Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Metabolism and hydrophilicity of the polarised ‘Janus face’ all-cis tetrafluorocyclohexyl ring, a candidate motif for drug discovery†
Andrea
Rodil
a,
Stefano
Bosisio
b,
Mohammed Salah
Ayoup
ad,
Laura
Quinn
c,
David B.
Cordes
 a,
Alexandra M. Z.
Slawin
a,
Alexandra M. Z.
Slawin
 a,
Cormac D.
Murphy
c,
Julien
Michel
*b and
David
O'Hagan
a,
Cormac D.
Murphy
c,
Julien
Michel
*b and
David
O'Hagan
 *a
*a
aEaStChem School of Chemistry, University of St Andrews, North Haugh, St Andrews, Fife KY16 9ST, UK. E-mail: do1@st-andrews.ac.uk
bEaStChem School of Chemistry, University of Edinburgh, Joseph Black Building, David Brewster Road, Edinburgh, EH9 3FJ, UK. E-mail: julien.michel@ed.ac.uk
cUCD School of Biomolecular and Biomedical Sciences, University College Dublin, Belfield, Dublin, Ireland. E-mail: cormac.d.murphy@ucd.ie
dDepartment of Chemistry, Faculty of Science, Alexandria University, P.B. 426 Ibrahimia, Egypt
First published on 19th February 2018
Abstract
The metabolism and polarity of the all-cis tetra-fluorocyclohexane motif is explored in the context of its potential as a motif for inclusion in drug discovery programmes. Biotransformations of phenyl all-cis tetra-, tri- and di- fluoro cyclohexanes with the human metabolism model organism Cunninghamella elegans illustrates various hydroxylated products, but limited to benzylic hydroxylation for the phenyl all-cis tetrafluorocyclohexyl ring system. Evaluation of the lipophilicities (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) indicates a significant and progressive increase in polarity with increasing fluorination on the cyclohexane ring system. Molecular dynamics simulations indicate that water associates much more closely with the hydrogen face of these Janus face cyclohexyl rings than the fluorine face owing to enhanced hydrogen bonding interactions with the polarised hydrogens and water.
P) indicates a significant and progressive increase in polarity with increasing fluorination on the cyclohexane ring system. Molecular dynamics simulations indicate that water associates much more closely with the hydrogen face of these Janus face cyclohexyl rings than the fluorine face owing to enhanced hydrogen bonding interactions with the polarised hydrogens and water.
Introduction
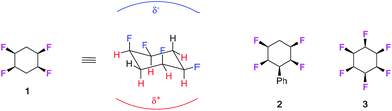
Approximately one third of drugs on the market or in development contain at least one fluorine atom1 and around a third of herbicides historically,2 and half the commercial pesticides introduced in the last period (2010–2016)3 contain fluorine atoms. The element is also important in organic materials with applications in next generation displays4 and high value materials.5 The investigation of new products bearing fluorinated moieties is an ever expanding field, given the particular properties that fluorine bestows on organic compounds.6 We have recently synthesised all-cis 1,2,4,5-tetrafluorocyclohexane ring systems such as 1 and 2 as a novel motif in organic chemistry.7 This tetrafluorocyclohexane isomer displays a particular polar property across the cyclohexane, largely because all of the fluorines are on one face of the ring and there are two 1,3-diaxial C–F bonds, with dipoles orientated parallel to each other.As an extreme example, we extended this concept to the preparation and analysis of all-cis hexafluorocyclohexane 3 which is even more polar due to a cyclohexane ring accommodating three axial C–F bonds.8a This cyclohexane has been referred to as a ‘Janus’-like molecule,8b because of its very well differentiated faces, and recent experimental and theoretical studies have indicated that these rings will coordinate cations to the fluorine face and anions to the hydrogen face, consistent with the electrostatic polarity of the ring system.9 The conformational and polar properties of these multi-vicinal fluorinated aliphatics is beginning to attract the attention of the synthesis community and new methods are emerging for their preparation, for example, from the Gilmour,9 Jacobsen10 and Carreira11 laboratories. For the cyclohexanes, a recent report from Glorius's laboratory12 has demonstrated the direct catalytic hydrogenation of fluorinated aromatics to generate all-cis fluorinated cyclohexanes in a single step, and this methodology promises to make compounds such as cyclohexanes 1 and 3 much more accessible to the organic chemistry community. With these developments in synthesis methods, we believe the cyclohexane motif merits exploration as a candidate substituent for agrochemicals or pharmaceutical drug discovery programmes.
