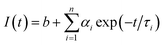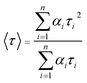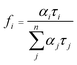Open Access Article
Open Access ArticleTunable syngas production from photocatalytic CO2 reduction with mitigated charge recombination driven by spatially separated cocatalysts†
Ang
Li‡
,
Tuo
Wang‡
 ,
Xiaoxia
Chang
,
Zhi-Jian
Zhao
,
Chengcheng
Li
,
Zhiqi
Huang
,
Piaoping
Yang
,
Guangye
Zhou
and
Jinlong
Gong
,
Xiaoxia
Chang
,
Zhi-Jian
Zhao
,
Chengcheng
Li
,
Zhiqi
Huang
,
Piaoping
Yang
,
Guangye
Zhou
and
Jinlong
Gong
 *
*
Key Laboratory for Green Chemical Technology of Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Weijin Road 92, Tianjin 300072, China. E-mail: jlgong@tju.edu.cn
First published on 25th May 2018
Abstract
Photocatalytic CO2 reduction represents a sustainable route to generate syngas (the mixture of CO and H2), which is a key feedstock to produce liquid fuels in industry. Yet this reaction typically suffers from two limitations: unsuitable CO/H2 ratio and serious charge recombination. This paper describes the production of syngas from photocatalytic CO2 reduction with a tunable CO/H2 ratio via adjustment of the components and surface structure of CuPt alloys and construction of a TiO2 mesoporous hollow sphere with spatially separated cocatalysts to promote charge separation. Unlike previously reported cocatalyst-separated hollow structures, we firstly create a reductive outer surface that is suitable for the CO2 reduction reaction. A high evolution rate of 84.2 μmol h−1 g−1 for CO and a desirable CO/H2 ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 are achieved. The overall solar energy conversion yield is 0.108%, which is higher than those of traditional oxide and sulfide based catalysts (generally about 0.006–0.042%). Finally, density functional theory calculations and kinetic experiments by replacing H2O with D2O reveal that the enhanced activity is mainly determined by the reduction energy of CO* and can be affected by the stability of COOH*.
2 are achieved. The overall solar energy conversion yield is 0.108%, which is higher than those of traditional oxide and sulfide based catalysts (generally about 0.006–0.042%). Finally, density functional theory calculations and kinetic experiments by replacing H2O with D2O reveal that the enhanced activity is mainly determined by the reduction energy of CO* and can be affected by the stability of COOH*.
Introduction
Fossil fuels play a pivotal role in energy supply and production, while such sources are unsustainable because of the finite amounts and the massive emission of CO2 (the main contributor to the greenhouse effect). Generally, there are two approaches to adjust the concentration of CO2 in the carbon cycle, including the storage of CO2 and the conversion of CO2 to useful chemicals.1 The photocatalytic CO2 reduction reaction (CRR) represents a promising approach for CO2 conversion, where the energy required to drive the uphill reaction directly come from solar irradiation.2,3 For instance, N-doped carbon@NiCo2O4 double-shelled nano-boxes and CuO–TiO2−xNx hollow nanocubes were used as absorption-enhanced photocatalysts, which can efficiently reduce CO2 to CH4 and CO.4,5 ZnIn2S4–In2O3 nanotubes and TiO2/graphene hollow structures with mitigated charge recombination were also synthesized for an effective photocatalytic CRR to produce CO and CH4.6,7 Typically, the CRR can produce various valuable chemical fuels, among which syngas (synthesis gas, the mixture of CO and H2) is a critical feedstock to produce synthetic liquid fuels via an established industrial process (e.g., Fischer–Tropsch synthesis).8 However, in industrial processes, unsuitable CO/H2 ratios often limit the practical applications of synthesis. Thus it is critical to precisely control the CO/H2 ratio from the photocatalytic CRR. To achieve this goal, appropriate catalysts with suitable chemical composition and surface properties are desired. Furthermore, a fundamental understanding of reaction processes and energetic changes in every elementary step is also significant.8,9Another limitation for the photocatalytic CRR lies in the severe recombination of photo-induced charge carriers (electrons and holes). Many strategies have been developed to solve such an ineluctable problem in photocatalytic reactions. Typically, junctions between different materials,10,11 phases12 or crystallographic planes13 have been constructed to enhance charge separation. However, interfaces between different materials will create recombination centers, which greatly restrict the efficiency of charge separation.14 Alternatively, the loading of cocatalysts can serve as traps of charges to promote their separation.14,15 Typically, cocatalysts are particles of metals, metal oxides or sulfides deposited on semiconductors, which can be classified into reduction and oxidation cocatalysts. Metal particles (such as Pt, Ag, and alloys)13,16,17 often act as reduction cocatalysts and electron shuttles when their Fermi level lies below the CB of the semiconductor and above the redox potential of the target reaction. On the other hand, metal oxides or sulfides (MnOx, PbO2, PbS etc.)13,18,19 can trap holes and are often used as oxidation cocatalysts. Generally, the co-deposition of both reduction and oxidation cocatalysts can trap electrons and holes separately and improve the reduction and oxidation reactions simultaneously. Nevertheless, the simple addition of cocatalysts with a random distribution may increase the possibility of recombination and lead to severe back reactions.15 One possible solution is to create a catalyst with spatially separated oxidation (hole traps) and reduction cocatalysts (electron traps) based on hollow structures to facilitate charge separation.14,17,20–23 For such structures with spatially separated cocatalysts, the overall photocatalytic activity is mainly determined by the properties of the outer surface, because active sites loaded inside will obstruct mass transportation and may cause severe cross reactions.14,17 However, in most previously reported structures, the outer surfaces are loaded with oxidation cocatalysts,14,17,20–23 creating oxidative outer surfaces that are only suitable for oxidation reactions.24 This is because loading oxidation cocatalysts inside uniformly with high dispersion is a great challenge due to the incompatibility between the precursor and template.14,17,20–23 Therefore, it is urgent to develop a structure with a reductive outer surface suitable for reduction reactions including the CRR.
