q. He, K. Wang, L. Duan, Y. Cui and J. Li, Angew. Chem., 2007, 119(14), 2483–2486 CrossRef.
- H. Kim, S. M. Jeong and J. W. Park, J. Am. Chem. Soc., 2011, 133(14), 5206–5209 CrossRef CAS PubMed.
- Y. Wu, S. Liu, Y. Tao, C. Ma, Y. Zhang, J. Xu and Y. Wei, ACS Appl. Mater. Interfaces, 2014, 6(3), 1470–1480 CAS.
- L. Cao, C. Gong and J. Yang, Macromol. Rapid Commun., 2016, 37(4), 343–350 CrossRef CAS PubMed.
- O. A. Bell, G. Wu, J. S. Haataja, F. Brömmel, N. Fey, A. M. Seddon, R. L. Harniman, R. M. Richardson, O. Ikkala, X. Zhang and C. F. J. Faul, J. Am. Chem. Soc., 2015, 137(45), 14288–14294 CrossRef CAS PubMed.
- C. U. Udeh, P. Rannou, B. P. Brown, J. O. Thomas and C. F. J. Faul, J. Mater. Chem. C, 2013, 1(39), 6428–6437 RSC.
- Z. Yang, X. Wang, Y. Yang, Y. Liao, Y. Wei and X. Xie, Langmuir, 2010, 26(12), 9386–9392 CrossRef CAS PubMed.
- Y. Wang, H. D. Tran, L. Liao, X. Duan and R. B. Kaner, J. Am. Chem. Soc., 2010, 132(30), 10365–10373 CrossRef CAS PubMed.
- Z. Wei and C. F. J. Faul, Macromol. Rapid Commun., 2008, 29(4), 280–292 CrossRef CAS.
- Y. Wang, J. A. Torres, A. Z. Stieg, S. Jiang, M. T. Yeung, Y. Rubin, S. Chaudhuri, X. Duan and R. B. Kaner, ACS Nano, 2015, 9(10), 9486–9496 CrossRef CAS PubMed.
- Q. Chen, X. Yu, Z. Pei, Y. Yang, Y. Wei and Y. Ji, Chem. Sci., 2017, 8(1), 724–733 RSC.
- Y. Yan, N. Sun, F. Li, X. Jia, C. Wang and D. Chao, ACS Appl. Mater. Interfaces, 2017, 9(7), 6497–6503 CAS.
- T. G. Kim, C. Kim and J. W. Park, Macromolecules, 2017, 50(20), 8185–8191 CrossRef CAS.
- Y. Hu, B. T. Miles, Y. L. D. Ho, M. P. C. Taverne, L. Chen, H. Gersen, J. G. Rarity and C. F. J. Faul, Adv. Opt. Mater., 2017, 5(3), 1600458 CrossRef.
- H. Gao, J. Zhang, W. Yu, Y. Li, S. Zhu, Y. Li, T. Wang and B. Yang, Sens. Actuators, B, 2010, 145(2), 839–846 CrossRef CAS.
- T. Smart, H. Lomas, M. Massignani, M. V. Flores-Merino, L. R. Perez and G. Battaglia, Nano Today, 2008, 3(3–4), 38–46 CrossRef CAS.
- D. A. Balazs and W. Godbey, J. Drug Delivery, 2011, 2011, 326497 Search PubMed.
- X. Zhao, Q. Wang, Y. I. Lee, J. Hao and H. G. Liu, Chem. Commun., 2015, 51(93), 16687–16690 RSC.
- Z. Jia, N. P. Truong and M. J. Monteiro, Polym. Chem., 2013, 4(2), 233–236 RSC.
- C. Po, A. Y. Y. Tam and V. W. W. Yam, Chem. Sci., 2014, 5(7), 2688–2695 RSC.
- Z. Shao, P. Rannou, S. Sadki, N. Fey, D. M. Lindsay and C. F. J. Faul, Chem.–Eur. J., 2011, 17(44), 12512–12521 CrossRef CAS PubMed.
- R. A. Khalil and A. H. A. Zarari, Appl. Surf. Sci., 2014, 318, 85–89 CrossRef CAS.
- R. Zana and E. W. Kaler, Giant micelles: properties and applications, CRC Press, 2007, Vol. 140 Search PubMed.
- www.sasview.org .
- http://www.isis.stfc.ac.uk .
- R. K. Heenan, S. E. Rogers, D. Turner, A. E. Terry, J. Treadgold and S. M. King, J. Neutron Res., 2011, 22(2), 19–21 CrossRef.
- http://www.mantidproject.org .
- G. D. Wignall and F. S. Bates, J. Appl. Crystallogr., 1987, 20(1), 28–40 CrossRef CAS.
- R. K. Heenan, J. Penfold and S. M. King, J. Appl. Crystallogr., 1997, 30(6), 1140–1147 CrossRef CAS.
- Here, both spectra samples were diluted from 4 mM to 1 mM due to high the absorption intensity in the 4 mM sample.
- W. Lyu, J. Feng, W. Yan and C. F. J. Faul, J. Mater. Chem. C, 2015, 3(45), 11945–11952 RSC.
- B. M. Mills, Z. Shao, S. R. Flynn, P. Rannou, D. M. Lindsay, N. Fey and C. F. J. Faul, 2018, submitted.
- W. Lv, J. Feng, W. Yan and C. F. J. Faul, J. Mater. Chem. B, 2014, 2(29), 4720–4725 RSC.
- Change in volume or concentration induced dilution de-doped self-assembly phenomena see ESI.†.
- Similar sol-gel transition phenomena for 2 mM and 4 mM TANI(TFA)2-PTAB samples were observed.
- D. F. Evans and H. Wennerstrom, The colloidal domain: where physics, chemistry, biology, and technology meet, Wiley-VCH, 1999 Search PubMed.
- O. T. Ikkala, L. Pietila, L. Ahjopalo, H. Osterholm and P. J. Passiniemi, J. Chem. Phys., 1995, 103, 9855–9863 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8sc00068a |
| This journal is © The Royal Society of Chemistry 2018 |