Open Access Article
Open Access ArticleNear infrared excitation and emission in rare earth MOFs via encapsulation of organic dyes†
Chong
Liu
 a,
Svetlana V.
Eliseeva
a,
Svetlana V.
Eliseeva
 b,
Tian-Yi
Luo
b,
Tian-Yi
Luo
 a,
Patrick F.
Muldoon
a,
Stéphane
Petoud
a,
Patrick F.
Muldoon
a,
Stéphane
Petoud
 *ab and
Nathaniel L.
Rosi
*ab and
Nathaniel L.
Rosi
 *a
*a
aDepartment of Chemistry, University of Pittsburgh, Pittsburgh, PA 15260, USA. E-mail: nrosi@pitt.edu
bCentre de Biophysique Moléculaire, CNRS UPR 4301, 45071 Orléans, France. E-mail: stephane.petoud@inserm.fr
First published on 27th August 2018
Abstract
We successfully demonstrate that metal–organic frameworks (MOFs) can be designed to be excited and emit within the biological diagnostic window (650–1450 nm). An isoreticular series of anionic rare earth MOFs with fcu topology was synthesized using 10 different rare earth elements (Y3+, Eu3+, Gd3+, Tb3+, Dy3+, Ho3+, Er3+, Tm3+, Yb3+, Lu3+) and common linear ditopic ligands. Five different cationic dye molecules were incorporated into the MOFs via ion exchange. When LDS 750, which exhibits low-energy absorption in the range 450–770 nm, is loaded into an Yb3+-MOF, it can be used as an antenna to sensitize the near-infrared (NIR) emission of Yb3+ centred at 980 nm.
Introduction
Biological tissues exhibit low autofluorescence and low absorption in the so-called biological diagnostic window (650–1450 nm). Biological imaging agents that both absorb and emit in this biological window can allow achievement of (i) higher sensitivity because their signals can be easily discriminated from the background and (ii) deeper tissue penetration due to minimal or no overlap of their excitation window with tissue absorption.1–3Trivalent rare earth (RE3+) based compounds emitting in the near-infrared (NIR) offer complementary advantages for biological imaging applications.4 RE3+ emitters exhibit narrow emission bands, long luminescence lifetimes (μs-ms range), and high resistance to photobleaching. In addition, their emission signals are not affected by environmental factors and experimental conditions. However, the design of RE3+ compounds with intense luminescence can be challenging because: (i) the direct excitation of RE3+ is inefficient due to the low absorbance of free RE3+ as most of the f → f transitions are forbidden by the Laporte rule; and (ii) overtones of C–H, O–H, and N–H vibrations can cause non-radiative deactivation and decreased intensities of RE3+ signals. To overcome these challenges, RE3+ electronic states must be indirectly populated by a photonic converter, such as an organic chromophore with high extinction coefficient, in a sensitizing process named the ‘antenna effect’.5,6 Antenna molecules possessing suitable electronic structures must be placed in sufficiently close proximity to RE3+ to provide efficient sensitization through energy transfer, as well as to ensure good protection against the vibrational overtones from external molecules. For biological imaging applications, especially in vivo, a combination of both NIR-emitting RE3+ and antennae that absorb in the lower energy range (visible or NIR) would be necessary.