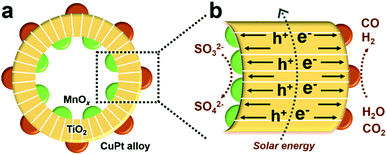
This paper describes the design and fabrication of a structure with reductive outer surfaces, decorated with spatially separated cocatalysts, where oxidation cocatalysts are uniformly dispersed on inner surfaces, which is efficient for the photocatalytic CRR. Besides, the CO/H2 ratio can be adjusted in a large range including the desired 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 by changing the component and properties of reduction cocatalysts.8,9,25 Specifically, TiO2 hollow spheres, MnOx and CuPt alloys act as the main catalysts, oxidation and reduction cocatalysts, respectively, and the final product is denoted as MnOx@TiO2@CuPt alloy mesoporous hollow spheres (MTCP-MSs, Fig. 1a). Driven by the spatially separated MnOx particles and CuPt alloys, holes and electrons will flow in opposite directions (Fig. 1b) to enhance the charge-separation efficiency. When these well-separated electrons reach the surfaces of reduction cocatalysts (CuPt alloy) with optimized Cu content, a high catalytic activity with a desired CO/H2 ratio will be achieved by adjusting the energy of CO2 activation and CO* desorption during the photocatalytic CRR, which can be explained by density functional theory (DFT) calculations and kinetic experiments.
2 by changing the component and properties of reduction cocatalysts.8,9,25 Specifically, TiO2 hollow spheres, MnOx and CuPt alloys act as the main catalysts, oxidation and reduction cocatalysts, respectively, and the final product is denoted as MnOx@TiO2@CuPt alloy mesoporous hollow spheres (MTCP-MSs, Fig. 1a). Driven by the spatially separated MnOx particles and CuPt alloys, holes and electrons will flow in opposite directions (Fig. 1b) to enhance the charge-separation efficiency. When these well-separated electrons reach the surfaces of reduction cocatalysts (CuPt alloy) with optimized Cu content, a high catalytic activity with a desired CO/H2 ratio will be achieved by adjusting the energy of CO2 activation and CO* desorption during the photocatalytic CRR, which can be explained by density functional theory (DFT) calculations and kinetic experiments.
Results and discussion
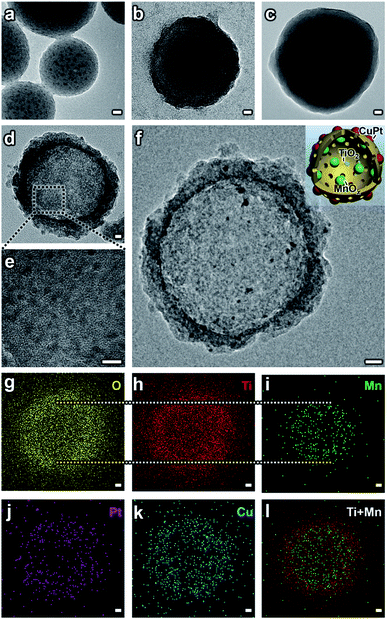
A hard-templating method (using SiO2 spheres as templates and dividers to disperse small particles) was used to synthesize the MTCP-MSs.14,17,26–28 Before the formation of the final structure, the catalysts mainly went through five stages with different morphologies. (1) SiO2–MnOx nanospheres (SM-NSs). A modified Stöber method was used to prepare the SiO2 nanospheres loaded with MnOx particles.17 Under alkaline conditions, KMnO4 was chosen as the precursor and it would be slowly reduced by ethanol to form MnOx (Fig. S2†), ensuring the uniform distribution of MnOx particles (Fig. 2a, S3 and S1a and b†). The high resolution transmission electron microscopy (HRTEM) image further confirms the existence of MnOx particles (Fig. S3d†). (2) SiO2–MnOx@TiO2 core–shell nanospheres (SMT-NSs). Subsequently, a layer of TiO2 was uniformly coated on SM-NSs by the hydrolysis of titanium tert-butoxide (TBOT) (Fig. 2b and S4†). (3) SiO2–MnOx@TiO2@SiO2 core–shell nanospheres (SMTS-NSs). Another SiO2 layer was then coated on the outer surface of SMT-NSs for protection. Without the protective layer, TiO2 shells would be destroyed during the subsequent calcination step (Fig. S1c†).26 The smooth surfaces shown in Fig. 2c and S5† suggest the successful coating of SiO2 layers. Uniformly distributed MnOx particles can also be observed. (4) MnOx@TiO2 mesoporous hollow spheres (MT-MSs). SMT-NSs were then calcined to create a mesoporous structure with a highly crystallized anatase phase (a crystal type of TiO2 suitable for photocatalysis).26 Typically, the crystallization processes from amorphous to anatase and from anatase to rutile occur in the temperature ranges of 450–550 °C and 600–700 °C,26 respectively. Thus the temperature we adopted at 500 °C can ensure the formation of a pure anatase phase, which can also be evidenced by XRD patterns (Fig. S8†). NaOH was used to etch all the SiO2 layers to form MT-MSs (Fig. 2d and S6†). Uniform MnOx particles are loaded on TiO2 shells (Fig. 2e), which could be confirmed by HRTEM (Fig. S6c†). (5) MnOx@TiO2@CuPt alloy mesoporous hollow spheres (MTCP-MSs). This final structure was formed after the loading of the CuPt alloy (Fig. 2f). To selectively load CuPt particles uniformly on the outer surface without agglomeration (Fig. S1a†), a photodeposition method (Fig. S7†) was adopted29 under the protection of a N2 atmosphere in methanolic solvent, using platinum acetylacetonate and copper acetylacetonate as precursors. Energy dispersive spectroscopy (EDS) mapping (Fig. 2g–l) and HRTEM (Fig. S9a†) exhibit the relative position and components of every material. It is obvious that MnOx and CuPt particles are selectively loaded on the inner and outer surfaces of TiO2 hollow spheres. X-Ray Diffraction (XRD) patterns (Fig. S8†) evidenced the formation of TiO2 and the CuPt alloy. For