Immediate questions which arise are how will these selectively fluorinated cyclohexane rings be metabolised and how lipophilic are these ring systems. It is commonly understood that increasing the level of fluorination of an organic motif will generally result in increasing its resistance to metabolism at certain sites.13 Also, the prevailing dogma is that increased levels of fluorination render a motif more lipophilic and, thus, its introduction would have a tendency to raise log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values in a manner detrimental to judicious selection in medicinal chemistry. However, it is more complex than that, and Müller and Carreira have exemplified this extensively in recent contributions e.g. mapping log
P values in a manner detrimental to judicious selection in medicinal chemistry. However, it is more complex than that, and Müller and Carreira have exemplified this extensively in recent contributions e.g. mapping log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ps of RCH3 compounds through progressive fluorination to RCF3, where intermediate fluorinations (RCH2F & RCF2H) decrease lipophilicity.14 It is a feature of these ring systems,15 where the fluorines have a relative stereochemistry such that they are all on one face of the cyclohexane, that the rings become polar, and thus increasing fluorination could reasonably increase hydrophilicity. Thus we set out to explore the nature of these ring systems in the context of their properties and potential as a novel motif for inclusion in bioactive research programmes. To that end we focus on phenylcyclohexane 2, because it is readily prepared7c and has been shown to be amenable to a range of synthetic transformations and diversification.16,7b The study compared the metabolism of 2 to close analogues 4–7 by incubation with the human metabolism model organism Cunninghamella elegans.17 Lipophilicity trends (log
Ps of RCH3 compounds through progressive fluorination to RCF3, where intermediate fluorinations (RCH2F & RCF2H) decrease lipophilicity.14 It is a feature of these ring systems,15 where the fluorines have a relative stereochemistry such that they are all on one face of the cyclohexane, that the rings become polar, and thus increasing fluorination could reasonably increase hydrophilicity. Thus we set out to explore the nature of these ring systems in the context of their properties and potential as a novel motif for inclusion in bioactive research programmes. To that end we focus on phenylcyclohexane 2, because it is readily prepared7c and has been shown to be amenable to a range of synthetic transformations and diversification.16,7b The study compared the metabolism of 2 to close analogues 4–7 by incubation with the human metabolism model organism Cunninghamella elegans.17 Lipophilicity trends (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) were also explored comparing cyclohexanes with four, three, two and no fluorine atoms. Lastly, a molecular dynamics simulation study was carried out to elucidate the structural basis of the observed lipophilicity trends.
P) were also explored comparing cyclohexanes with four, three, two and no fluorine atoms. Lastly, a molecular dynamics simulation study was carried out to elucidate the structural basis of the observed lipophilicity trends.
Results and discussion
Biotransformations with Cunninghamella elegans
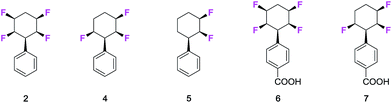
The fungus Cunninghamella elegans represents a well-established model for drug metabolism in mammals due to its ability to biotransform and degrade a wide range of xenobiotics.18 The organism contains a range of cytochrome P450 enzymes and this gives an oxidative metabolic profile which mimics phase-I oxidative metabolism. In order to investigate how the tetrafluorocyclohexyl motif may be metabolised, we have explored the incubation of phenyl tetrafluorocyclohexanes and also compounds with three and two fluorine atoms. Five compounds were investigated in total, three of which were the phenyl derivatives 2, 4 and 5, and two were the benzoic acid derivatives 6 and 7.7d Incubations with C. elegans were carried out in triplicate in submerged liquid cultures. In each case, the incubations were worked up after three days and products were extracted and analysed.Phenylcyclohexane 2 gave rise to only one obvious metabolite in a conversion of around 30%. This product arose by direct hydroxylation at the benzylic position of 2 to give benzyl alcohol 8. Only one product as a single isomer could be detected, with the hydroxyl group configured anti to the adjacent fluorine atoms of the cyclohexane ring. The identity of 8 and its stereochemistry was confirmed by X-ray structure analysis.