We have been developing RE3+-based metal–organic framework (MOF) materials to study and harness their luminescent properties in the field of biological applications, motivated by the ultimate goal of in vivo imaging.7–11 MOFs are intriguing materials for harnessing RE3+ luminescence because they can provide: (i) a high density arrangement of RE3+ emitters and sensitizing species in proximity to one another; (ii) macroscopic entities and stable coordination spheres with spatially constrained RE3+ and ligands that effectively decrease undesirable interactions with extraneous species; and (iii) a highly diverse collection of structures with functional handles that can be exploited for a broad range of applications.12–14
Specifically, our research in this area has focused on the exploration of different strategies for optimizing the sensitization of RE3+-based luminescent MOFs, with the aim of achieving excitation in the biological diagnostic window. We have prepared a NIR-emitting Yb3+-based in vitro imaging agent using nano-sized MOFs comprising phenylene-based antennae and Yb3+.10 More recently, we established a new strategy for expanding the π conjugation of the MOF linkers via covalent postsynthetic modification in order to red-shift the antennae absorption and thus the RE3+ excitation wavelengths.11 Herein, we report a new strategy to red-shift the excitation wavelength of RE3+ MOFs via incorporation of organic molecular chromophores into MOF channels.15–17
Results and discussion
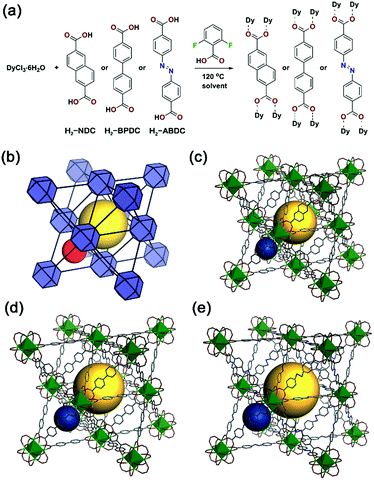
An isoreticular family of anionic fcu MOFs based on RE3+6 clusters was selected for the present study.18 Pioneered by Eddaoudi and co-workers, these MOFs are typically constructed using monofluorinated dicarboxylates, which direct the formation of the RE63+ clusters.19–22 Here, we used 2,6-difluorobenzoic acid (DFBA) as a modulator to effect formation of the clusters and analogous fcu MOFs using non-fluorinated ligands including 2,6-naphthalenedicarboxylic acid (H2-NDC), 1,1′-biphenyl-4,4′-dicarboxylic acid (H2-BPDC), and 4,4′-azobenzenedicarboxylic acid (H2-ABDC) (Fig. 1), namely Dy3+-NDC, Dy3+-BPDC, and Dy3+-ABDC. These modulated MOF syntheses yielded large single crystals suitable for single crystal X-ray diffraction (SC-XRD) (Fig. S1†), and structures of the Dy3+-MOFs were successfully determined (Fig. 1c–e). Phase purity of the as-synthesized Dy3+-MOFs was assessed and confirmed by comparing simulated diffraction patterns to those collected via powder X-ray diffraction (PXRD) (Fig. S5†). The chemical formulae of the as-synthesized MOFs were determined by a combination of elemental microanalysis and thermogravimetric analysis (see ESI Section 2.8 for details). It is noteworthy that the as-synthesized MOFs do not contain DFBA modulator, as determined via1H nuclear magnetic resonance (NMR) spectroscopy of dissolved MOF samples (Fig. S9†). Although activation procedures were not optimized, N2 adsorption experiments revealed that Dy3+-NDC, Dy3+-BPDC, and Dy3+-ABDC were each permanently porous (Fig. S10†).DFBA-modulated conditions were found to be compatible with 9 other RE3+ (Y3+, Eu3+, Gd3+, Tb3+, Ho3+, Er3+, Tm3+, Yb3+, Lu3+) in addition to Dy3+. Isostructural MOFs were formed in pure phases, as determined by comparing their respective PXRD patterns with those of Dy3+-MOFs and simulated patterns from SC-XRD structures (Fig. S11–S13†). Such results confirm that the synthesized MOFs are a versatile platform for the development of luminescent materials with different functional properties.
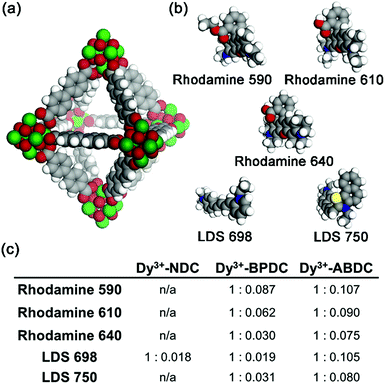
The fcu RE3+-MOFs are anionic with dimethylammonium cations residing in the pores balancing the framework charges (see ESI Section 2.8 for details†). Therefore, it is expected that the native cations can be exchanged with other positively charged species. In addition to the MOF ligands, a broader sensitization of the RE3+ centres could be obtained if cationic dyes absorbing at longer wavelengths were incorporated into the MOF channels. Ion exchange experiments were carried out with Dy3+-NDC, Dy3+-BPDC, and Dy3+-ABDC, each having different pore dimensions, and five cationic dyes (Rhodamine 590, Rhodamine 610, Rhodamine 640, LDS 698, LDS 750). Space filling models of Dy3+-BPDC and the five dyes are shown in Fig. 2a and b. After similar treatment with various dye solutions (Fig. S14†), the MOF crystallinity was retained in all cases, as indicated by PXRD patterns (Fig. S15–S17†). Different degrees of loading (Fig. 2c) were observed in Dy3+-NDC, Dy3+-BPDC, and Dy3+-ABDC, as determined from 1H NMR analysis of the dissolved products (Fig. S18–S31†).