a low loading and small particle size, the single of MnOx is not obvious, while can be clearly seen in the HRTEM (Fig. S9a†). The loading of the CuPt alloy can also be evidenced by the result of X-ray photoelectron spectroscopy (XPS), which shows obviously peaks at the banding energies of 74 eV, 71 eV and 932 eV, representing the Pt 4f5/2, Pt 4f7/2 and Cu 2p3/2 of the CuPt alloy (Fig. S10†), matching well with references,9 indicating the successful loading of the CuPt alloy on the outer surfaces. EDS analysis (Fig. S9b†) focused on a single CuPt particle (within the dotted circle in Fig. S9a†)30 quantitatively revealed the microscopic composition of the CuPt alloy (Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt = 3.33), consistent with the result of inductively coupled plasma optical emission spectroscopy (ICP-OES) (Cu
Pt = 3.33), consistent with the result of inductively coupled plasma optical emission spectroscopy (ICP-OES) (Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt = 3.17). Thus, the catalysts can be finally denoted as MTC3.17P-MSs according to the ICP-OES result. HRTEM of MTC3.17P-MSs with a higher resolution are provided (Fig. S9†) to show the composition and relative position of the CuPt alloy and MnOx. By changing the feed ratio of precursors, CuPt alloys with different component ratios were synthesized. The results of EDS at a single point (Cu
Pt = 3.17). Thus, the catalysts can be finally denoted as MTC3.17P-MSs according to the ICP-OES result. HRTEM of MTC3.17P-MSs with a higher resolution are provided (Fig. S9†) to show the composition and relative position of the CuPt alloy and MnOx. By changing the feed ratio of precursors, CuPt alloys with different component ratios were synthesized. The results of EDS at a single point (Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt = 0.35, 1.48, 8.80) (Fig. S11†) are also consistent with the respective ICP-OES results (Cu
Pt = 0.35, 1.48, 8.80) (Fig. S11†) are also consistent with the respective ICP-OES results (Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt = 0.31, 1.13, 8.72), and the corresponding catalysts can be denoted as MTC0.31P-MSs, MTC1.13P-MSs and MTC8.72P-MSs, respectively.
Pt = 0.31, 1.13, 8.72), and the corresponding catalysts can be denoted as MTC0.31P-MSs, MTC1.13P-MSs and MTC8.72P-MSs, respectively.
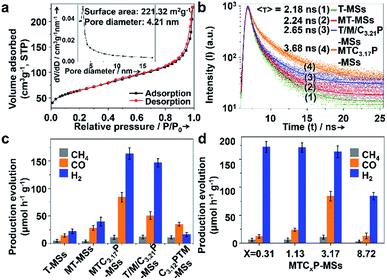
The Brunauer–Emmett–Teller (BET) surface areas and pore structures of MTCP-MSs were measured by nitrogen adsorption at 77 K. A type IV (defined by IUPAC in 1984 (ref. 31)) isotherm along with two hysteresis loops at relative pressures (P/Po) of 0.40–0.66 and 0.86–0.99 was observed (Fig. 3a), indicating the presence of mesopores in this sample.32 The BET surface area was calculated to be about 221.32 m2 g−1, which can provide abundant catalytically active sites for heterogeneous photocatalysis.24,33–36 The average pore size was determined to be 4.21 nm, which greatly improves the penetration and transportation of reactants and products.14,17,27,37–41
The light absorption for photocatalysts can be characterized by ultraviolet-visible (UV-Vis) spectroscopy (Fig. S12†). The UV-Vis of T-MSs consisted of pure TiO2 shows a strong absorption within the UV region (λ < 400 nm) while almost no absorption in the visible region (λ > 400 nm). For MT-MSs, a slight absorption of visible light can be observed. Furthermore, after the loading of the CuPt alloy to form MTCP-MSs, the absorption of visible light can be greatly enhanced, which may be attributed to the localized surface plasmon resonance of Cu and Pt.17,42 To further clarify the role of Cu, MT-MSs-Pt was synthesized by loading pure Pt onto MT-MSs via photoreduction. Compared with MT-MSs-Pt, MTCP-MSs exhibit stronger absorption of visible light, indicating that the Cu particles could act as a sensitizer to expand the light response of TiO2 to the visible region.17,42
To confirm the enhancement of charge separation and CRR activity by spatially separated cocatalysts (MTC3.17P-MSs), reference catalysts including pure TiO2 mesoporous hollow spheres (T-MSs, Fig. S13†) and TiO2/MnOx/Cu3.21Pt mesoporous hollow spheres (T/M/C3.21P-MSs, Fig. S14†) were also synthesized. T/M/C3.21P-MSs stand for T-MSs with MnOx and CuPt alloys (Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt = 3.21, detected by ICP-OES) randomly distributed on both inner and outer surfaces, constructed by a direct immersion method.13 Catalysts with the same components but oxidative outer surfaces constructed by loading CuPt inside while MnOx outside were also synthesized, denoted as C3.12PTM-MSs. It should be noted that the Cu contents of the CuPt alloy in MTC3.17P-MSs (with separated distributed cocatalysts), T/M/C3.21P-MSs (with randomly distributed cocatalysts) and C3.12PTM-MSs (with oxidation outer surfaces) are all around 76% (molar content). Thus, with similar compositions, the only differences between these catalysts is the distribution of cocatalysts, which should be responsible for the difference of activities.