Phenyl trifluorocyclohexane 4 was similarly incubated with the fungus and it too gave rise to the analogous benzyl hydroxylated product 9. The extent of microbial conversion was approximately 50% after the three day incubation. The residual 4 was assayed for enantiomeric purity by chiral HPLC, and it was shown to be almost racemic, thus there is no indication that the microbial hydroxylation was significantly enantioselective. Finally in this series, difluorocyclohexane 5 was subject to a similar incubation with C. elegans. This compound was completely and extensively metabolised, and it generated a much greater product profile of which compounds 10–13 were isolated. Compounds 11–13 were characterised by X-ray crystallography as illustrated in Fig. 1. Monohydroxylated products 10–12, can be rationalised by direct methylene P450 type hydroxylations, however the monofluorinated cyclohexanol 13 is less easily rationalised and presumably arises from a series of biotransformations involving fluoride elimination. More generally, it is clear that removal of two of the ring fluorines from positions 2 and 3 of the phenyl all-cis tetracyclohexyl ring system has rendered the aliphatic ring much more susceptible to metabolism.
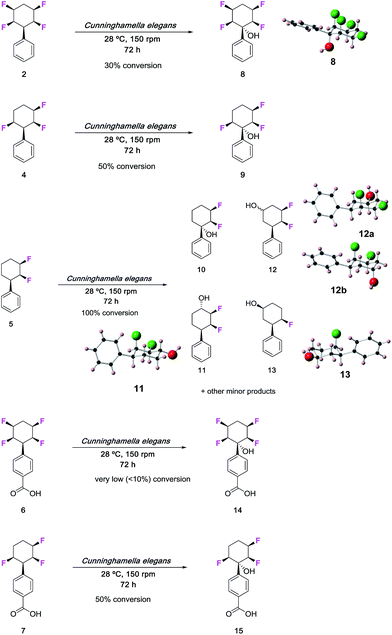 | ||
| Fig. 1 Biotransformations of selectively fluorinated phenyl fluorocyclohexanes 2 and 4–7 by C. elegans. Some of the products were crystalline and amenable to X-ray structure analysis. | ||
The benzoic acids 6 and 7 were also incubated with C. elegans. The tetrafluorocyclohexyl benzoic acid 6 was poorly biotransformed and only a very low conversion to alcohol 14 was obvious after the three day incubation. Trifluorocyclohexyl benzoic acid 7 was more readily transformed, but only to benzylalcohol 15 (∼50% conversion). This product was isolated and crystallised and X-ray analysis confirmed its structure. Again, in order to explore any enantioselectivity for this biotransformation, the methyl ester of the residual carboxylic acid 7 was analysed by chiral HPLC and this indicated a very low enantioselectivity, thus in a similar outcome to substrate 4, there was no obvious selectivity for 7 by the hydroxylation enzyme involved.
Lipophilicity study of selectively fluorinated phenyl cyclohexanes
An important measure of the druggability of a substituent is its lipophilicity,19 and given the polarity of the phenyl all-cis tetrafluorocyclohexyl moiety it was of interest to explore the relative log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ps of various analogous compounds. log
Ps of various analogous compounds. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P's were measured by reverse phase HPLC (AcCN 60%: water 40%, with TFA 0.05%), as previously described.20 The measured log
P's were measured by reverse phase HPLC (AcCN 60%: water 40%, with TFA 0.05%), as previously described.20 The measured log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P's of a series of phenyl fluorocyclohexane derivatives are summarised in Fig. 2 and against a series of compounds, of known log
P's of a series of phenyl fluorocyclohexane derivatives are summarised in Fig. 2 and against a series of compounds, of known log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values, which were re-measured for comparison, including biphenyl 16 and phenylcyclohexane 17.
P values, which were re-measured for comparison, including biphenyl 16 and phenylcyclohexane 17.