Dye-loaded RE3+-BPDC was chosen for luminescence studies because it could adsorb a quantifiable amount of dye and because the dye molecules remain within the MOF channels after several cycles of washing. In contrast, only trace amounts of dyes could be incorporated into RE3+-NDC and significant quantities of dyes leached out of RE3+-ABDC upon washing, which could be due to unfavourable interactions between the dyes and the RE3+-ABDC MOFs. As previously articulated, having both antennae absorption and RE3+ emission in the biological diagnostic window is ideal for optical imaging experiments and diagnostics on biological materials, especially when carried out in vivo. On the basis of this criterion, LDS 750 was chosen as the sensitizer and was incorporated into Yb3+-BPDC. The ratio of Yb3+ to LDS 750 can be finely controlled by varying the loading condition (e.g. concentration, reaction time), as confirmed by 1H NMR quantification (Fig. S32†) and summarized in Table S4.†
LDS 750@Yb3+-BPDC (Yb3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LDS 750 = 1
LDS 750 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
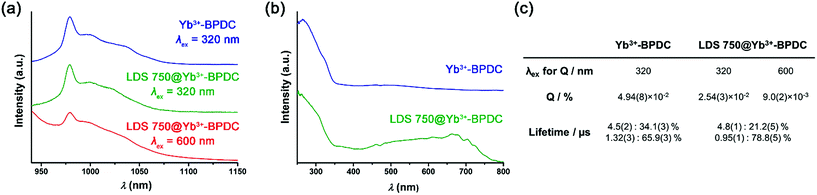
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.003) was then studied by optical spectroscopy. The emission spectrum recorded upon an excitation wavelength corresponding to low energy (600 nm, Fig. 3a, red curve) reveals the presence of a sharp emission band with an apparent maximum at 980 nm that can be attributed to Yb3+ located in the MOF structure. As the Yb3+ cation does not possess any accepting level below 980 nm, the sensitization can only take place through the organic chromophores. LDS 750@Yb3+-BPDC contains two different chromophores that could potentially act as sensitizers of the Yb3+ emission, through the ‘antenna effect’: (i) the BPDC linkers that constitute the MOF scaffold; and (ii) the LDS 750 encapsulated inside the MOF pores. The comparison of the excitation spectra recorded upon monitoring emission at 980 nm of Yb3+-BPDC and LDS 750@Yb3+-BPDC (Fig. 3b) revealed no bands above 600 nm for the former, while a broad and intense excitation band extended up to 800 nm was observed for the latter. Therefore, upon 600 nm excitation of LDS 750@Yb3+-BPDC, only LDS 750 can act as the sensitizer of the characteristic Yb3+ emission. These results confirm the validity of the approach and the creation of a new MOF system capable of sensitizing NIR-emitting RE3+ with an extended range of excitation wavelengths through the selection of encapsulated chromophores.
0.003) was then studied by optical spectroscopy. The emission spectrum recorded upon an excitation wavelength corresponding to low energy (600 nm, Fig. 3a, red curve) reveals the presence of a sharp emission band with an apparent maximum at 980 nm that can be attributed to Yb3+ located in the MOF structure. As the Yb3+ cation does not possess any accepting level below 980 nm, the sensitization can only take place through the organic chromophores. LDS 750@Yb3+-BPDC contains two different chromophores that could potentially act as sensitizers of the Yb3+ emission, through the ‘antenna effect’: (i) the BPDC linkers that constitute the MOF scaffold; and (ii) the LDS 750 encapsulated inside the MOF pores. The comparison of the excitation spectra recorded upon monitoring emission at 980 nm of Yb3+-BPDC and LDS 750@Yb3+-BPDC (Fig. 3b) revealed no bands above 600 nm for the former, while a broad and intense excitation band extended up to 800 nm was observed for the latter. Therefore, upon 600 nm excitation of LDS 750@Yb3+-BPDC, only LDS 750 can act as the sensitizer of the characteristic Yb3+ emission. These results confirm the validity of the approach and the creation of a new MOF system capable of sensitizing NIR-emitting RE3+ with an extended range of excitation wavelengths through the selection of encapsulated chromophores.