Pt = 3.21, detected by ICP-OES) randomly distributed on both inner and outer surfaces, constructed by a direct immersion method.13 Catalysts with the same components but oxidative outer surfaces constructed by loading CuPt inside while MnOx outside were also synthesized, denoted as C3.12PTM-MSs. It should be noted that the Cu contents of the CuPt alloy in MTC3.17P-MSs (with separated distributed cocatalysts), T/M/C3.21P-MSs (with randomly distributed cocatalysts) and C3.12PTM-MSs (with oxidation outer surfaces) are all around 76% (molar content). Thus, with similar compositions, the only differences between these catalysts is the distribution of cocatalysts, which should be responsible for the difference of activities.
The charge separation can be monitored by time-resolved photoluminescence (TR-PL) spectroscopy (Fig. 3b). Electrons and holes with long lifetimes would show slow PL decay, indicating efficient charge separation.14,43–46 The decay curves are obtained by fitting the observed data (the dots in Fig. 3b) according to the extended exponential function47
The fractional contribution fi of each decay component was estimated by
In particular, in our research, to reflect the situation of charge recombination during the reaction, catalysts are dispersed in water and the measurement was performed in air. The samples were excited at 355 nm, and the photoluminescence was monitored at 480 nm. The observed data points are indicated by dots, while the fitting curve are shown as smooth curves in Fig. 3b. The results of fitting parameters such as α and τ are summarized in Table 1.
| Catalysts | Pre-exponential factors | Decay life timeb/ns | Fractional contribution | ||||
|---|---|---|---|---|---|---|---|
| α 1 | α 2 | τ 1 | τ 2 | 〈τ〉 | f 1 | f 2 | |
| a The double exponential fitting (n = 2) was used according to the shape of decay curves. b τ 1 and τ2 are short and long PL lifetimes, respectively. The double exponential PL decay curve suggests that the two recombination processes exist. The fast component of the exponential decay of the TR-PL could be correlated with the band-to-band transition in the high injection regime and the slow component is due to the recombination of minority carriers.48 | |||||||
| T-MSs | 0.54 | 0.46 | 0.42 | 2.52 | 2.18 | 0.16 | 0.84 |
| MT-MSs | 0.53 | 0.47 | 0.46 | 2.59 | 2.24 | 0.17 | 0.83 |
| T/M/C3.21P-MSs | 0.52 | 0.48 | 0.49 | 3.03 | 2.65 | 0.15 | 0.85 |
| MTC3.17P-MSs | 0.35 | 0.65 | 0.63 | 3.94 | 3.68 | 0.08 | 0.92 |
As shown in Fig. 3b and Table 1, T-MSs exhibit the fastest PL decay (Fig. 3b, curve 1), indicating the most severe charge recombination.14,43–46 The MT-MSs show a slower PL decay (Fig. 3b, curve 2), suggesting the enhancement of charge separation after the loading of MnOx. The decay time is further prolonged after the selective loading of the CuPt alloy on the outer surface (Fig. 3b, curve 4), indicating a better inhibition of charge recombination. In addition, T/M/C3.21P-MSs (Fig. 3b, curve 3) with randomly distributed cocatalysts show a faster PL decay than MTC3.17P-MSs, confirming the critical role of spatial separation of catalysts (Cu contents of T/M/C3.21P-MSs and MTC3.17P-MSs are very close, and thus the difference between Cu contents is negligible). To further explore charge recombination, steady state fluorescence (PL) spectra were adopted with the wavelength from 280 to 750 nm. The excitation of catalysts with monochromatic light will lead to fluorescence as a result of the recombination of photogenerated charges. Thus, more severe charge recombination will result in stronger fluorescence intensity. The results show that the fluorescence intensity is decreased following the sequence of T-MSs > MT-MSs > T/M/C3.21P-MSs > MTC3.17P-MSs (Fig. S15†), supporting the conclusion that structures with separated cocatalysts will enhance the charge separation. The enhanced charge separation can be attributed to the spatially separated MnOx and CuPt alloy, which act as hole and electron traps to drive different charge carriers to flow in different directions. The photocatalytic CRR activity of every catalyst was detected in the solvent of KHCO3 (0.1 M, to enhance the solubility of CO2) and Na2SO3 (0.1 M, acting as a hole sacrificial agent) aqueous solution under AM 1.5G illumination. As a hole sacrificial agent, Na2SO3 can rapidly eliminate holes to ensure the smooth proceeding of CO2 reduction reactions driven by electrons. In the contrastive experiment with the absence of Na2SO3 over MTC3.17P-MSs, the generation of both CO and H2 is obviously reduced by about 80% (Fig. S16†), indicating the significant role of Na2SO3. The results of the photocatalytic CRR show increased activities (Fig. 3c) following the same trend of increasing charge separation (the trend of activity: MTC3.17P-MSs > T/M/C3.21P-MSs > MT-MSs > T-MSs). To reflect the conversion efficiency from solar to chemical energy, the overall conversion yields (η)49 were supplied based on the generation rate of the product, irradiation intensity of the light source and the change of Gibbs free energy during the reaction (see section 5 of methods in the ESI†). The η values (ref. 49) of MTC3.17P-MSs, T/M/C3.21P-MSs, MT-MSs and T-MSs are calculated to be 0.108%, 0.065%, 0.036% and 0.018%, respectively, which also coincide with the trend of charge separation. The best η of 0.108% over MTC3.17P-MSs is higher than those of general oxide and sulfide based catalysts (generally about 0.006–0.042% (ref. 50 and 51)). Given the similar Cu content (ca. 76%) in both structures with separated and randomly loaded cocatalysts, the influence of surface reactions can be ruled out, and thus the enhanced activity can be attributed to the improvement of charge separation. additionally, compared with oxidative outer surfaces (C3.12PTM-MSs), reductive outer surfaces (MTC3.17P-MSs) show obvious advantages in the photocatalytic CRR (Fig. 3c). During the reaction, the influences of carbon contamination should be ruled out. To verify that carbon in products comes from CO2 instead of possible carbon containing contaminants, we design a contrastive experiment which was performed without the injection of CO2 with other situations unchanged over MTC3.17P-MSs. The results show that (Fig. S16†) without CO2 injection, negligible carbon containing products can be detected, indicating that carbon in products originates from CO2.