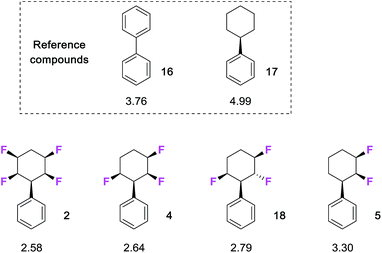 | ||
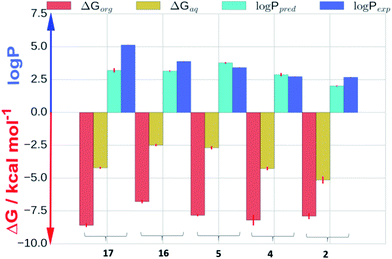
Fig. 2 Measured20 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values for compounds selectively fluorinated phenylcyclohexanes and reference compounds 16 and 17. Increasing fluorination lowers log P values for compounds selectively fluorinated phenylcyclohexanes and reference compounds 16 and 17. Increasing fluorination lowers log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P consistent with increasing hydrophilicity. P consistent with increasing hydrophilicity. | ||
It is clear that there is a significant reduction in log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P with increasing fluorination. Phenyldifluorocyclohexane 5 (log
P with increasing fluorination. Phenyldifluorocyclohexane 5 (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 3.30) is significantly more polar than the phenylcyclohexane (log
P 3.30) is significantly more polar than the phenylcyclohexane (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 4.99), and then both the tri-and tetra- fluoro cyclohexanes progressively increase in polarity (log
P 4.99), and then both the tri-and tetra- fluoro cyclohexanes progressively increase in polarity (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ps of 2.64 and 2.58 respectively) with additional fluorine atoms. An interesting comparison on log
Ps of 2.64 and 2.58 respectively) with additional fluorine atoms. An interesting comparison on log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ps can be made with the two trifluorinated stereoisomers 4 and 18. Compound 4 is more polar, and this presumably arises as it has a preferred diaxial arrangement of the C2 and C6 C–F bonds.7c This parallel alignment can be expected to increase the molecular dipole relative to isomer 18 which has one of these fluorines lying in an equatorial orientation.
Ps can be made with the two trifluorinated stereoisomers 4 and 18. Compound 4 is more polar, and this presumably arises as it has a preferred diaxial arrangement of the C2 and C6 C–F bonds.7c This parallel alignment can be expected to increase the molecular dipole relative to isomer 18 which has one of these fluorines lying in an equatorial orientation.
The study extended to substituted aryls of the benzoic acids 6 and 7 and the anilines 21 and 22(ref. 7b) as illustrated in Fig. 3. In each case both the trifluoro- and tetrafluoro- cyclohexanes are around two log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P units more lipophilic than the nonfluorinated cyclohexanes 20 and 24, whereas the phenyl derivatives 19 and 23 lie in between. There is a clear trend that selective fluorinations around the ring increases the polarity of the cyclohexane.
P units more lipophilic than the nonfluorinated cyclohexanes 20 and 24, whereas the phenyl derivatives 19 and 23 lie in between. There is a clear trend that selective fluorinations around the ring increases the polarity of the cyclohexane.
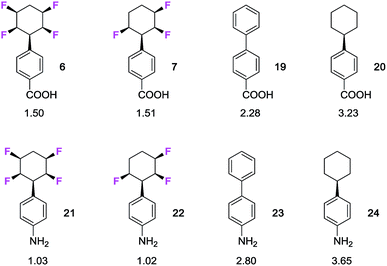 | ||
Fig. 3 Measured20 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values for benzoic acid and aniline derivatives of selectively fluorinated cyclohexanes. P values for benzoic acid and aniline derivatives of selectively fluorinated cyclohexanes. | ||
Computational analysis of lipophilicity trends
Molecular dynamics (MD) simulations were carried out for phenylcyclohexanes 2, 4, 5, 16 and 17 to clarify the mechanisms by which progressive fluorination decreases lipophilicity. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ps were predicted by computing absolute solvation free energies in aqueous and organic phases using explicit solvent molecular dynamics simulations.21Fig. 4 shows a comparison of calculated (log
Ps were predicted by computing absolute solvation free energies in aqueous and organic phases using explicit solvent molecular dynamics simulations.21Fig. 4 shows a comparison of calculated (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ppred) and measured (log
Ppred) and measured (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pexp) log
Pexp) log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values, as well as calculated solvation free energies in aqueous (ΔGaq) and cyclohexane (ΔGorg) phases. Overall, the log
P values, as well as calculated solvation free energies in aqueous (ΔGaq) and cyclohexane (ΔGorg) phases. Overall, the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P calculations are in good agreement with the experimental data (Kendall tau 0.5 ± 0.1 and mean unsigned error 0.77 ± 0.07 log
P calculations are in good agreement with the experimental data (Kendall tau 0.5 ± 0.1 and mean unsigned error 0.77 ± 0.07 log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P units). Inspection of the solvation free energies shows that the trend for decreased log
P units). Inspection of the solvation free energies shows that the trend for decreased log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P upon increased fluorination is due to a more rapid decrease in solvation free energies in the aqueous phase (from ca. −2.5 to −5.2 kcal mol−1 for 16 and 2 respectively) vs. the cyclohexane phase (ca. −7.5 kcal mol−1 for all compounds).