It is also worth noting that despite the low amount of LDS 750 located in the MOF pores, it was sufficient to generate an Yb3+ signal that is intense enough to be not only monitored but also quantified. Yb3+-centred quantum yields recorded upon two excitation wavelengths corresponding to low and high energies as well as luminescence lifetimes were collected on both Yb3+-BPDC and LDS 750@Yb3+-BPDC (Fig. 3c) to quantify the effect of LDS 750 inside the MOF. Luminescence lifetime values allow us to obtain information about the environment around Yb3+. A more quenching environment implies a shorter luminescence lifetime. The results shown in Fig. 3c can be rationalized by the presence of two different environments around the Yb3+: the longer luminescence lifetime value corresponds to the Yb3+ located at the core of the MOF crystals that may benefit from a higher level of protection from the environment (i.e. solvent molecules). The second, smaller lifetime component can be attributed to the Yb3+ that are present at or near the exterior of the MOF crystals which are more exposed to sources of non-radiative deactivation. It should be noted that the incorporation of LDS 750 inside the pores of Yb3+-BPDC has no significant impact on the Yb3+ luminescence lifetimes (i.e. the longer lifetime component remains the same, within experimental error), while the shorter lifetime component decreases by 1.4 times. Such results point out the similarities of the coordination environments around the Yb3+ in both MOFs.
Yb3+-centred quantum yield (Q) values determined under excitation at 320 and 600 nm (Fig. 3a) reflect quantitatively the combination of the protection of Yb3+ against non-radiative deactivations and the sensitization efficiency of the chromophores (Fig. 3c). We have previously concluded from the luminescence lifetime measurements that the environments around Yb3+ are not strongly affected by the presence of LDS 750. Quantum yields will therefore be mainly dependent on the sensitization efficiencies of the BPDC linker and LDS 750. Upon excitation at 320 nm (BPDC chromophore), the quantum yield of LDS 750@Yb3+-BPDC is lower by 1.9 times compared to the value recorded for the Yb3+-BPDC. Therefore, the incorporation of LDS 750 inside the pores of Yb3+-BPDC decreases the sensitization efficiency of BPDC towards Yb3+ emission. Quantum yield value of LDS 750@Yb3+-BPDC recorded upon excitation at 600 nm (LDS 750 chromophore) decreases to 9.0(2) × 10−3%, suggesting that the sensitization efficiency of LDS 750 is lower than that of BPDC. Inefficient energy transfer from LDS 750 to Yb3+ is further supported by the observation of residual broad-band emission along with sharp Yb3+ 2F5/2 → 2F7/2 transition centred at 980 nm in the emission spectrum of the LDS 750@Yb3+-BPDC upon 600 nm excitation (Fig. 3a, red curve). Nevertheless, we demonstrated that by using our approach, the excitation wavelength of Yb3+-BPDC MOF can be tuned by straightforward incorporation of an appropriate chromophore through non-covalent interactions.
Conclusions
This work constitutes a major achievement towards obtaining RE3+-based NIR-emitting MOFs with controllable luminescent properties. The originality of this approach lies in the non-covalent encapsulation of chromophores in the pores for the sensitization of RE3+ that are part of the MOF scaffold, which broadens the range of chromophore selection for RE3+ sensitization in systems where covalent or dative attachment is difficult or impossible. We have shown that such sensitization was sufficiently efficient with even a small amount of sensitizer to generate a NIR signal from Yb3+ that can be both monitored and quantified. Using this strategy, we have been able to create a MOF that not only emits, but also absorbs in the biological window, which represents a significant breakthrough in the realm of biological imaging with MOFs: thus far, all MOFs, including nanoMOFs that we have tested, absorb in the UV or high energy visible range. Excitation light with such low energy is favourable to biological systems as it minimizes interaction with sensitive samples.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The MOF synthesis and development work in this project received partial support from the Defense Threat Reduction Agency-Joint Science and Technology Office for Chemical and Biological Defense Basic Research (Grant no. HDTRA1-16-1-0044, NLR). In addition, this work is partially supported by La Ligue Contre le Cancer, La Region Centre, Agence Nationale de la Recherche (NIRA ANR-13-BS08-0011 and LUMZIF ANR-12-BS07-0012). S. P. acknowledges support from the Institut National de la Santé et de la Recherche Médicale (INSERM). The authors thank the Petersen Nano Fabrication and Characterization Facility at the University of Pittsburgh for access to PXRD instrumentation. The authors thank Dr Kristy Gogick for helpful discussion and Ms. Disi Wang for figure preparation.References
- R. Weissleder, Nat. Biotechnol., 2001, 19, 316 CrossRef PubMed.