The activity and composition of syngas production can also be influenced by the Cu content of the CuPt alloy (Fig. 3d). With a low Cu content (MTC0.31P-MSs and MTC1.13P-MSs), H2 is the main product, while the production of CO is low (12.42 μmol h−1 g−1 and 23.93 μmol h−1 g−1, respectively). η values (ref. 49) for MTC0.31P-MSs and MTC1.13P-MSs are calculated to be 0.016% and 0.031%, respectively. With a high Cu content (MTC8.72P-MSs), the catalyst also exhibits relatively low activity for CO (13.16 μmol h−1 g−1) with an η of 0.017%. Higher CO activity was achieved with a moderate Cu content (MTC3.17P-MSs) (84.18 μmol h−1 g−1) with an η of 0.108%. A 0.08 g catalyst was used in the photocatalytic reaction, and the obtained production evolution rate without normalization by catalyst weight shows the same trend over various catalysts (Fig. S17 and Table S1†). The desirable CO/H2 ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (ref. 8) is also obtained when using MTC3.17P-MSs, and it can be concluded that the CO/H2 ratio can be adjusted in a large range between 1
2 (ref. 8) is also obtained when using MTC3.17P-MSs, and it can be concluded that the CO/H2 ratio can be adjusted in a large range between 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 and 1
14 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 by changing the Cu content. To investigate the stability of the catalysts, photocatalytic reactions were repeated 4 times under similar conditions within 12 h. The results show that the generation of products decreases by no more than 10% over every catalyst for either CO or H2 (Fig. S18†), indicating that MTCP-MSs remain stable during the photocatalytic reaction. The morphology of MTC3.17P-MSs can also be maintained after the photocatalytic reaction (Fig. S19†). The enhancement of activity and the control of the CO/H2 ratio are attributed to the balance of every elementary process. With different Cu contents, the surface composition and properties of CuPt cocatalysts are changed, which directly affect the adsorption energy and reaction barrier for all reactants, intermediates and products.52 Only with an appropriate ratio of Cu/Pt can we obtain the optimal reaction process and achieve the desirable product, which will be elaborately discussed with the help of DFT calculations (see Section 7 of methods in the ESI†).
2 by changing the Cu content. To investigate the stability of the catalysts, photocatalytic reactions were repeated 4 times under similar conditions within 12 h. The results show that the generation of products decreases by no more than 10% over every catalyst for either CO or H2 (Fig. S18†), indicating that MTCP-MSs remain stable during the photocatalytic reaction. The morphology of MTC3.17P-MSs can also be maintained after the photocatalytic reaction (Fig. S19†). The enhancement of activity and the control of the CO/H2 ratio are attributed to the balance of every elementary process. With different Cu contents, the surface composition and properties of CuPt cocatalysts are changed, which directly affect the adsorption energy and reaction barrier for all reactants, intermediates and products.52 Only with an appropriate ratio of Cu/Pt can we obtain the optimal reaction process and achieve the desirable product, which will be elaborately discussed with the help of DFT calculations (see Section 7 of methods in the ESI†).