P upon increased fluorination is due to a more rapid decrease in solvation free energies in the aqueous phase (from ca. −2.5 to −5.2 kcal mol−1 for 16 and 2 respectively) vs. the cyclohexane phase (ca. −7.5 kcal mol−1 for all compounds).
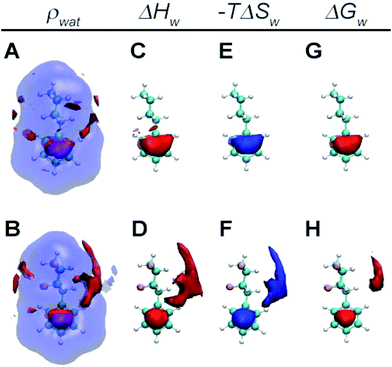
Further insights were investigated to help rationalise the calculated differences in hydration free energies by grid-cell theory (GCT) analyses of the MD simulation trajectories.22 GCT is a MD trajectory post-processing method that spatially resolves the water contribution to enthalpies, entropies and free energies of the hydration for small molecules, host/guests and protein-ligands complexes.23
Fig. 5 depicts spatially resolved hydration thermodynamics around the non-fluorinated cyclohexane 17 and the tetrafluorinated cyclohexane 2. Comparison of water density contours show water structuring above and below the π-cloud of the phenyl ring due to the expected weak hydrogen bonding interactions in this region. In addition the four fluorine atoms in 2 induce further structuring of water around the cyclohexyl moiety, with a more pronounced effect around the hydrogen face of the cyclohexane (panels A and B). Owing to the different polarities of the cyclohexyl ring in 2, water near the fluorine-face preferentially orients hydrogen atoms towards the ring, whereas water near the hydrogen-face preferentially orients oxygen atoms towards the ring. Water near the hydrogen-face is more enthalpically stabilised and entropically destabilised with respect to bulk, whereas the energetics are not significantly different from the bulk in the vicinity of the fluorine face (panels C and D and E and F). Overall favourable enthalpic contributions offset unfavourable entropic contributions for water near the hydrogen face and water in this region makes additional favourable contributions to the hydration free energy (panels G and H). Therefore the decreased lipophilicity of 2 with respect to 17 is attributed to enhanced hydrogen bonding interactions between water and the hydrogen face of the all-cis tetrafluorocyclohexane ring.
Conclusions
The all-cis tetrafluorocyclohexane motif has been recognised to have particularly polar properties and the ease of synthesis of the phenyl derivative 2 has prompted us to investigate it properties further as it emerges as a building block for the introduction of this new motif into medicinal chemistry and other bioactives discovery programmes. The metabolism of the phenyl cyclohexane derivatives 2, 4–7 with varying levels of fluorination was explored in incubations with Cunninghamella elegans. This fungus has been used as a microbial model for mammalian metabolism. In the present study we observed that increasing the degree of fluorination of cyclohexyl ring leads to a more stable xenobiotic. The phenyl all-cis tetrafluorocyclohexane 2 was significantly less metabolised than the trifluoro-4 and then difluoro-5, the latter of which was extensively metabolised. In the case of 2, 4, 6 and 7 metabolism is confined to benzylic hydroxylation.A systematic log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P evaluation of these ring systems shows an increase in hydrophilicity with increasing fluorination, and for the phenyl all-cis tetrafluorocyclohexanes (including anilines and benzoic acids) there is a maximal effect. These ring systems are at least two full log
P evaluation of these ring systems shows an increase in hydrophilicity with increasing fluorination, and for the phenyl all-cis tetrafluorocyclohexanes (including anilines and benzoic acids) there is a maximal effect. These ring systems are at least two full log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P units (100 fold) more hydrophilic than their non-fluorinated cyclohexane counterparts.