- V. J. Pansare, S. Hejazi, W. J. Faenza and R. K. Prud'homme, Chem. Mater., 2012, 24, 812–827 CrossRef PubMed.
- I. Martinić, S. V. Eliseeva and S. Petoud, J. Lumin., 2017, 189, 19–43 CrossRef.
- S. V. Eliseeva and J.-C. G. Bünzli, Chem. Soc. Rev., 2010, 39, 189–227 RSC.
- S. I. Weissman, J. Chem. Phys., 1942, 10, 214–217 CrossRef.
- H. Uh and S. Petoud, C. R. Chim., 2010, 13, 668–680 CrossRef.
- K. A. White, D. A. Chengelis, M. Zeller, S. J. Geib, J. Szakos, S. Petoud and N. L. Rosi, Chem. Commun., 2009, 4506–4508, 10.1039/b909658b.
- K. A. White, D. A. Chengelis, K. A. Gogick, J. Stehman, N. L. Rosi and S. Petoud, J. Am. Chem. Soc., 2009, 131, 18069–18071 CrossRef PubMed.
- J. An, C. M. Shade, D. A. Chengelis-Czegan, S. Petoud and N. L. Rosi, J. Am. Chem. Soc., 2011, 133, 1220–1223 CrossRef PubMed.
- A. Foucault-Collet, K. A. Gogick, K. A. White, S. Villette, A. Pallier, G. Collet, C. Kieda, T. Li, S. J. Geib, N. L. Rosi and S. Petoud, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 17199–17204 CrossRef PubMed.
- T.-Y. Luo, C. Liu, S. V. Eliseeva, P. F. Muldoon, S. Petoud and N. L. Rosi, J. Am. Chem. Soc., 2017, 139, 9333–9340 CrossRef PubMed.
- J. Della Rocca, D. Liu and W. Lin, Acc. Chem. Res., 2011, 44, 957–968 CrossRef PubMed.
- P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Férey, R. E. Morris and C. Serre, Chem. Rev., 2012, 112, 1232–1268 CrossRef PubMed.
- Y. Cui, Y. Yue, G. Qian and B. Chen, Chem. Rev., 2012, 112, 1126–1162 CrossRef PubMed.
- D. T. de Lill, A. de Bettencourt-Dias and C. L. Cahill, Inorg. Chem., 2007, 46, 3960–3965 CrossRef PubMed.
- C. Yuanjing, S. Ruijing, Y. Jiancan, L. Min, W. Ziqi, W. Chuande, Y. Yu, W. Zhiyu, C. Banglin and Q. Guodong, Adv. Mater., 2015, 27, 1420–1425 CrossRef PubMed.
- X. Lian and B. Yan, RSC Adv., 2016, 6, 11570–11576 RSC.
- D. X. Xue, A. J. Cairns, Y. Belmabkhout, L. Wojtas, Y. Liu, M. H. Alkordi and M. Eddaoudi, J. Am. Chem. Soc., 2013, 135, 7660–7667 CrossRef PubMed.
- V. Guillerm, L. Weselinski, Y. Belmabkhout, A. J. Cairns, V. D'Elia, L. Wojtas, K. Adil and M. Eddaoudi, Nat. Chem., 2014, 6, 673–680 CrossRef PubMed.
- D. Alezi, A. M. P. Peedikakkal, Ł. J. Weseliński, V. Guillerm, Y. Belmabkhout, A. J. Cairns, Z. Chen, Ł. Wojtas and M. Eddaoudi, J. Am. Chem. Soc., 2015, 137, 5421–5430 CrossRef PubMed.
- Y. J. Li, Y. L. Wang and Q. Y. Liu, Inorg. Chem., 2017, 56, 2159–2164 CrossRef PubMed.
- M. L. Gao, W. J. Wang, L. Liu, Z. B. Han, N. Wei, X. M. Cao and D. Q. Yuan, Inorg. Chem., 2017, 56, 511–517 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details, general characterization, X-ray crystallography, photoluminescence studies, additional figures and tables. CCDC 1840493–1840495. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8sc03168a |
| This journal is © The Royal Society of Chemistry 2018 |