The DFT models of CuPt alloys are established based on similar Cu contents (CuPt3, CuPt, Cu3Pt and Cu7Pt, respectively) (Fig. S20 and Table S2†). According to previous research,49,53 the formation of CO from the photocatalytic CRR mainly occurs through the carboxyl process, which can also be proved by the successful detection of adsorbed carboxyl groups (COOH*) during the reaction by using an in situ infrared spectrometer (Section 6 of methods in the ESI and Fig. S21†). The carboxyl process can be mainly divided into the following three steps:49,53 (1) the activation of carbon dioxide (CO2 + H+ + e− → COOH*) (Fig. 4a and b), (2) the removal of the hydroxyl radical (COOH* + H+ + e− → CO* + H2O, where CO* stands for the adsorbed carbon monoxide) and (3) the desorption of carbon monoxide (CO* → CO). Unlike step 2, steps 1 and 3 are endothermic processes (Fig. 4b), which are thus dominant for the overall activity. Our previous study has shown that the local structure of Pt in the CuPt alloy has a strong effect on the CO* binding strength.52 With the increase of surface Pt, the binding strength of CO* is weakened, while subsurface Pt behaves oppositely. Additional calculations indicate a similar trend for COOH* adsorption. Genetic algorithm based global optimization indicates that complete Pt skins exist over the CuPt alloy (111) surface until the Pt/Cu ratio is lower than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Thus, the weakest adsorption of CO* and COOH* over Pt sites has been observed on CuPt(111) (a thermodynamically stable facet created by the final calcination during synthesis, which can be observed in Fig. S9a†). Strong CO* binding will inhibit its desorption (step 3)54 and thus limits the CO formation rate, while too weak binding destabilizes COOH* and thus suppresses the first hydrogenation step (step 1). Therefore, suitable CO* and COOH* binding strength is needed to achieve the best performance for CO2 reduction. In addition, DFT shows that the binding energy (defined in Section 7 of methods in the ESI†) of CO on Pt, CuPt3, CuPt, Cu3Pt and Cu7Pt is 1.32, 1.05, 0.75, 1.18 and 1.02, respectively, indicating that the adsorption of CO on the CuPt alloy is weakened. Thus, the poisoning of Pt can be weakened by the introduction of Cu. The results of photocatalysis (Fig. 3d) also indicate that increased Cu content will inhibit the competitive H2 evolution reaction (HER). By investigating the interaction between Cu and Pt based on DFT and XPS, we found that the addition of Cu will enrich the electron density around Pt, which results in a greater energy barrier to inhibit the HER (Fig. S23†).55–59
1. Thus, the weakest adsorption of CO* and COOH* over Pt sites has been observed on CuPt(111) (a thermodynamically stable facet created by the final calcination during synthesis, which can be observed in Fig. S9a†). Strong CO* binding will inhibit its desorption (step 3)54 and thus limits the CO formation rate, while too weak binding destabilizes COOH* and thus suppresses the first hydrogenation step (step 1). Therefore, suitable CO* and COOH* binding strength is needed to achieve the best performance for CO2 reduction. In addition, DFT shows that the binding energy (defined in Section 7 of methods in the ESI†) of CO on Pt, CuPt3, CuPt, Cu3Pt and Cu7Pt is 1.32, 1.05, 0.75, 1.18 and 1.02, respectively, indicating that the adsorption of CO on the CuPt alloy is weakened. Thus, the poisoning of Pt can be weakened by the introduction of Cu. The results of photocatalysis (Fig. 3d) also indicate that increased Cu content will inhibit the competitive H2 evolution reaction (HER). By investigating the interaction between Cu and Pt based on DFT and XPS, we found that the addition of Cu will enrich the electron density around Pt, which results in a greater energy barrier to inhibit the HER (Fig. S23†).55–59
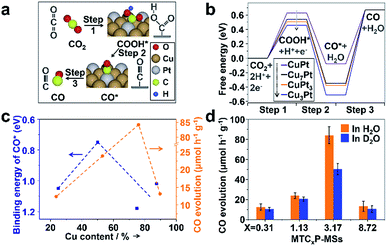 | ||
| Fig. 4 (a) The main processes and intermediate species for CO2 reduction to CO. (b) The calculated free energy of the main intermediate species in every step. Models of every steps over each catalyst are shown in Fig. S22.† (c) The calculated binding energy of CO* and the overall activity for CO generation over each catalyst. (d) CO evolution rates of each catalyst in H2O and D2O. The rate ratios of r(H2O)/r(D2O) over MTC0.31P-MSs, MTC1.13P-MSs, MTC3.17P-MSs and MTC8.72P-MSs are 1.20, 1.16, 1.67 and 1.26, respectively, suggesting that the photocatalytic activity over MTC8.72P-MSs are greatly affected by D2O. | ||
Specifically, with increasing Cu content in our catalysts, the volcano curve of activity is well consistent with the volcano curve of CO* binding energy in Fig. 4c, suggesting that the enhanced activity over the optimal CuPt alloy is mainly caused by the weakening of CO* binding energy. It should be noted that there is a shift of peak positions between the two volcano curves. The shift is mainly caused by the un-uniform distribution of the Pt/Cu ratio in the synthesized nanoparticles, and the influence of COOH* formation. To be more elaborate, a kinetic isotope experiment was performed to investigate the shifted peak position. Interestingly, only MTC3.17P-MSs show a notable kinetic isotope effect after replacing H2O with D2O (Fig. 4d), indicating that the COOH* formation step influences the whole conversion only over MTC3.17P-MSs because hydrogen element only takes part in step 1 instead of step 3, and thus the isotope of hydrogen can only affect step 1. Less stable COOH* was accompanied by weaker binding of CO* (Fig. 4b), consistent with the trend analysis shown in Fig. 4c. Therefore, the different activity is mainly determined by the energy of CO*, which is affected by the stability of COOH*.
Conclusions
In summary, to overcome the main limitations of syngas production by the photocatalytic CRR (unsuitable CO/H2 ratio and serious charge recombination), we construct a structure with spatially separated cocatalysts and firstly introduced a reductive outer surface to avoid back and cross reactions. The CO/H2 ratio can be adjusted in a wide range including the desirable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 by changing the component of reduction cocatalysts. Furthermore, with the help of DFT calculations and kinetic experiments, we disclose the diversity in activity mainly determined by the energy of CO* desorption and CO2 activation. Under optimal conditions, the evolution rate of CO reaches 84.2 μmol h−1 g−1 and the overall conversion yield reaches 0.108%, which is higher than those of traditional oxide and sulfide based catalysts (generally about 0.006–0.042%).49–51 This work opens up opportunities to develop sustainable and carbon neutral syngas production from the reduction of CO2 and H2O using solar energy with rationally designed heterostructures.
2 by changing the component of reduction cocatalysts. Furthermore, with the help of DFT calculations and kinetic experiments, we disclose the diversity in activity mainly determined by the energy of CO* desorption and CO2 activation. Under optimal conditions, the evolution rate of CO reaches 84.2 μmol h−1 g−1 and the overall conversion yield reaches 0.108%, which is higher than those of traditional oxide and sulfide based catalysts (generally about 0.006–0.042%).49–51 This work opens up opportunities to develop sustainable and carbon neutral syngas production from the reduction of CO2 and H2O using solar energy with rationally designed heterostructures.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the National Key R&D Program of China (2016YFB0600901), the National Natural Science Foundation of China (21525626, U1463205, and 21722608), and the Program of Introducing Talents of Discipline to Universities (B06006) for financial support.Notes and references
- Y. Fang and X. Wang, Chem. Commun., 2018, 54, 5674–5687 RSC.