P units (100 fold) more hydrophilic than their non-fluorinated cyclohexane counterparts.
Molecular dynamics simulations reproduce the experimental trends and suggest that the decreased lipophilicity of 2 is due to enhanced hydrogen bonding interactions of water molecules with the hydrogen face of the cyclohexane ring with respect to bulk water. The orientation of the water near this face of the ring was consistent with the hydrogen bonding donor ability of the polarised hydrogens of the ring.
This contrasts with the energetics of water near the fluorine face of the ring which are comparable to bulk water. Altogether these studies indicate that metabolism of the all-cis tetrafluorocyclohexyl motif is slow, and that the ring system is significantly hydrophilic for an aliphatic motif. These factors add to the unique facially polarised aspect of this motif and make it an attractive option for inclusion in medicinal chemistry or crop protection studies.
Conflicts of interest
There are no conflicts of interest.Acknowledgements
This work was supported by the Initial Training Network, FLUOR21, funded by the FP7 Marie Curie Actions of the European Commission (FP7-PEOPLE-2013-ITN-607787). JM is supported by a University Research Fellowship from the Royal Society. The research leading to these results has received funding from the European Research Council under the EU 7th Framework Programme (FP7/2007-2013)/ERC No 336289. We acknowledge the EPSRC UK National Mass Spectrometry Facility at Swansea University. We also thank Drs Guillaume Berthon, Adam Burris and Andreas Beck of Syngenta Crop Protection AG, Stein Switzerland for assistance in log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P determinations.
P determinations.
Notes and references
- (a) J. Wang, M. Sanchez-Rosello, J. L. Acena, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2014, 114, 2432–2506 CrossRef CAS PubMed; (b) D. O'Hagan and D., J. Fluorine Chem., 2010, 131, 1071–1081 CrossRef; (c) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320–330 RSC; (d) J.-P. Begue and D. Bonnet-Delpon, J. Fluorine Chem., 2006, 127, 992–1012 CrossRef CAS; (e) K. Müller, C. Faeh and F. Diederich, Science, 2007, 317, 1881–1886 CrossRef PubMed; (f) W. K. Hagmann, J. Med. Chem., 2008, 51, 4359–4369 CrossRef CAS PubMed; (g) K. L. Kirk, J. Fluorine Chem., 2006, 127, 1013–1029 CrossRef CAS.
- T. Fujiwara and D. O'Hagan, J. Fluorine Chem., 2014, 167, 16–29 CrossRef CAS.
- P. Jeschke, Pest Manage. Sci., 2017, 73, 1053–1066 CrossRef CAS PubMed.
- (a) M. Alaasar, M. Prehm, S. Poppe and C. Tschierske, Chem.–Eur. J., 2017, 23, 5541–5556 CrossRef CAS PubMed; (b) P. Kirsch, J. Fluorine Chem., 2015, 177, 29–36 CrossRef CAS; (c) N. Al-Maharik, P. Kirsch, A. M. Z. Slawin and D. O'Hagan, Tetrahedron, 2014, 70, 4626–4630 CrossRef CAS; (d) M. Bremer, P. Kirsch, M. Klasen-Memmer and K. Tarumi, Angew. Chem., Int. Ed., 2013, 52, 8880–8896 CrossRef CAS PubMed.
- (a) W. Feng, P. Long, Y. Feng and Y. Li., Adv. Sci., 2016, 3, 1500413 CrossRef PubMed; (b) R. J. Kashtiban, M. A. Dyson, R. R. Nair, R. Zan, S. L. Wong, Q. Ramasse, A. K. Geim, U. Bangert and J. Sloan, Nat. Commun., 2014, 5, 5545 CrossRef CAS.
- (a) C. Thiehoff, Y. P. Rey and R. Gilmour, Isr. J. Chem., 2017, 57, 92–100 CrossRef CAS; (b) D. L. Orsi and and R. A. Altman, Chem. Commun., 2017, 53, 7168–7181 RSC.
- (a) A. J. Durie, A. M. Z. Slawin, T. Lebl, P. Kirsch and D. O'Hagan, Chem. Commun., 2012, 48, 9643–9645 RSC; (b) A. J. Durie, T. Fujiwara, R. Cormanich, M. Bühl, A. M. Z. Slawin and D. O'Hagan, Chem.–Eur. J., 2014, 20, 6259–6263 CrossRef CAS PubMed; (c) A. J. Durie, T. Fujiwara, N. Al-Maharik, A. M. Z. Slawin and D. O'Hagan, J. Org. Chem., 2014, 79, 8228–8233 CrossRef CAS PubMed; (d) M. S. Ayoup, D. B. Cordes, A. M. Z. Slawin and D. O'Hagan, Org. Biomol. Chem., 2015, 13, 5621–5624 RSC; (e) M. S. Ayoup, D. B. Cordes, A. M. Z. Slawin and D. O'Hagan, Beilstein J. Org. Chem., 2015, 11, 2671–2676 CrossRef CAS PubMed.
- (a) N. S. Keddie, A. M. Z. Slawin, T. Lebl, D. Philp and D. O'Hagan, Nat. Chem., 2015, 7, 483–488 CrossRef CAS PubMed; (b) N. Santschi and R. Gilmour, Nat. Chem., 2015, 7, 467–468 CrossRef CAS PubMed.
- (a) F. Scheidt, P. Selter, N. Santschi, M. C. Holland, D. V. Dudenko, C. Daniliuc, C. Mück-Lichtenfeld, M. R. Hansen and R. Gilmour, Chem.–Eur. J., 2017, 23, 6142–6149 CrossRef CAS PubMed; (b) I. G. Molinár and R. Gilmour, J. Am. Chem. Soc., 2016, 138, 5004–5007 CrossRef PubMed; (c) I. G. Molnár, C. Thiehoff, M. C. Holland and R. Gilmour, ACS Catal., 2016, 6, 7167–7173 CrossRef.
- S. M. Banik, J. W. Medley and E. N. Jacobsen, J. Am. Chem. Soc., 2016, 138, 5000–5003 CrossRef CAS PubMed.
- S. Fischer, N. Huwyler, S. Wolfrum and E. M. Carreira, Angew. Chem., Int. Ed., 2016, 55, 2555–2558 CrossRef CAS PubMed.
- M. P. Wiesenfeldt, Z. Nairoukh, W. Li and F. Glorius, Science, 2017, 357, 908–912 CrossRef CAS PubMed.
- (a) T. Bright, F. Dalton, V. L. Elder, C. D. Murphy, N. K. O'Connor and G. Sandford, Org. Biomol. Chem., 2013, 11, 1135–1142 RSC; (b) M. J. Shaughnessy, A. Harsanyi, J. Li, T. Bright, C. D. Murphy and G. Sandford, ChemMedChem, 2014, 9, 733–736 CrossRef CAS PubMed.
- (a) Q. A. Huchet, N. Trapp, B. Kuhn, B. Wagner, H. Fischer, N. A. Kratochwil, E. M. Carreira and K. Müller, J. Fluorine Chem., 2017, 198, 34–46 CrossRef CAS; (b) R. Vorberg, N. Trapp, D. Zimmerli, B. Wagner, H. Fischer, N. A. Kratochwil, M. Kansy, E. M. Carreira and K. M. Müller, ChemMedChem, 2016, 11, 2216–2239 CrossRef CAS PubMed.
- (a) B. E. Ziegler, M. Lecours, R. A. Marta, J. Featherstone, E. Fillion, W. S. Hopkins, V. Steinmetz, N. S. Keddie, D. O'Hagan and T. B. McMahon, J. Am. Chem. Soc., 2016, 138, 7460–7463 CrossRef CAS PubMed; (b) M. J. Lecours, R. A. Marta, V. Steinmetz, N. S. Keddie, E. Fillion, D. O'Hagan, T. B. McMahon and W. S. Hopkins, J. Phys. Chem. Lett., 2017, 8, 109–113 CrossRef CAS PubMed.
- (a) T. Bykova, N. Al-Maharik, A. M. Z. Slawin and D. O'Hagan, Beilstein J. Org. Chem., 2017, 13, 728–733 CrossRef CAS PubMed; (b) T. Bykova, N. Al-Maharik, A. M. Z. Slawin and D. O'Hagan, Org. Biomol. Chem., 2016, 14, 1117–1123 RSC.
- M. Hezari and P. J. Davis, Drug Metab. Dispos., 1993, 21, 259–267 CAS.
- (a) W. Palmer-Brown, B. Dunne, Y. Ortin, M. A. Fox, G. Sandford and C. D. Murphy, Xenobiotica, 2017, 47, 763–770 CrossRef CAS PubMed; (b) J. Amadio, E. Casey and C. D. Murphy, Appl. Microbiol. Biotechnol., 2013, 97, 5955–5963 CrossRef CAS PubMed; (c) J. Amadio and C. D. Murphy, Appl. Microbiol. Biotechnol., 2010, 86, 345–351 CrossRef CAS PubMed; (d) L. Quinn, R. Dempsey, E. Casey, A. Kane and C. D. Murphy, J. Ind. Microbiol. Biotechnol., 2015, 42, 799–806 CrossRef CAS PubMed.
- (a) C. A. Lipinski, Adv. Drug Delivery Rev., 2016, 101, 34–41 CrossRef CAS PubMed; (b) C. A. Lipinski, F. Lombardo, B. W. Dominy and P. J. Feeney, Adv. Drug Deliv. Rev., 1997, 23, 3–25 CrossRef CAS.
- (a) R. Tomita, N. Al-Maharik, A. Rodil, M. Bühl and D. O'Hagan, Org. Biomol. Chem., 2018, 16, 1113–1117 RSC; (b) C. Giaginis and A. Tsantili-Kakoulidou, J. Liq. Chromatogr. Relat. Technol., 2008, 31, 79–96 CrossRef CAS; (c) C. My Du, K. Valko, C. Bevan, D. Reynolds and M. H. Abraham, Anal. Chem., 1998, 70, 4228–4234 CrossRef.
- (a) S. Bosisio, A. S. J. S. Mey and M. J. Michel, J. Comput.-Aided Mol. Des., 2016, 30, 1101–1114 CrossRef CAS PubMed; (b) C. C. Bannan, K. H. Burley, M. Chiu, M. R. Shirts, M. K. Gilson and D. L. Mobley, J. Comput.-Aided Mol. Des., 2016, 30, 927–944 CrossRef CAS PubMed; (c) J. P. M Jämbeck, F. Mocci, A. P. Lyubartsev and A. Laaksonen, J. Comput. Chem., 2013, 34, 187–197 CrossRef PubMed.
- G. Gerogiokas, G. Calabro, R. H. Henchman, M. W. Y. Southey, R. J. Law and J. Michel, J. Chem. Theory Comput., 2013, 10, 35–48 CrossRef PubMed.
- (a) J. Michel, R. H. Henchman, G. Gerogiokas, M. W. Southey, M. P. Mazanetz and R. J. Law, J. Chem. Theory Comput., 2014, 10, 4055–4068 CrossRef CAS PubMed; (b) G. Gerogiokas, M. W. Y. Southey, M. P. Mazanetz, A. Heifetz, M. Bodkin, R. J. Law and M. J. Michel, Phys. Chem. Chem. Phys., 2015, 17, 8416–8426 RSC; (c) G. Gerogiokas, M. W. Y. Southey, M. P. Mazanetz, A. Heifetz, M. Bodkin, R. J. Law, R. H. Henchman and M. J. Michel, J. Phys. Chem. B, 2016, 120, 10442–10452 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1817386–1817390. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8sc00299a |
| This journal is © The Royal Society of Chemistry 2018 |