- A. Manzi, T. Simon, C. Sonnleitner, M. Doblinger, R. Wyrwich, O. Stern, J. K. Stolarczyk and J. Feldmann, J. Am. Chem. Soc., 2015, 137, 14007–14010 CrossRef PubMed.
- G. A. Olah, G. K. Prakash and A. Goeppert, J. Am. Chem. Soc., 2011, 133, 12881–12898 CrossRef PubMed.
- S. Wang, B. Y. Guan and X. W. Lou, Energy Environ. Sci., 2018, 11, 306–310 Search PubMed.
- S. I. In, D. D. Vaughn 2nd and R. E. Schaak, Angew. Chem., Int. Ed., 2012, 51, 3915–3918 CrossRef PubMed.
- S. Wang, B. Y. Guan and X. W. D. Lou, J. Am. Chem. Soc., 2018, 140, 5037–5040 CrossRef PubMed.
- W. Tu, Y. Zhou, Q. Liu, Z. Tian, J. Gao, X. Chen, H. Zhang, J. Liu and Z. Zou, Adv. Funct. Mater., 2012, 22, 1215–1221 CrossRef.
- S. Chu, S. Fan, Y. Wang, D. Rossouw, Y. Wang, G. A. Botton and Z. Mi, Angew. Chem., Int. Ed., 2016, 55, 14262–14266 CrossRef PubMed.
- X. Zhao, B. Luo, R. Long, C. Wang and Y. Xiong, J. Mater. Chem. A, 2015, 3, 4134–4138 Search PubMed.
- Y. Hou, F. Zuo, A. Dagg and P. Feng, Nano Lett., 2012, 12, 6464–6473 CrossRef PubMed.
- X. Chang, T. Wang, P. Zhang, J. Zhang, A. Li and J. Gong, J. Am. Chem. Soc., 2015, 137, 8356–8359 CrossRef PubMed.
- J. Liu, X. Yu, Q. Liu, R. Liu, X. Shang, S. Zhang, W. Li, W. Zheng, G. Zhang, H. Cao and Z. Gu, Appl. Catal. B Environ., 2014, 158–159, 296–300 CrossRef.
- R. Li, F. Zhang, D. Wang, J. Yang, M. Li, J. Zhu, X. Zhou, H. Han and C. Li, Nat. Commun., 2013, 4, 1432 CrossRef PubMed.
- A. Li, X. Chang, Z. Huang, C. Li, Y. Wei, L. Zhang, T. Wang and J. Gong, Angew. Chem., Int. Ed., 2016, 55, 13734–13738 CrossRef PubMed.
- S. Obregón and G. Colón, Appl. Catal. B Environ., 2014, 144, 775–782 CrossRef.
- H. Yan, J. Yang, G. Ma, G. Wu, X. Zong, Z. Lei, J. Shi and C. Li, J. Catal., 2009, 266, 165–168 CrossRef.
- A. Li, T. Wang, X. Chang, W. Cai, P. Zhang, J. Zhang and J. Gong, Chem. Sci., 2016, 7, 890–895 RSC.
- L. Huang, X. Wang, J. Yang, G. Liu, J. Han and C. Li, J. Phys. Chem. C, 2013, 117, 11584–11591 Search PubMed.
- Y. Qu and X. Duan, Chem. Soc. Rev., 2013, 42, 2568–2580 RSC.
- J. Zhang, Z. Yu, Z. Gao, H. Ge, S. Zhao, C. Chen, S. Chen, X. Tong, M. Wang, Z. Zheng and Y. Qin, Angew. Chem., Int. Ed., 2017, 56, 816–820 CrossRef PubMed.
- D. Zheng, X. N. Cao and X. Wang, Angew. Chem., Int. Ed., 2016, 55, 11512–11516 CrossRef PubMed.
- D. Wang, T. Hisatomi, T. Takata, C. Pan, M. Katayama, J. Kubota and K. Domen, Angew. Chem., Int. Ed., 2013, 52, 11252–11256 CrossRef PubMed.
- Z. Wang, W. Wu, Q. Xu, G. Li, S. Liu, X. Jia, Y. Qin and Z. L. Wang, Nano Energy, 2017, 38, 518–525 CrossRef.
- J. Zhang, T. Wang, X. Chang, A. Li and J. Gong, Chem. Sci., 2016, 7, 6381–6386 RSC.
- W. Zhu, L. Zhang, P. Yang, X. Chang, H. Dong, A. Li, C. Hu, Z. Huang, Z. J. Zhao and J. Gong, Small, 2018, 14, 1703314 CrossRef PubMed.
- J. B. Joo, Q. Zhang, I. Lee, M. Dahl, F. Zaera and Y. Yin, Adv. Funct. Mater., 2012, 22, 166–174 CrossRef.
- A. Li, P. Zhang, X. Chang, W. Cai, T. Wang and J. Gong, Small, 2015, 11, 1892–1899 CrossRef PubMed.
- X. Wang, Y. Zhao, K. Mølhave and H. Sun, Nanomaterials, 2017, 7, 382 CrossRef PubMed.
- S. Farsinezhad, H. Sharma and K. Shankar, Phys. Chem. Chem. Phys., 2015, 17, 29723–29733 RSC.
- Z. Luo, C. Li, S. Liu, T. Wang and J. Gong, Chem. Sci., 2017, 8, 91–100 RSC.
- K. S. Sing, Pure Appl. Chem., 1985, 57, 603–619 CrossRef.
- D. Chen, L. Cao, F. Huang, P. Imperia, Y.-B. Cheng and R. A. Caruso, J. Am. Chem. Soc., 2010, 132, 4438–4444 CrossRef PubMed.
- S. Wang, B. Y. Guan, Y. Lu and X. W. Lou, J. Am. Chem. Soc., 2017, 139, 17305–17308 CrossRef PubMed.
- C. Li, A. Li, Z. Luo, J. Zhang, X. Chang, Z. Huang, T. Wang and J. Gong, Angew. Chem., Int. Ed., 2017, 56, 4150–4155 CrossRef PubMed.
- P. Zhang, A. Li and J. Gong, Particuology, 2015, 22, 13–23 CrossRef.
- S. Wang, C. Li, T. Wang, P. Zhang, A. Li and J. Gong, J. Mater. Chem. A, 2014, 2, 2885 Search PubMed.
- Z. Huang, Y. Liu, Q. Zhang, X. Chang, A. Li, L. Deng, C. Yi, Y. Yang, N. M. Khashab, J. Gong and Z. Nie, Nat. Commun., 2016, 7, 12147 CrossRef PubMed.
- J. Lu, P. Zhang, A. Li, F. Su, T. Wang, Y. Liu and J. Gong, Chem. Commun., 2013, 49, 5817–5819 RSC.
- L. Yu, H. Hu, H. B. Wu and X. W. D. Lou, Adv. Mater., 2017, 29, 1604563 CrossRef PubMed.
- L. Yu, H. B. Wu and X. W. D. Lou, Acc. Chem. Res., 2017, 50, 293–301 CrossRef PubMed.
- S. Wang, B. Y. Guan, L. Yu and X. W. D. Lou, Adv. Mater., 2017, 29, 1702724 CrossRef PubMed.
- A. J. Haes and R. P. Van Duyne, J. Am. Chem. Soc., 2002, 124, 10596–10604 CrossRef PubMed.
- S. D. Stranks, G. E. Eperon, G. Grancini, C. Menelaou, M. J. Alcocer, T. Leijtens, L. M. Herz, A. Petrozza and H. J. Snaith, Science, 2013, 342, 341–344 CrossRef PubMed.
- J. Zhang, M. Zhang, R. Q. Sun and X. Wang, Angew. Chem., Int. Ed., 2012, 51, 10145–10149 CrossRef PubMed.
- G. E. Eperon, S. D. Stranks, C. Menelaou, M. B. Johnston, L. M. Herz and H. J. Snaith, Energy Environ. Sci., 2014, 7, 982–988 Search PubMed.
- L. Liu, Z. Ji, W. Zou, X. Gu, Y. Deng, F. Gao, C. Tang and L. Dong, ACS Catal., 2013, 3, 2052–2061 CrossRef.
- K. Das and S. De, J. Lumin., 2009, 129, 1015–1022 CrossRef.
- S. Shirakata and T. Nakada, Thin Solid Films, 2007, 515, 6151–6154 CrossRef.
- X. Jiao, X. Li, X. Jin, Y. Sun, J. Xu, L. Liang, H. Ju, J. Zhu, Y. Pan, W. Yan, Y. Lin and Y. Xie, J. Am. Chem. Soc., 2017, 139, 18044–18051 CrossRef PubMed.
- L. Liu, C. Zhao, D. Pitts, H. Zhao and Y. Li, Catal. Sci. Technol., 2014, 4, 1539–1546 Search PubMed.
- L. Liu, C. Zhao and Y. Li, J. Phys. Chem. C, 2012, 116, 7904–7912 Search PubMed.
- Z. J. Zhao, R. Mu, X. Wang and J. Gong, Langmuir, 2017, 33, 8700–8706 CrossRef PubMed.
- S. Kattel, W. Yu, X. Yang, B. Yan, Y. Huang, W. Wan, P. Liu and J. G. Chen, Angew. Chem., Int. Ed., 2016, 55, 7968–7973 CrossRef PubMed.
- J. Liu, F. R. Lucci, M. Yang, S. Lee, M. D. Marcinkowski, A. J. Therrien, C. T. Williams, E. C. Sykes and M. Flytzani-Stephanopoulos, J. Am. Chem. Soc., 2016, 138, 6396–6399 CrossRef PubMed.
- Z. W. Seh, J. Kibsgaard, C. F. Dickens, I. Chorkendorff, J. K. Norskov and T. F. Jaramillo, Science, 2017, 355 Search PubMed.
- S. Zhang, P. Kang and T. J. Meyer, J. Am. Chem. Soc., 2014, 136, 1734–1737 CrossRef PubMed.
- A. J. Medford, A. C. Lausche, F. Abild-Pedersen, B. Temel, N. C. Schjødt, J. K. Nørskov and F. Studt, Top. Catal., 2013, 57, 135–142 CrossRef.
- M. D. Marcinkowski, M. T. Darby, J. Liu, J. M. Wimble, F. R. Lucci, S. Lee, A. Michaelides, M. Flytzani-Stephanopoulos, M. Stamatakis and E. C. H. Sykes, Nat. Chem., 2018, 10, 325–332 CrossRef PubMed.
- Z. Chen, Y. Song, J. Cai, X. Zheng, D. Han, Y. Wu, Y. Zang, S. Niu, Y. Liu, J. Zhu, X. Liu and G. Wang, Angew. Chem., Int. Ed., 2018, 57, 5076–5080 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8sc01812j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |